Abstract
We made a coupled genetic reporter that detects rare transcription misincorporation errors to measure RNA polymerase transcription fidelity in Escherichia coli. Using this reporter, we demonstrated in vivo that the transcript cleavage factor GreA, but not GreB, is essential for proofreading of a transcription error where a riboA has been misincorporated instead of a riboG. