Abstract
A role of the nuclear receptor Rev-erbα in the regulation of transcription pathways involving other nuclear receptors is emerging. Indeed, Rev-erbα is a negative regulator of transcription by binding to overlapping response elements shared with various nuclear receptors, including the peroxisome proliferator-activated receptors and the retinoid-related orphan receptor α (RORα). Here, we show that Rev-erbα is expressed in primary human macrophages and that its expression is induced by synthetic ligands for the liver X receptors (LXRs), which control cholesterol homeostasis, inflammation, and the immune response in macrophages. LXRα binds to a specific response element in the human Rev-erbα promoter, thus inducing Rev-erbα transcriptional expression. Interestingly, Rev-erbα does not influence basal or LXR-regulated cholesterol homeostasis. However, Rev-erbα overexpression represses the induction of toll-like receptor (TLR)-4 by LXR agonists, whereas Rev-erbα silencing by short interfering RNA results in enhanced TLR-4 expression upon LXR activation. Electrophoretic mobility shift, chromatin immunoprecipitation, and transient transfection experiments demonstrate that Rev-erbα represses human TLR-4 promoter activity by binding as a monomer to a RevRE site overlapping with the LXR response element site in the TLR-4 promoter. These data identify Rev-erbα as a new LXR target gene, inhibiting LXR-induction of TLR-4 in a negative transcriptional feedback loop, but not cholesterol homeostasis gene expression.
NUCLEAR RECEPTORS (NRs) are transcription factors that translate physiological signals into gene regulation. For a long time, Rev-erbα had no identified ligands and was referred to as an orphan NR (1). Very recently, the porphyrin heme has been identified as a physiological ligand for Rev-erbα (2, 3). Structural analysis of Rev-erbα has revealed a highly hydrophobic surface (due to the absence of helix 12) through which corepressors are recruited. As a consequence, Rev-erbα acts as a negative regulator of transcription after binding either as a monomer to a response element composed of the consensus half-site motif (A/G)GGTCA preceded by an A/T-rich 5′ sequence (RevRE), or as a homodimer to a direct repeat of the core motif spaced by two nucleotides (RevDR-2) (4). Rev-erbα is highly expressed in adipose tissue, skeletal muscle, liver and also in endothelial (EC) and smooth muscle (SMC) cells (5, 6, 7). Recently, the expression of this NR has been reported in murine macrophages and in human peripheral blood mononuclear cells (8, 9). Although its biological function remains largely elusive, Rev-erbα has been identified as a regulator of lipid and glucose metabolism, adipogenesis, muscle physiology, and vascular inflammation (3, 7, 10, 11, 12, 13). In addition, Rev-erbα is a critical regulator of circadian rhythm and a clock target gene, its expression being induced by the Bmal1/Clock heterodimer and inhibited by the Per/Cry complex (14, 15, 16). Interestingly, alterations in circadian rhythmicity have been recently implicated in the dysregulation of many physiological processes including metabolism and inflammation (17, 18).
Because NRs regulate the expression of genes involved in reproduction, development, and metabolism, fine-tuned time- and tissue-specific regulation of their activity is crucial. Ligand-independent regulation mechanisms have been identified, among which cross talk between different NRs (for review see Ref. 19). Such NR cross talk can occur via different mechanisms, including the formation of inactive complexes via protein-protein interaction, competition for coactivators (squelching) or binding to neighboring or overlapping sites in target gene promoters. Rev-erbα has been shown to cross talk with different transcription factors (for review see Ref. 20), often by competitively binding to shared response elements with other NRs, such as the retinoid-related orphan receptor (ROR)α and the peroxisome proliferator-activated receptors (PPARs), resulting in opposite effects on gene transcription (10, 11, 21, 22, 23, 24, 25, 26, 27, 28, 29).
The liver X receptors (LXRα and LXRβ) are NRs activated by oxysterols, which regulate the expression of genes controlling lipid metabolism and inflammation (30). LXRs bind as heterodimers with the retinoid X receptors (RXRs) to specific response elements termed LXR response elements (LXREs) in the promoters of their target genes. LXREs usually consist of a direct repeat of the (A/G)GGTCA motif spaced by four nucleotides (DR-4). LXR activation in macrophages induces the expression of several genes involved in lipid homeostasis including those encoding the ATP-binding cassette transporters (ABC)A1, ABCG1/ABCG4, apolipoprotein (apo) E and the Niemann Pick C proteins (NPC)1/NPC2 (31, 32, 33, 34, 35). In addition to their well-established role as cholesterol sensors, LXRs regulate transcriptional programs involved in macrophage immune responses. Indeed, mice lacking LXRα are highly susceptible to infection with the gram-negative bacteria Salmonella typhimurium and exhibit a defective bacterial clearance due to the loss of regulation of the antiapoptotic factor AIM, a direct target gene of LXRs in murine macrophages (36, 37, 38). In human macrophages, LXR activation also increases the response against bacteria via induction of toll-like receptor (TLR)-4 expression (39). TLR-4 is the receptor of lipopolysaccharide (LPS), a chief pathogen-associated molecular pattern from gram-negative bacteria (40). Activation of the TLR-4 signaling pathway by LPS induces antibacterial effects by secreting cytokines, which recruit and/or activate neighboring cells to eliminate pathogens (40). In addition to an enhanced antibacterial response, LXRs also exert antiinflammatory responses once the inflammatory stimulus is present (41, 42).
Here, we demonstrate that Rev-erbα gene is expressed in human macrophages where its expression is regulated by LPS and cholesterol content. Because LXRs control inflammatory/immune responses and cholesterol homeostasis in macrophages, we investigated whether a potential cross talk between these NRs exists. Interestingly, we identify Rev-erbα as a new LXR target gene in human macrophages. Rev-erbα does not affect macrophage cholesterol homeostasis. However, Rev-erbα acts as a negative regulator of LXR transactivation on TLR-4 expression by binding to the same response element as LXRs. These findings provide evidences for a novel cross talk of Rev-erbα with other NRs and identify Rev-erbα as a molecular link driving a transcriptional feedback loop on select LXR-induced pathways.
RESULTS
Rev-erbα Is Expressed in Primary Human Macrophages and Its Expression Is Regulated by LXR Activation
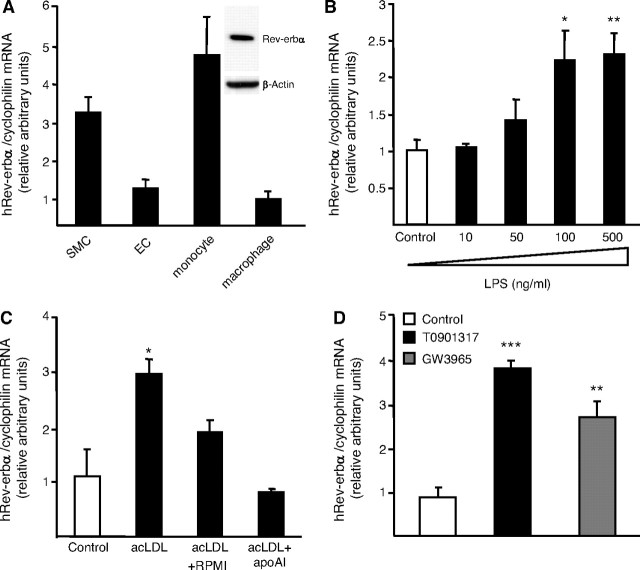
Recently, Rev-erbα has been reported to be expressed in human peripheral blood mononuclear cells (9). Here, we show for the first time Rev-erbα mRNA and protein expression in primary human macrophages (Fig. 1A). As described previously (7), Rev-erbα is also expressed in other cell types of the vascular wall such as SMCs and ECs (Fig. 1A). In addition, Rev-erbα mRNA levels were induced in a dose-dependent manner when macrophages were treated with increasing concentrations of LPS (Fig. 1B). Interestingly, treatment with other proinflammatory stimuli such as TNFα or IL-1β did not influence Rev-erbα expression (data not shown). Rev-erbα mRNA expression was also regulated by macrophage cholesterol content because its expression increased when macrophages were cholesterol-loaded with acetylated LDL (acLDL) and conversely decreased after cholesterol efflux using apoAI (Fig. 1C). Because LXRs are NRs controlling cholesterol homeostasis (31, 32, 33, 34, 35) as well as the LPS response (39) in human macrophages and because Rev-erbα expression is under the control of many NRs (11, 28, 29, 43), we next investigated whether LXR activation affects Rev-erbα expression in primary human macrophages. To this aim, macrophages were treated by either T0901317 (1 μm) or GW3965 (1 μm), two synthetic LXR ligands for 24 h. Both LXR agonists strongly increased Rev-erbα mRNA levels (Fig. 1D) and this regulation occurred in a dose-dependent manner (data not shown). By contrast, Rev-erbα gene expression was not regulated by LXR agonists in mouse macrophages, pointing to a species-specific regulation of Rev-erbα by LXRs (data not shown).
Fig. 1.
Rev-erbα Is Expressed in Primary Human Macrophages and Is Regulated by LPS Stimulation, Cholesterol Content, and LXR Activation
A, Rev-erbα mRNA from human aortic SMCs, HUVECs, primary human monocytes and macrophages were quantified by Q-PCR. Nuclear protein extracts were prepared from human primary macrophages and Rev-erbα and β-actin protein levels were measured by western blot analysis. B, Primary human macrophages were stimulated with increasing concentrations of LPS during 24 h. C, Primary human macrophages were cholesterol-loaded with AcLDL (50 μg/ml) for 24 h and subsequently incubated with RPMI 1640 medium with or without apoAI (10 ng/ml). D, Primary human macrophages were incubated with either T0901317 (1 μm) or GW3965 (1 μm). Rev-erbα mRNA levels were quantified by Q-PCR and normalized to cyclophilin mRNA levels. The range of cycle threshold (Ct) measured in cDNA equivalent to 10 ng RNA extracted from primary human macrophages is 24–30 for Rev-erbα compared with 19–21 for cyclophilin. Results are representative of those obtained from three independent macrophage preparations and are expressed relative to the levels in untreated cells set as 1. Each bar is the mean value ± sd of triplicate determinations. Statistically significant differences between treatments and control are indicated (t test; *, P < 0.05; **, P < 0.01; ***, P < 0.001).
LXRα Binds as a Heterodimer with RXRα to the Human Rev-erbα Promoter
Next, it was investigated whether LXRα directly regulates Rev-erbα transcription. Bio-informatic analysis of the human Rev-erbα gene promoter identified a potential LXR-binding site highly similar to the consensus LXRE (cons LXRE) located between nucleotides −1038 and −1053 from the transcription initiation site (Fig. 2A). EMSAs demonstrated that this Rev-erbα LXRE was able to compete for LXRα/RXRα binding to the consensus LXRE (Fig. 2B, lanes 5–7). In addition, a specific DNA-protein complex was formed when in vitro-synthesized LXRα and RXRα proteins were incubated with the 32P-labeled probe covering the Rev-erbα LXRE (Fig. 2B lane 11). RXRα or LXRα alone did not bind to this site, confirming that LXRα does not bind DNA as a monomer. LXRα/RXRα binding to the probe was specific because it was competed, to a similar extent, by an excess of the unlabeled consensus LXRE oligonucleotide (cons LXRE) (Fig. 2B, lanes 12–14) as well as by an excess of the unlabeled Rev-erbα LXRE oligonucleotide (Fig. 2B, lanes 15–17). Furthermore, no binding was observed on the mutated Rev-erbα LXRE (Fig. 2B, lane 21). A chromatin immunoprecipitation (ChIP) assay was also performed to determine whether LXRα binds to the endogenous Rev-erbα promoter in human primary macrophages (Fig. 2C). The anti-LXRα antibody precipitated DNA fragments containing the region of the Rev-erbα promoter with the (−1038/−1053) LXRE. PCR amplification with primers specific for the β-actin gene did not result in any significant signal, thus demonstrating the specificity of immunoprecipitation and PCR amplification reactions. Altogether, these data demonstrate that LXRα binds as a heterodimer with RXRα to the (−1038/−1053) LXRE present in the promoter of the human Rev-erbα gene.
Fig. 2.
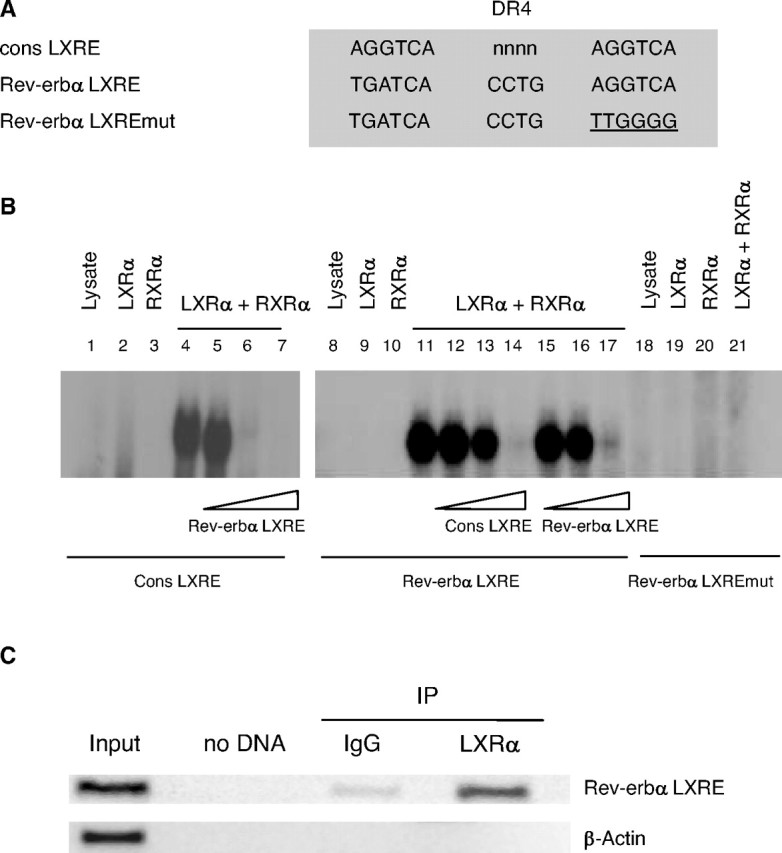
LXRα Binds as a Heterodimer with RXRα to a LXRE Site in the Human Rev-erbα Promoter
A, Sequences of consensus (cons LXRE), wild-type (Rev-erbα LXRE), and mutated (Rev-erbα LXRE mut) LXRE are shown. Mutations are underlined. B, EMSA was performed using the indicated end-labeled oligonucleotides in the presence of in vitro-translated hLXRα, hRXRα, or unprogrammed reticulocyte lysate. Competition experiments were performed by adding 1-, 10-, and 100-fold excess of cold Rev-erbα LXRE or cons LXRE oligonucleotides. C, ChIP assays were performed using chromatin isolated from human primary macrophages treated with T0901317 (1 μm) for 12 h. Cross-linked cell lysates were immunoprecipitated with goat IgG (control) or polyclonal LXRα-specific antibody. DNA precipitates were isolated and then subjected to PCR by using primer pairs covering either the (−1038/−1053) Rev-erbα gene promoter (top) or the β-actin gene (bottom) region. Control PCRs were performed without DNA (no DNA) or with nonimmunoprecipitated genomic DNA (input).
The Heterodimer LXRα/RXRα Induces Human Rev-erbα Promoter Activity
To assess whether LXRα/RXRα activates transcription from the (−1038/−1053) Rev-erbα LXRE site, three copies of this element were cloned in front of the herpes simplex virus thymidine kinase (Tk) promoter to obtain the Tk-pGl3(Rev-erbα LXRE)3 luciferase reporter vector. Cotransfection of the hLXRα and hRXRα expression vectors followed by treatment with the LXR ligand T0901317 (1 μm) led to significant induction of the transcriptional activity of the Tk-pGl3(Rev-erbα LXRE)3 in Cos-7 cells (Fig. 3A). A similar induction level was obtained when Tk-pGl3(cons LXRE)3 was transfected as positive control.
Fig. 3.
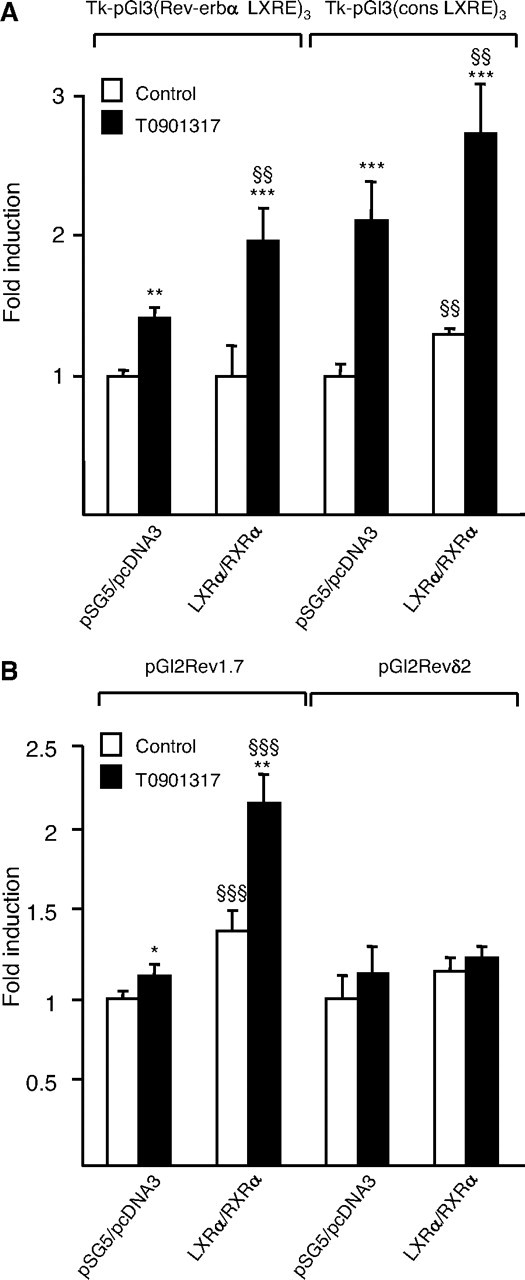
LXRα Induces Human Rev-erbα Promoter Activity
Cos-7 cells were transiently transfected with the indicated reporter constructs in the presence of pcDNA3-hLXRα, pSG5-hRXRα or empty vectors. Cells were treated with T0901317 (1 μm) or vehicle (Control) for 24 h, and luciferase activity was measured and normalized to activity of a cotransfected β-galactosidase expression vector. Results are representative of those obtained from three independent experiments and are set as 1 for each reporter construct. Each bar is the mean value ± sd of triplicate determinations. Statistically significant differences are indicated (ANOVA/t test; pSG5/pcDNA3 vs. LXRα/RXRα: §§, P < 0.01; §§§, P < 0.001 and control vs. T0901317: *, P < 0.05; **, P < 0.01; ***, P < 0.001). A, Effect of LXRα/RXRα activation on the activity of the Rev-erbα LXRE and cons LXRE sites cloned in three copies upstream of the Tk promoter. B, Effect of LXRα/RXRα activation on the activity of a human Rev-erbα promoter fragment containing (Rev1.7) or not (Revδ2) the Rev-erbα LXRE site.
To further investigate whether LXRα activates the Rev-erbα promoter, transfection assays were carried out using luciferase reporter constructs driven by the Rev-erbα 1.7-kb promoter or by the 5′ deleted Rev-erbα δ2 promoter lacking the Rev-erbα LXRE site (43). Rev-erbα 1.7kb promoter activity was induced by LXRα/RXRα cotransfection, an effect that was enhanced by the presence of T0901317 (1 μm) (Fig. 3B). By contrast, Rev-erbα δ2 promoter activity was not induced neither by LXRα/RXRα cotransfection nor by T0901317 (1 μm) treatment. These results demonstrate that the Rev-erbα human promoter activity is induced by activated LXRα.
Rev-erbα Antagonizes TLR-4 Transactivation by LXRs
In human macrophages, LXRs have been shown to regulate genes involved in macrophage cholesterol homeostasis as well as in the inflammatory/immune response (31, 33, 35, 39, 42). To address the functional role of the Rev-erbα/LXR cross talk, we investigated whether Rev-erbα regulates LXR target genes in primary human macrophages. Two different approaches were developed to study the functional activities of Rev-erbα in macrophages. First, human Rev-erbα was cloned into an adenoviral vector (ad-Rev-erbα) and primary human macrophages were infected with this adenovirus or with the corresponding empty adenovirus expressing only the green fluorescent protein (ad-GFP). At a multiplicity of infection of 100, more than 80% of cells were infected after 12 h of incubation as assessed by fluorescent microscopy (data not shown). A strong increase in Rev-erbα gene expression was observed in cells infected with the ad-Rev-erbα compared with ad-GFP expression vector-infected cells (Fig. 4A). As control, no variation in the level of cyclophilin mRNA was observed. Secondly, a short interfering RNA (siRNA) approach was used to reduce Rev-erbα gene expression in primary human macrophages. Rev-erbα siRNA significantly suppressed both gene and protein expression of Rev-erbα when compared with scrambled siRNA transfected cells (Fig. 4B). As expected, Rev-erbα mRNA level was still induced by LXR activation, but the maximal level was significantly lower than in T0901317-treated scrambled siRNA transfected cells (Fig. 4B).
Fig. 4.
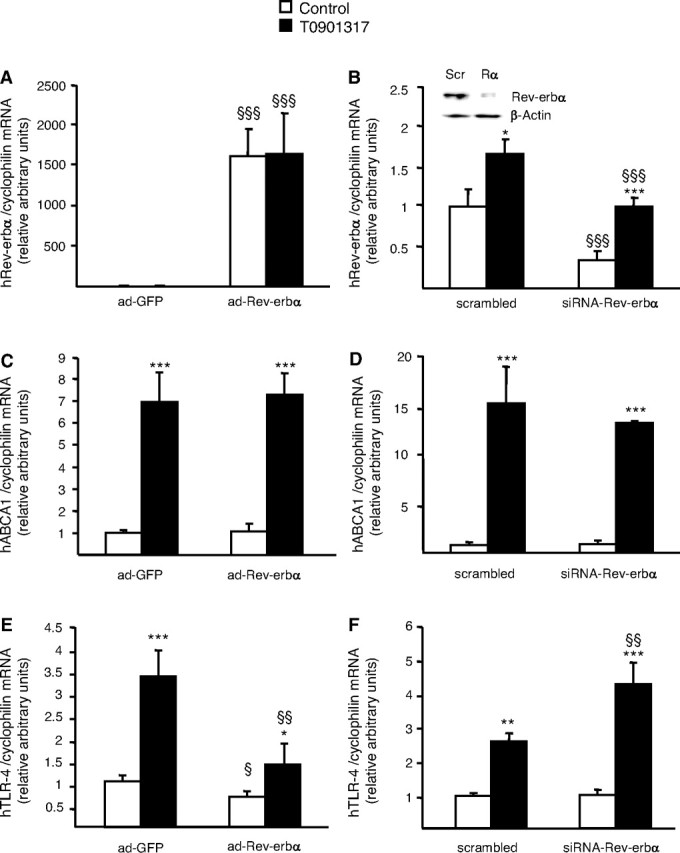
Rev-erbα Antagonizes LXR-Mediated TLR-4 Induction
A, C, and E, Primary human macrophages were infected with a Rev-erbα expressing adenovirus (ad-Rev-erbα) or a GFP (ad-GFP) expressing adenovirus and subsequently treated with T0901317 (1 μm) or vehicle (Control) for 24 h. B, D, and F, Primary human macrophages transfected with siRNA against the hRev-erbα sequence or with the scrambled control. Nuclear protein extracts were prepared and Rev-erbα and β-actin protein levels were measured by western blot analysis. Scr, Scrambled; Rα, Rev-erbα (B). Cells were treated with T0901317 (1 μm) or vehicle (Control) for 24 h and total RNA was extracted and treated with deoxyribonuclease I. mRNA levels of Rev-erbα (A and B), ABCA1 (C and D) and TLR-4 (E and F) were quantified by Q-PCR and normalized to cyclophilin mRNA levels. Results are representative of those obtained from three independent macrophage preparations and are expressed relative to the levels in untreated cells set as 1. Each bar is the mean value ± sd of triplicate determinations. Statistically significant differences are indicated (ANOVA/t test; adGFP vs. ad-Rev-erbα or scramble vs. siRNA-Rev-erbα: §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 and control vs. T0901317: *, P < 0.05; **, P < 0.01; ***, P < 0.001). Based on two-way ANOVA analysis, Rev-erbα expression significantly affected TLR-4 mRNA induction by T0901317 treatment.
The effect of ectopic Rev-erbα on known LXR target gene expression was subsequently measured in cells treated or not with the LXR agonist T0901317 (1 μm) for 24 h. The expression of different LXR target genes such as ABCA1, ABCG1, apoE, and NPC1/2 involved in macrophage cholesterol homeostasis was not affected by Rev-erbα overexpression and their induction by T0901317 was completely preserved (e.g. ABCA-1 (Fig. 4C) and data not shown). In addition, apoAI-specific cholesterol efflux as well as cholesteryl ester formation, two processes regulated by LXRs (35), were unaltered in ad-Rev-erbα infected cells (data not shown). Moreover, knockdown with Rev-erbα siRNA did not affect basal or T0901317-induced ABCA1 mRNA levels (Fig. 4D). These data indicate that Rev-erbα does not influence regulation of cholesterol homeostasis by LXRs in human macrophages.
The effect of Rev-erbα overexpression or silencing was then examined on TLR-4 expression. As previously described (39), LXR activation led to an increase of TLR-4 mRNA levels in macrophages (Fig. 4, E and F). Interestingly, adenovirus-mediated Rev-erbα over-expression led to a significant reduction of basal and LXR-induced TLR-4 gene expression (3.3- vs. 1.8-fold, P <0,001) (Fig. 4E), whereas Rev-erbα silencing resulted in an enhanced response of the TLR-4 gene to LXR activation (2.6- vs. 4.4-fold increase, P <0.005) (Fig. 4F). This result was reproduced using another siRNA sequence specific for Rev-erbα (data not shown). Similarly, the induction of TLR-4 protein levels by T0901317 was inhibited by Rev-erbα overexpression (Fig. 5A), whereas the response to LXR activation was enhanced by Rev-erbα knockdown (Fig. 5B). Together, these data demonstrate that Rev-erbα acts as a negative regulator of LXR-induced TLR-4 gene expression in human macrophages.
Fig. 5.
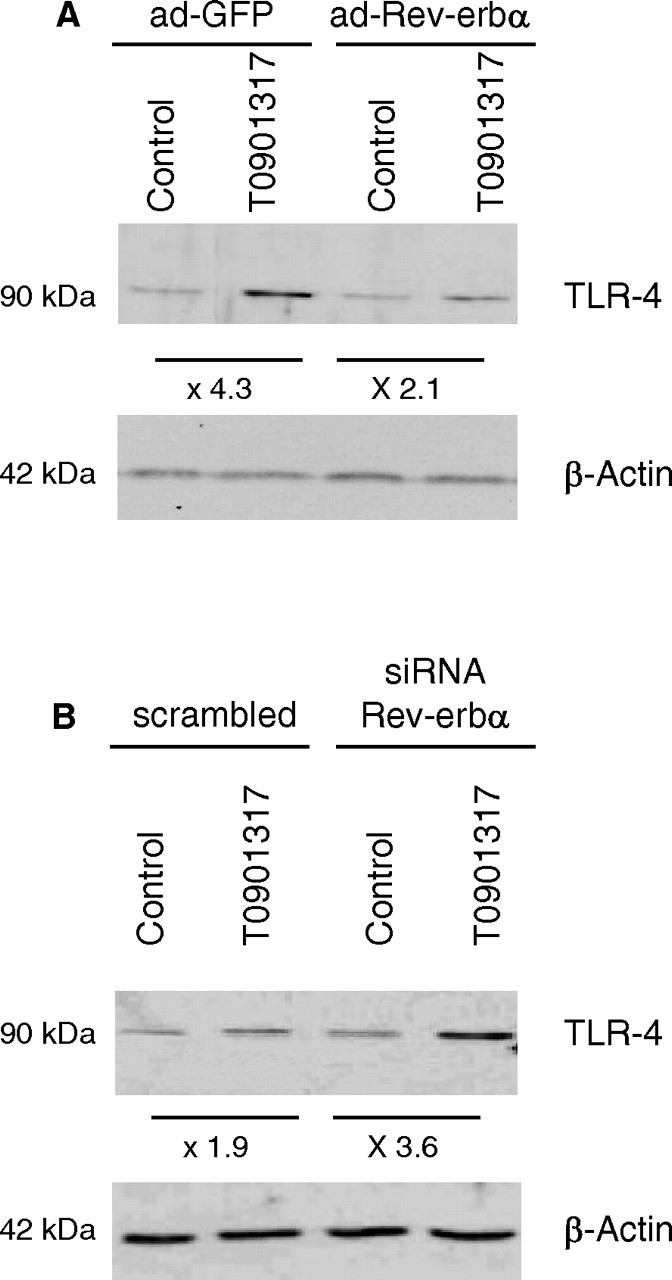
Rev-erbα Inhibits TLR-4 Protein Induction by LXR Activation
TLR-4 and β-actin proteins were measured by Western blot analysis. A, Primary human macrophages were infected with a Rev-erbα expressing adenovirus (ad-Rev-erbα) or a GFP (ad-GFP) expressing control vector and then treated with T0901317 (1 μm) or vehicle (Control) for 48 h. B, Primary human macrophages transfected with siRNA against hRev-erbα or with scrambled control were treated with T0901317 (1 μm) or vehicle (Control) for 48 h.
To study whether such regulation occurs also in mice, macrophages from WT and Rev-erbα KO mice were treated with T0901317 for 24 h. Neither absence of Rev-erbα expression nor LXR activation induced changes in TLR-4 expression in murine macrophages, illustrating the species-specific regulation of TLR-4 by LXRs and Rev-erbα (data not shown).
Rev-erbα and LXRα/RXRα Bind to the Same Response Element in the TLR-4 Promoter
Next, it was determined whether Rev-erbα could directly regulate TLR-4 transcription. Therefore, the human TLR-4 promoter was analyzed for the presence of potential Rev-erbα binding sites. Rev-erbα binds as a monomer to a hexameric core motif consisting of the consensus sequence (A/G)GGTCA preceded by a 5′ A/T-rich sequence (43) (Fig. 6A). A potential RevRE was identified in the human TLR-4 promoter overlapping the previously identified LXRE (39). To determine whether Rev-erbα binds to the native TLR-4 promoter, a ChIP assay was performed in human primary macrophages. The genomic DNA region encompassing the RevRE/LXRE of the TLR-4 gene was immunoprecipitated with a polyclonal anti-Rev-erbα antibody (Fig. 6B). PCR amplification with primers specific for the β-actin gene did not result in any significant signal, thus demonstrating the specificity of immunoprecipitation and PCR amplification reactions. This result shows that in human macrophages, Rev-erbα binds to the RevRE/ LXRE sequence of the TLR-4 gene similarly as previously reported for LXRα (Ref. 39 and Fig. 6B).
Fig. 6.
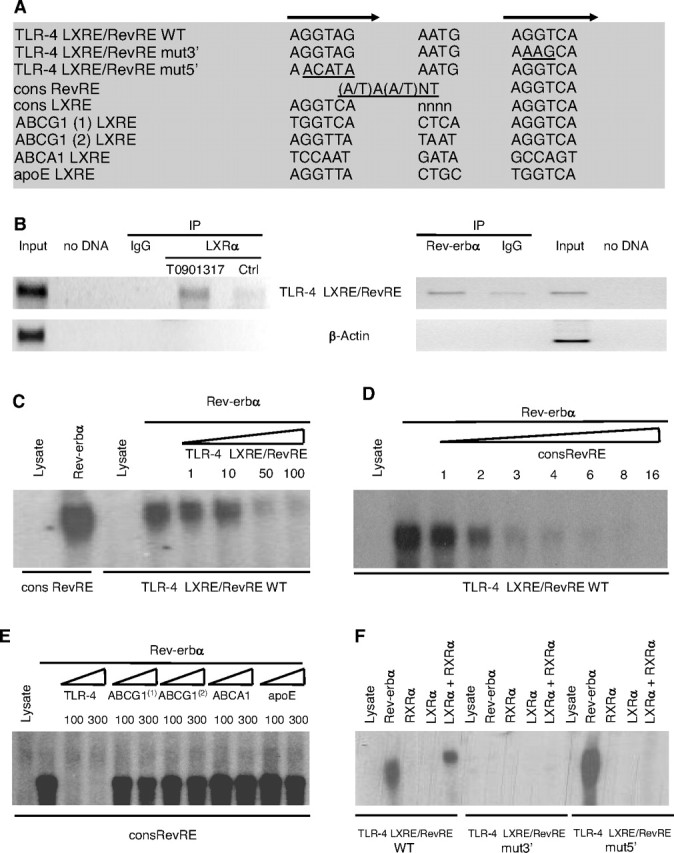
The Heterodimer LXRα/RXRα and the Monomer Rev-erbα Bind to Overlapping Response Elements in the Human TLR-4 Promoter
A, Sequences of the WT and mutated overlapping LXRE and RevRE in the human TLR-4 promoter as well as LXRE sequences present in the promoters of genes involved in cholesterol homeostasis are shown. Half-site sequences are indicated by arrows and mutations are underlined. B, ChIP assays were performed using chromatin isolated from differentiated human macrophages treated or not with T0901317 (1 μm) for 12 h. Cross-linked cell lysates were immunoprecipitated with IgG (control), a LXRα-specific antibody (left panel) or a Rev-erbα-specific antibody (right panel). DNA precipitates were isolated and then subjected to PCR by using primer pairs covering either the −555/−349 TLR-4 gene promoter (top) or β-actin gene (bottom) region. Control PCRs were performed without DNA or with nonimmunoprecipitated genomic DNA (input). C–F, EMSAs were performed in the presence of in vitro-translated hLXRα, hRXRα, hRev-erbα or unprogrammed reticulocyte lysate and the WT (C–F) or mutated (F) overlapping LXRE/RevRE site in the human TLR-4 promoter. Competition experiments were performed by adding the indicated fold excess of cold LXRE/RevRE WT (C), consensus RevRE (D) oligonucleotides or indicated LXRE sequences present in the promoters of genes involved in cholesterol homeostasis (E).
As assessed by EMSA, Rev-erbα directly binds to this site as a monomer displaying a migration pattern similar to the one obtained with the RevRE consensus site (Fig. 6C). The binding of Rev-erbα on the TLR-4 LXRE/RevRE site was specific because it could be competed by increasing amounts of unlabeled wild-type probe (Fig. 6C), as well as increasing amounts of unlabeled consensus RevRE sequence (Fig. 6D). Interestingly, none of the LXRE sequences present in the promoters of genes involved in cholesterol homeostasis competed for Rev-erbα binding to the RevRE consensus site (Fig. 6E), reinforcing the absence of cross talk between Rev-erbα and the ABC/cholesterol pathway. Moreover, mutation in the 3′ half-site of the TLR-4 LXRE/RevRE completely abolished Rev-erbα binding, whereas Rev-erbα was still able to bind the 5′ half-site mutated TLR-4 LXRE/RevRE (Fig. 6F) indicating that Rev-erbα binds on the 3′ half-site recognized by LXR.
Rev-erbα Represses TLR-4 Promoter Activity
Transient transfection assays using the Rev-erbα expression vector and human TLR-4 promoter-driven reporter plasmids were next performed to test whether Rev-erbα could modulate TLR-4 promoter activity. In line with the EMSA results, Rev-erbα cotransfection repressed human TLR-4 promoter (pGl3promTLR-4-620bp) activity (Fig. 7A). Interestingly, this repression was lost when a 5′ deleted promoter construct (pGl3promTLR-4-480bp) lacking the RevRE site was transfected (Fig. 7A). In addition, introduction of specific mutations of the RevRE in the context of the human TLR-4 promoter completely abolished the repression by Rev-erbα (Fig. 7A). In agreement with these results, the activity of the wild-type TLR-4 LXRE/RevRE site cloned in three copies in front of the Tk promoter was decreased by cotransfection of Rev-erbα, whereas the repression was abolished when the TLR-4 LXRE/RevRE mut3′ site was tested (Fig. 7B). Taken together, the results from EMSA and transfection experiments demonstrate that Rev-erbα represses human TLR-4 promoter activity by binding as a monomer to a RevRE site overlapping with the previously described LXRE site (39).
Fig. 7.
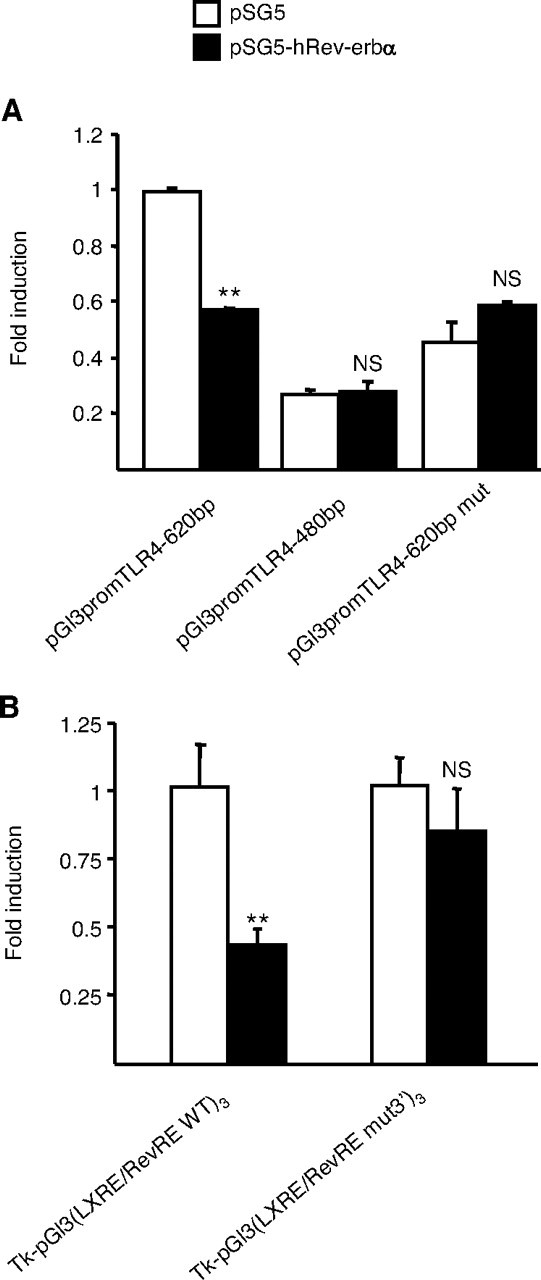
Rev-erbα Represses Human TLR-4 Promoter Activity
Cos-7 cells were transiently transfected with the indicated reporter constructs in the presence of pSG5-hRev-erbα or pSG5 vectors during 24 h. Cells were lysed and luciferase activity was measured as described in Materials and Methods. Results are representative of those obtained from three independent experiments and are expressed relative to the levels in untreated cells set as 1. Each bar is the mean value ± sd of triplicate determinations. Statistically significant differences are indicated (t test; **, P < 0.01). A, Effects of Rev-erbα on the activity of the indicated human TLR-4 promoter fragments. B, Effects of Rev-erbα on a construct containing the TLR-4 LXRE/RevRE or TLR-4 LXRE/RevRE mut3′ sites cloned in three copies upstream of the Tk promoter. NS, Not significant.
Rev-erbα Modulates the Response to LPS in LXR-Activated Human Macrophages
To determine the functional consequences of TLR-4 regulation by Rev-erbα, the production of inflammatory cytokines in response to LPS, the TLR-4 ligand, was analyzed by ELISA (44, 45, 46, 47). As previously described, MCP-1 and TNFα secretion in response to LPS was significantly enhanced in macrophages pretreated for 48 h with T0901317 due to the induction of TLR-4 expression (39). Rev-erbα overexpression decreased the induction of cytokine production by LPS both in untreated and in LXR activated-macrophages (Fig. 8, A and B). Moreover, Rev-erbα silencing enhanced the induction of MCP-1 and TNFα secretion in response to LPS in LXR agonist pretreated macrophages (Fig. 8, C and D).
Fig. 8.
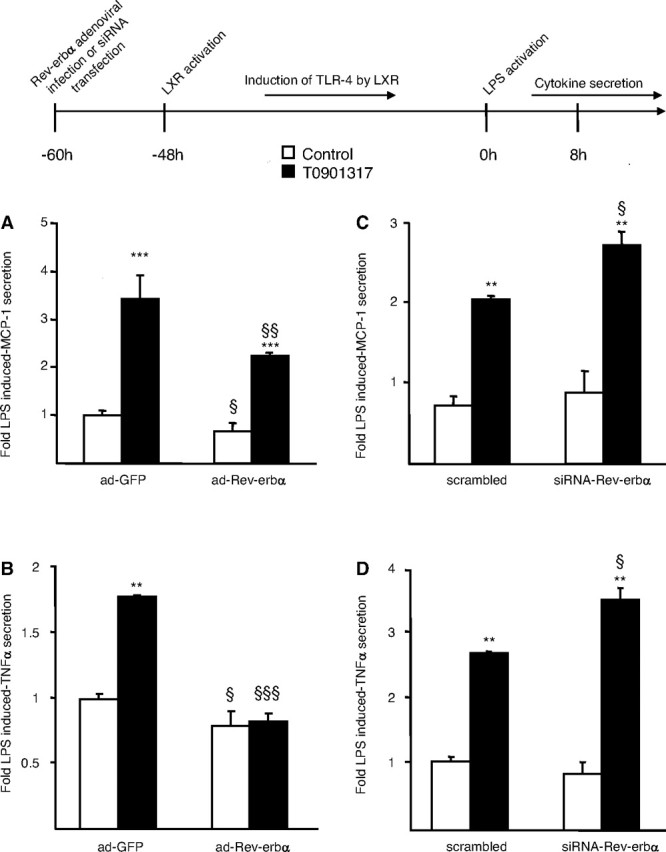
Rev-erbα Negatively Interferes with the Induction of MCP-1 and TNFα Secretion by LPS in LXR-Agonist-Treated Macrophages
A and B, Primary human macrophages were infected with a Rev-erbα adenovirus (ad-Rev-erbα) or a GFP (ad-GFP) expressing control adenovirus and then treated with T0901317 (1 μm) or vehicle (Control) for 48 h. C and D, Primary human macrophages transfected with anti-hRev-erbα or scrambled control siRNA were treated with T0901317 (1 μm) or vehicle (Control) for 48 h. Cells were incubated with LPS (100 ng/ml) during 8 h and MCP-1 (A and C) and TNFα (B and D) secretion were analyzed by ELISA. Results are representative of those obtained from three independent macrophage preparations and are expressed relative to the levels in LPS-stimulated cells set as 1. Each bar is the mean value ± sd of triplicate determinations. Statistically significant differences are indicated (ANOVA/t test; adGFP vs. adRev-erbα or scramble vs. siRNA-Rev-erbα: §, P < 0.05; §§, P < 0.01; §§§, P < 0.001 and control vs. T0901317: **, P < 0.01; ***, P < 0.001). Based on two-way ANOVA analysis, Rev-erbα expression significantly affected induction of cytokine response to LPS after T0901317 treatment.
DISCUSSION
Rev-erbα is an orphan NR expressed in different tissues including liver, adipose tissue, muscle, brain, and cell types such as macrophages and human peripheral blood mononuclear cells (8, 9). Rev-erbα participates in the regulation of different biological processes such as circadian rhythm and cellular differentiation (11, 15). However, its regulation is still poorly documented and the physiological role of Rev-erbα remains largely unexplored. Here, we show for the first time that Rev-erbα is expressed in primary differentiated human macrophages.
For a long time, Rev-erbα had no identified ligands and was referred to as an orphan NR (1). Very recently, the porphyrin heme has been identified as a physiological ligand for Rev-erbα (2, 3). In addition to ligand binding, regulation of Rev-erbα expression constitutes a crucial level for receptor activity control. Rev-erbα expression is controlled by a variety of stimuli (e.g. circadian rhythmicity, hormonal status, cellular differentiation… ). Here, we show that Rev-erbα expression is regulated both by cholesterol content and LPS stimulation in human macrophages. Furthermore, Rev-erbα expression is under the control of NRs such as PPARγ in adipose tissue, and PPARα, RORα and the glucocorticoid receptor in the liver (28, 29, 48). We identify the LXRs, NRs involved in the regulation of macrophage cholesterol homeostasis and inflammatory/immune response, as regulators of Rev-erbα expression in human macrophages. EMSA and transient transfection experiments revealed that the regulation of human Rev-erbα expression by LXRs occurs at the transcriptional level via LXR binding to a LXRE site present in the human Rev-erbα gene promoter.
Many cross talks between Rev-erbα and other NRs have been described. They are governed by two mechanisms. On the one hand, Rev-erbα could mediate certain actions of other NRs. For instance, it has been proposed that PPARα may negatively regulate genes such as rat apoA-I (25) via an indirect mechanism implicating the induction of Rev-erbα. In addition, Rev-erbα acts as an enhancer of adipogenesis acting downstream of PPARγ (11). On the other hand, Rev-erbα often binds to response elements shared with other NRs, such as RORα and PPARs, resulting in opposite effects on gene transcription (10, 21, 22, 23, 24, 25, 26, 27). Indeed, Rev-erbα acts as a transcriptional repressor, whereas binding of RORs and PPARs increase transcription of their target genes via these sites. As a consequence, Rev-erbα competes with these NRs for the regulation of common target genes. For instance, Rev-erbα modulates the expression of the hydratase-dehydrogenase (21) and the microsomal cytochrome P450 fatty acid ω-hydroxylase (22) genes by inhibiting PPARα-dependent transactivation via competition on the same response element. Rev-erbα also acts as a negative regulator of RORα transactivation of the α-fetoprotein (23), apoCIII (10, 24), rat apoAI (25, 26), and Bmal1 (27) genes. Moreover, Rev-erbα suppresses its own transcription via a Rev-DR2 element, which is also essential for the up-regulation of Rev-erbα by PPARα, PPARγ and RORα (11, 28, 29). The results reported here show that LXRα and Rev-erbα also bind to a shared response element in the TLR-4 promoter, regulating its transcriptional activity in an opposite manner. This LXRE/RevRE site is not conserved between the human and mouse TLR-4 promoters (49), likely explaining the species-specific regulation of TLR-4 by both LXR and Rev-erbα.
The LXR-mediated induction of ABCA1, ABCG1, apoE, NPC1/2 expression, which are involved in macrophage cholesterol homeostasis, was not affected by Rev-erbα. In addition, macrophage cholesterol homeostasis was unaltered by Rev-erbα (data not shown). These data indicated that LXR-induction of Rev-erbα in human macrophages results in a negative transcriptional feedback loop on the selective LXR-induced TLR-4 pathway involved in the immune/inflammatory response (Fig. 9). The induction of TLR-4, which occurs after long treatment with LXR agonists, prepares macrophages to an increased antibacterial response, whereas LXR activation results in antiinflammatory effects under acute situations of inflammation (42). Rev-erbα thus likely acts as a temporal switch inhibiting LXR-induction of TLR-4 expression. Interestingly, Rev-erbα expression is also induced after LPS stimulation in human macrophages, suggesting a role for this receptor also in the desensitization of macrophages to LPS via repression of its receptor TLR-4. Furthermore, Rev-erbα was previously shown to be down-regulated by glucocorticoids (48), whereas these immunomodulatory agents induce TLR-4 expression in peripheral blood mononuclear cells (50). It is tempting to speculate that the decrease in Rev-erbα expression could relieve TLR-4 inhibition and thus, mediates, at least in part, the glucocorticoid effect.
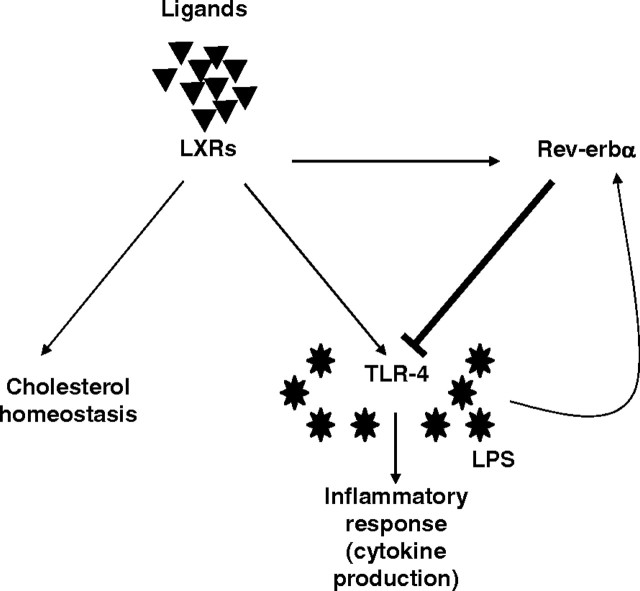
Fig. 9.
Negative Transcriptional Feedback Loop by Rev-erbα on the Selective LXR-Induced TLR-4 Pathway
Rev-erbα is a LXR target gene in human macrophages and represses LXR-induced-TLR-4 expression by binding to a RevRE overlapping with the previously described LXRE site. This cross talk between Rev-erbα and LXR together with the induction of Rev-erbα expression by LPS identifies Rev-erbα as a molecular link driving transcriptional feedback loops and as a crucial factor in the macrophage response to LPS.
Rev-erbα plays a key role in the regulation of circadian rhythm as a molecular link between the positive and negative regulatory limbs of the clock system (15, 48). Rev-erbα expression is directly activated by the Bmal1-Clock heterodimer and Rev-erbα itself represses expression of these two proteins (15, 51). Accordingly, expression of Rev-erbα itself oscillates diurnally in the suprachiasmatic nucleus as well as in peripheral tissues (14). Rev-erbα expression was recently shown to be constant or to display very low amplitude of circadian variation in human peripheral blood mononuclear cells (9). However, peripheral blood mononuclear cells are a heterogeneous population of different cell types and whether Rev-erbα oscillates in monocyte/macrophages is currently unknown. The inflammatory response is modulated by a circadian rhythm because LPS-stimulated human whole blood cytokine production exhibits diurnal rhythmicity (52). Thus, it is tempting to speculate that Rev-erbα may serve to integrate immunomodulatory functions of LXRs and circadian signals in human macrophages.
In conclusion, we identified the nuclear receptor Rev-erbα as a novel LXR target gene in human macrophages. Rev-erbα represses human TLR-4 promoter activity by binding as a monomer to a RevRE site overlapping with the LXRE site. This cross talk between Rev-erbα and LXRs constitutes another example in which Rev-erbα acts as a molecular relayer of a negative transcription feedback loop.
MATERIALS AND METHODS
Cell Culture
Mononuclear cells were isolated from blood of healthy normolipidemic donors (thrombopheresis residues). After Ficoll gradient centrifugation, the monocytes were suspended in RPMI 1640 medium containing gentamycin (40 μg/ml), glutamine (0,05%) (Sigma) and 10% pooled human serum. Cells were cultured at a density of 2 × 106 cells/well in six-well plastic culture dishes (Primaria, Polylabo, Strasbourg, France). Differentiation of monocytes into macrophages occurred spontaneously by adhesion of cells to the culture dish. Mature monocyte-derived macrophages were used for experiments after 10 d of culture. For treatment with different ligands medium was changed to RPMI 1640 medium without serum. Cos-7 cells were grown in DMEM supplemented with glutamine (2 mm) and 10% fetal calf serum. For experiments, cells medium was replaced by DMEM supplemented with glutamine (2 mm) without serum.
RNA Extraction and Analysis
Total cellular RNA from macrophages was extracted using Trizol (Life Technologies, Cergy Pontoise, France) and reverse transcription was performed using random hexameric primers and Superscript reverse transcriptase (Life Technologies). cDNA were quantified by quantitative PCR on a MX 4000 apparatus (Stratagene, La Jolla, CA) using specific primers for hRev-erbα: 5′-GACATGACGACCCTGGACTC-3′ and 5′-GCTGCCATTGGAGTTGTCAC-3′, hTLR-4: 5′-AAGCCGAAAGGTGATTGTTG-3′ and 5′-CTGAGCAGGGTCTTCTCCAC-3′, hABCA1: 5′-AAGGTCTTGTTCACCTCAGCCATCAC-3′ and 5′-GTGAACAGCTCCAGCTCCTCCAC-3′ and cyclophilin: 5′-GCATACGGGTCCTGGCATCTTGTCC-3′ and 5′-ATGGTGATCTTCTTGCTGGTCTTGC-3′. PCR amplification was performed in a volume of 20 μl containing 100 nm of each primer, 4 mm MgCl2, the Brilliant Q-PCR Core Reagent Kit mix as recommended by the manufacturer (Stratagene) and SYBR Green 0.33× (Sigma-Aldrich, St. Louis, MO). The conditions were 95 C for 10 min, followed by 40 cycles of 30 sec at 95 C, 30 sec at 55 C and 30 sec at 72 C. mRNA levels were subsequently normalized to cyclophilin mRNA.
EMSAs
In vitro-synthesized human Rev-erbα, LXRα and RXRα were incubated for 10 min at room temperature in a total volume of 20 μl containing 0.5 μg poly(deoxyinosine-deoxycytosine) and 0.5 μg herring sperm DNA in the following binding buffer: HEPES 30 mm, KCl 60 mm, dithiothreitol 1 mm, 0.1% Triton X-100, 0.5% glycerol, BSA 0.1%. The radiolabeled probes hRev-erbα LXRE: 5′-AACTCCTGACCTCAGGTGATCAACCCAC-3′, hTLR-4 RevRE/LXRE WT: 5′-AAAGAGGTATGTAAGGTAGAATGAGGTCATTATG-3′, hTLR-4 RevRE/LXRE mut3′: 5′-AAAGAGGTATGTAAGGTAGAATGAAAGCATTATG-3′, hTLR-4 RevRE/LXRE mut5′: 5′-AAAGAGGTATGTAAACATAAATGAGGTCATTATG-3′ were added and the binding reaction was incubated for a further 15 min at room temperature. The protein complexes were resolved by 6% nondenaturing polyacrylamide gel electrophoresis in 0.25× Tris-borate-EDTA at room temperature. Competition experiments were performed by adding indicated-fold excess of unlabeled oligonucleotides: human (h) ABCG1 LXRE1: 5′-GCT TTG GTC ACT CAA GTT CAA GTT-3′, h ABCG1 LXRE2: 5′-GGG AAG TTT ATA ATA GTT CAT ATA-3′, hABCA1 LXRE: 5′-AAA CTG GCT ATC ATT GGA GAC GCG-3′, hapoE LXRE: 5′-GTC AAT GAC CAG CAG TAA CCT CAG CAG CTT-3′.
ChIP Assays
ChIP assays were carried out as previously described (53) with slight modifications. Briefly, 107 primary human macrophages plated on 10 cm dishes were cross-linked with 1% formaldehyde at 37 C for 15 min. The reaction was stopped by 200 mm glycine. After ice-cold PBS washes, nuclei were extracted with 500 μl of extraction buffer [Tris-HCl, pH 8.0; 50 mm; KCl 85 mm; Nonidet P-40 0.5%; protease inhibitors (Roche, Indianapolis, IN)]. Cells were scraped and incubated at 4 C for 10 min. Nucleus were harvested by centrifugation at 4 C, for 5 min, at 6000 × g and lysed with 400 μl of lysis buffer [Tris-HCl, pH 8.0, 50 mm; EDTA 10 mm; sodium dodecyl sulfate (SDS) 1%; protease inhibitors]. DNA was sheared by sonication and nuclear lysates centrifuged for 10 min, 12,000 × g. Fifty microliters of supernatant were preserved as input-positive control. The rest of supernatant was diluted 10-fold in IP buffer (Tris-HCl, pH 8.0; 20 mm; NaCl 150 mm; EDTA 2 mm; Triton X-100 1%; protease inhibitors) and precleared with 200 μl of a 50% protein A Sepharose slurry (equilibrated in IP buffer supplemented with BSA 1 mg·ml−1 and herring sperm 100 μg·ml−1) at 4 C, for 2 h at least. Then, beads were removed by centrifugation and immune complexes were formed overnight with 2 μg of specific antibodies [anti-LXRα: P-20 (Santa Cruz); anti-Rev-Erbα (PPMX; Perseus, Tokyo, Japan) or IgG as negative control (Santa Cruz)]. Complexes were collected with 100 μl of 50% equilibrated protein A Sepharose slurry for 2 h at 4 C. Beads were washed for 10 min in buffer A (Tris-HCl, pH 8.0, 20 mm; NaCl 150 mm; EDTA 2 mm; Triton X-100 1%; SDS 0.1%), buffer B (Tris-HCl, pH 8.0, 20 mm; NaCl 500 mm; EDTA 2 mm; Triton X-100 1%; SDS 0.1%), buffer C (Tris-HCl, pH 8.0, 10 mm; LiCl 250 mm; EDTA 1 mm; Nonidet P-40 1%; sodium deoxycholate 1%) and twice in TE buffer (Tris-HCl, pH 8.0, 10 mm; EDTA 1 mm). Cross-linking was reversed with elution buffer (NaHCO3 0.1 m, SDS 1%) at 65 C, overnight. DNAs were purified with QIAquick Spin Kit (QIAGEN, Valencia, CA). The TLR-4 promoter or part of the β-actin gene as negative control were amplified by using previously described primers (39). The Rev-Erbα promoter was amplified with the following primers: 5′-ACG TGG TTT CAC CGT GTT G-3′ and 5′-TGG CTC ATG CCT GTA ATC C-3′. QPCR amplification reactions were carried out to ensure that DNA amplification reactions were in the linear range and that PCR products were specifics.
Plasmid Cloning
The hLXREwt and hLXREmut constructs were obtained by inserting three copies of the double-strand wild-type or mutated LXRE in the Tk-pGl3 plasmid. The hTLR-4pGl3 constructs were obtained by inserting 480- or 620-bp fragments of the human TLR-4 promoter in the multicloning site of the pGl3 vector. Fragments were obtained by PCR amplification using human genomic DNA as template and specific primers as previously described (39). Specific mutations in the RevRE in the context of the 620-bp TLR-4 promoter were generated by site-directed mutagenesis.
Transfection Experiments
Cos-7 cells, cultured in 24-well plates, were transfected with reporter plasmids and with expression vectors (pSG5, pcDNA3, pSG5-hRXRα, pcDNA3-hLXRα or pSG5-hRev-erbα and pCMV β-gal) using the cationic lipid RPR 120535B as previously described (10). Cells were subsequently incubated in medium containing 2% Ultroser (BioSepra, Villeneuve la Garenne, France) in the presence of the LXR ligand T0901317 (1 μm) or dimethylsulfoxide as vehicle for 24 h. At the end of the experiment, cells were lysed and luciferase and β-galactosidase assays were performed.
Adenovirus Preparation and Cell Infection
The pSG5-hRev-erbα vector was digested with BamHI/HindIII and the Rev-erbα cDNA was cloned into the pAdCMV5-IRES-GFP transfer vector (Q-Biogene, Illkirch, France). The construct was then linearized and cotransfected with the E1/E3-deleted Ad5 backbone viral Adeno LacZ. One positive adenovirus recombinant was purified and amplified on HEK293 cells. Sequencing and transient transfection assays showed that constructs were functional at the different steps of the construction process. For the infection experiments, primary human macrophages were seeded in six-well plates at a density of 106 cells/well and viral particles were added at a multiplicity of infection of 100 for 12 h. Thereafter, cells were washed three times with PBS and incubated in standard culture medium.
siRNA
RNA oligonucleotide derived from the human Rev-erbα (siRNA ID no. 940) sequence was purchased from Ambion (Courtaboeuf, France). The scrambled RNA oligonucleotide (QIAGEN) was used as control. The 10-d-old macrophages were transfected with siRNA using jetSI (Polyplus Transfection, Strasbourg, France) according to the manufacturer’s instructions.
Protein Extraction and Western Blot Analysis
Cells were lysed in buffer (50 mm Tris-HCl; 300 mm sucrose; 50 mm NaCl; proteinase inhibitor cocktail, pH 7.3). For the nuclear proteins extraction, nuclear and cytoplasmic fractions were separated by centrifugation for 10 min, 6000 × g, at 4 C. Nuclei were resuspended with 200 μl of hypertonic buffer (HEPES 10 mm, pH 7.8; NaCl 300 mm; MgCl2 1.5 mm; EDTA 0.2 mm; dithiothreitol 0.5 mm; glycerol 25%; complete protease inhibitors tablet from Roche). After 30 min of incubation at 4 C, nuclear extracts were clarified by centrifugation for 15 min, 16,000 × g, 4 C. Twenty micrograms of protein lysate or protein nuclear extract were separated by SDS-PAGE and transferred to nitrocellulose. Equal loading of the gel was verified by Ponceau red staining. Membranes were then subjected to immunodetection with polyclonal anti-TLR-4 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA; H-80) or anti-Rev-erbα (Cell Signaling Technology, Danvers, MA) and anti-β-actin (Santa Cruz I-19) antibodies. Immunoreactive bands were revealed using an ECL detection kit (Amersham Pharmacia Biotech, Piscataway, NJ).
Cytokine Secretion in Cell Culture Supernatants
Secreted MCP-1 and TNFα were quantified by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Peprotech) according to the manufacturer’s instructions.
Statistical Analysis
Statistical differences between groups were analyzed by ANOVA and Student’s t tests and were considered significant when P < 0.05.
Acknowledgments
B. Noel is kindly acknowledged for his technical help. We thank K. Bertrand (Genfit, Loos, France) for providing the T0901317 and GW3965 compounds.
NURSA Molecule Pages:
Ligands: GW 3965 | T0901317;
Nuclear Receptors: LXRα | REV-ERBα | RXRα.
Footnotes
This work was supported by grants from the Institut National de la Santé et de la Recherche Médicale, the Région Nord Pas de Calais/FEDER, the Fondation de France (to H.D. and B.S.) and is part of the project Novel Molecular Drug Targets for Obesity and Type 2 Diabetes (DIABESITY), supported by the European commission as an integrated project of the 6th Framework Program (Contract LSH-CT2003-503041).
Disclosure Statement: The authors have nothing to disclose.
First Published Online May 29, 2008
Abbreviations: ABC, ATP-binding cassette transporters; acLDL, acetylated LDL; adGFP, adenovirus expressing only the green fluorescent protein; apo, apolipoprotein; ChIP, chromatin immunoprecipitation; DR, direct repeat; EC, endothelial cell; h, human; LXRE, LXR response element; LPS, lipopolysaccharide; NPC, Niemann Pick C proteins; NR, nuclear receptor; PPAR, peroxisome proliferator-activated receptor; ROR, retinoid-related orphan receptor; RXR, retinoid X receptors; SDS, sodium dodecyl sulfate; siRNA, short interfering RNA; SMC, smooth muscle cell; Tk, thymidine kinase; TLR, toll-like receptor.
References
- 1.Lazar MA, Hodin RA, Darling DS, Chin WW 1989. A novel member of the thyroid/steroid hormone receptor family is encoded by the opposite strand of the rat c-erbA α transcriptional unit. Mol Cell Biol 9:1128–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raghuram S, Stayrook KR, Huang P, Rogers PM, Nosie AK, McClure DB, Burris LL, Khorasanizadeh S, Burris TP, Rastinejad F 2007. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat Struct Mol Biol 14:1207–1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yin L, Wu N, Curtin JC, Qatanani M, Szwergold NR, Reid RA, Waitt GM, Parks DJ, Pearce KH, Wisely GB, Lazar MA 2007. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 318:1786–1789 [DOI] [PubMed] [Google Scholar]
- 4.Harding HP, Lazar MA 1995. The monomer-binding orphan receptor Rev-Erb represses transcription as a dimer on a novel direct repeat. Mol Cell Biol 15:4791–4802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla A, Lazar MA 1993. Induction of Rev-ErbAα, an orphan receptor encoded on the opposite strand of the α-thyroid hormone receptor gene, during adipocyte differentiation. J Biol Chem 268:16265–16269 [PubMed] [Google Scholar]
- 6.Forman BM, Chen J, Blumberg B, Kliewer SA, Henshaw R, Ong ES, Evans RM 1994. Cross-talk among RORα1 and the Rev-erb family of orphan nuclear receptors. Mol Endocrinol 8:1253–1261 [DOI] [PubMed] [Google Scholar]
- 7.Migita H, Morser J, Kawai K 2004. Rev-erbα upregulates NF-κB-responsive genes in vascular smooth muscle cells. FEBS Lett 561:69–74 [DOI] [PubMed] [Google Scholar]
- 8.Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM 2005. A nuclear receptor atlas: macrophage activation. Mol Endocrinol 19:2466–2477 [DOI] [PubMed] [Google Scholar]
- 9.Teboul M, Barrat-Petit MA, Li XM, Claustrat B, Formento JL, Delaunay F, Levi F, Milano G 2005. Atypical patterns of circadian clock gene expression in human peripheral blood mononuclear cells. J Mol Med 83:693–699 [DOI] [PubMed] [Google Scholar]
- 10.Raspe E, Duez H, Mansen A, Fontaine C, Fievet C, Fruchart JC, Vennstrom B, Staels B 2002. Identification of Rev-erbα as a physiological repressor of apoC-III gene transcription. J Lipid Res 43:2172–2179 [DOI] [PubMed] [Google Scholar]
- 11.Fontaine C, Dubois G, Duguay Y, Helledie T, Vu-Dac N, Gervois P, Soncin F, Mandrup S, Fruchart JC, Fruchart-Najib J, Staels B 2003. The orphan nuclear receptor Rev-Erbα is a peroxisome proliferator-activated receptor (PPAR) γ target gene and promotes PPARγ-induced adipocyte differentiation. J Biol Chem 278:37672–37680 [DOI] [PubMed] [Google Scholar]
- 12.Laitinen S, Fontaine C, Fruchart JC, Staels B 2005. The role of the orphan nuclear receptor Rev-Erb α in adipocyte differentiation and function. Biochimie 87:21–25 [DOI] [PubMed] [Google Scholar]
- 13.Pircher P, Chomez P, Yu F, Vennstrom B, Larsson L 2005. Aberrant expression of myosin isoforms in skeletal muscles from mice lacking the rev-erbAα orphan receptor gene. Am J Physiol Regul Integr Comp Physiol 288:R482–R490 [DOI] [PubMed]
- 14.Preitner N, Brown S, Ripperger J, Le-Minh N, Damiola F, Schibler U 2003. Orphan nuclear receptors, molecular clockwork, and the entrainment of peripheral oscillators. Novartis Found Symp 253:89–99; discussion 99–109 [PubMed] [Google Scholar]
- 15.Preitner N, Damiola F, Lopez-Molina L, Zakany J, Duboule D, Albrecht U, Schibler U 2002. The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110:251–260 [DOI] [PubMed] [Google Scholar]
- 16.Ueda HR, Chen W, Adachi A, Wakamatsu H, Hayashi S, Takasugi T, Nagano M, Nakahama K, Suzuki Y, Sugano S, Iino M, Shigeyoshi Y, Hashimoto S 2002. A transcription factor response element for gene expression during circadian night. Nature 418:534–539 [DOI] [PubMed] [Google Scholar]
- 17.Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308:1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP 2004. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab 89:2119–2126 [DOI] [PubMed] [Google Scholar]
- 19.Gottlicher M, Heck S, Herrlich P 1998. Transcriptional cross-talk, the second mode of steroid hormone receptor action. J Mol Med 76:480–489 [DOI] [PubMed] [Google Scholar]
- 20.Fontaine C, Staels B 2007. The orphan nuclear receptor Rev-erbα: a transcriptional link between circadian rhythmicity and cardiometabolic disease. Curr Opin Lipidol 18:141–146 [DOI] [PubMed] [Google Scholar]
- 21.Kassam A, Capone JP, Rachubinski RA 1999. Orphan nuclear hormone receptor RevErbα modulates expression from the promoter of the hydratase-dehydrogenase gene by inhibiting peroxisome proliferator-activated receptor α-dependent transactivation. J Biol Chem 274:22895–22900 [DOI] [PubMed] [Google Scholar]
- 22.Hsu MH, Palmer CN, Song W, Griffin KJ, Johnson EF 1998. A carboxyl-terminal extension of the zinc finger domain contributes to the specificity and polarity of peroxisome proliferator-activated receptor DNA binding. J Biol Chem 273:27988–27997 [DOI] [PubMed] [Google Scholar]
- 23.Bois-Joyeux B, Chauvet C, Nacer-Cherif H, Bergeret W, Mazure N, Giguere V, Laudet V, Danan JL 2000. Modulation of the far-upstream enhancer of the rat α-fetoprotein gene by members of the ROR α, Rev-erb α, and Rev-erb β groups of monomeric orphan nuclear receptors. DNA Cell Biol 19:589–599 [DOI] [PubMed] [Google Scholar]
- 24.Coste H, Rodriguez JC 2002. Orphan nuclear hormone receptor Rev-erbα regulates the human apolipoprotein CIII promoter. J Biol Chem 277:27120–27129 [DOI] [PubMed] [Google Scholar]
- 25.Vu-Dac N, Chopin-Delannoy S, Gervois P, Bonnelye E, Martin G, Fruchart JC, Laudet V, Staels B 1998. The nuclear receptors peroxisome proliferator-activated receptor α and Rev-erbα mediate the species-specific regulation of apolipoprotein A-I expression by fibrates. J Biol Chem 273:25713–25720 [DOI] [PubMed] [Google Scholar]
- 26.Vu-Dac N, Gervois P, Grotzinger T, De Vos P, Schoonjans K, Fruchart JC, Auwerx J, Mariani J, Tedgui A, Staels B 1997. Transcriptional regulation of apolipoprotein A-I gene expression by the nuclear receptor RORα. J Biol Chem 272:22401–22404 [DOI] [PubMed] [Google Scholar]
- 27.Guillaumond F, Dardente H, Giguere V, Cermakian N 2005. Differential control of Bmal1 circadian transcription by REV-ERB and ROR nuclear receptors. J Biol Rhythms 20:391–403 [DOI] [PubMed] [Google Scholar]
- 28.Gervois P, Chopin-Delannoy S, Fadel A, Dubois G, Kosykh V, Fruchart JC, Najib J, Laudet V, Staels B 1999. Fibrates increase human REV-ERBα expression in liver via a novel peroxisome proliferator-activated receptor response element. Mol Endocrinol 13:400–409 [DOI] [PubMed] [Google Scholar]
- 29.Raspe E, Mautino G, Duval C, Fontaine C, Duez H, Barbier O, Monte D, Fruchart J, Fruchart JC, Staels B 2002. Transcriptional regulation of human Rev-erbα gene expression by the orphan nuclear receptor retinoic acid-related orphan receptor α. J Biol Chem 277:49275–49281 [DOI] [PubMed] [Google Scholar]
- 30.Zelcer N, Tontonoz P 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J Clin Invest 116:607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Costet P, Luo Y, Wang N, Tall AR 2000. Sterol-dependent transactivation of the ABC1 promoter by the liver X receptor/retinoid X receptor. J Biol Chem 275:28240–28245 [DOI] [PubMed] [Google Scholar]
- 32.Engel T, Lorkowski S, Lueken A, Rust S, Schluter B, Berger G, Cullen P, Assmann G 2001. The human ABCG4 gene is regulated by oxysterols and retinoids in monocyte-derived macrophages. Biochem Biophys Res Commun 288:483–488 [DOI] [PubMed] [Google Scholar]
- 33.Wang N, Lan D, Chen W, Matsuura F, Tall AR 2004. ATP-binding cassette transporters G1 and G4 mediate cellular cholesterol efflux to high-density lipoproteins. Proc Natl Acad Sci USA 101:9774–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laffitte BA, Repa JJ, Joseph SB, Wilpitz DC, Kast HR, Mangelsdorf DJ, Tontonoz P 2001. LXRs control lipid-inducible expression of the apolipoprotein E gene in macrophages and adipocytes. Proc Natl Acad Sci USA 98:507–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rigamonti E, Helin L, Lestavel S, Mutka AL, Lepore M, Fontaine C, Bouhlel MA, Bultel S, Fruchart JC, Ikonen E, Clavey V, Staels B, Chinetti-Gbaguidi G 2005. Liver X receptor activation controls intracellular cholesterol trafficking and esterification in human macrophages. Circ Res 97:682–689 [DOI] [PubMed] [Google Scholar]
- 36.Valledor AF 2005. The innate immune response under the control of the LXR pathway. Immunobiology 210:127–132 [DOI] [PubMed] [Google Scholar]
- 37.Valledor AF, Hsu LC, Ogawa S, Sawka-Verhelle D, Karin M, Glass CK 2004. Activation of liver X receptors and retinoid X receptors prevents bacterial-induced macrophage apoptosis. Proc Natl Acad Sci USA 101:17813–17818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, Hogenesch J, O'Connell R M, Cheng G, Saez E, Miller JF, Tontonoz P 2004. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell 119:299–309 [DOI] [PubMed] [Google Scholar]
- 39.Fontaine C, Rigamonti E, Nohara A, Gervois P, Teissier E, Fruchart JC, Staels B, Chinetti-Gbaguidi G 2007. Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ Res 101:40–49 [DOI] [PubMed] [Google Scholar]
- 40.Akira S, Uematsu S, Takeuchi O 2006. Pathogen recognition and innate immunity. Cell 124:783–801 [DOI] [PubMed] [Google Scholar]
- 41.Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P 2003. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med 9:213–219 [DOI] [PubMed] [Google Scholar]
- 42.Walcher D, Kummel A, Kehrle B, Bach H, Grub M, Durst R, Hombach V, Marx N 2006. LXR Activation reduces proinflammatory cytokine expression in human CD4-positive lymphocytes. Arterioscler Thromb Vasc Biol 5:1022–1028 [DOI] [PubMed] [Google Scholar]
- 43.Adelmant G, Begue A, Stehelin D, Laudet V 1996. A functional Rev-erb α responsive element located in the human Rev-erb α promoter mediates a repressing activity. Proc Natl Acad Sci USA 93:3553–3558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bosisio D, Polentarutti N, Sironi M, Bernasconi S, Miyake K, Webb GR, Martin MU, Mantovani A, Muzio M 2002. Stimulation of toll-like receptor 4 expression in human mononuclear phagocytes by interferon-γ: a molecular basis for priming and synergism with bacterial lipopolysaccharide. Blood 99:3427–3431 [DOI] [PubMed] [Google Scholar]
- 45.Chow JC, Young DW, Golenbock DT, Christ WJ, Gusovsky F 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J Biol Chem 274:10689–10692 [DOI] [PubMed] [Google Scholar]
- 46.Fu SL, Hsu YH, Lee PY, Hou WC, Hung LC, Lin CH, Chen CM, Huang YJ 2006. Dioscorin isolated from Dioscorea alata activates TLR4-signaling pathways and induces cytokine expression in macrophages. Biochem Biophys Res Commun 339:137–144 [DOI] [PubMed] [Google Scholar]
- 47.Xu Z, Huang CX, Li Y, Wang PZ, Ren GL, Chen CS, Shang FJ, Zhang Y, Liu QQ, Jia ZS, Nie QH, Sun YT, Bai XF 2007. Toll-like receptor 4 siRNA attenuates LPS-induced secretion of inflammatory cytokines and chemokines by macrophages. J Infect 55:e1–e9 [DOI] [PubMed]
- 48.Torra IP, Tsibulsky V, Delaunay F, Saladin R, Laudet V, Fruchart JC, Kosykh V, Staels B 2000. Circadian and glucocorticoid regulation of Rev-erbα expression in liver. Endocrinology 141:3799–3806 [DOI] [PubMed] [Google Scholar]
- 49.Thomas AV, Broers AD, Vandegaart HF, Desmecht DJ 2006. Genomic structure, promoter analysis and expression of the porcine (Sus scrofa) TLR4 gene. Mol Immunol 43:653–659 [DOI] [PubMed] [Google Scholar]
- 50.Galon J, Franchimont D, Hiroi N, Frey G, Boettner A, Ehrhart-Bornstein M, O'Shea JJ, Chrousos GP, Bornstein SR 2002. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J 16:61–71 [DOI] [PubMed] [Google Scholar]
- 51.Triqueneaux G, Thenot S, Kakizawa T, Antoch MP, Safi R, Takahashi JS, Delaunay F, Laudet V 2004. The orphan receptor Rev-erbα gene is a target of the circadian clock pacemaker. J Mol Endocrinol 33:585–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petrovsky N, Harrison LC 1997. Diurnal rhythmicity of human cytokine production: a dynamic disequilibrium in T helper cell type 1/T helper cell type 2 balance? J Immunol 158:5163–5168 [PubMed] [Google Scholar]
- 53.Lefebvre B, Ozato K, Lefebvre P 2002. Phosphorylation of histone H3 is functionally linked to retinoic acid receptor β promoter activation. EMBO Rep 3:335–340 [DOI] [PMC free article] [PubMed] [Google Scholar]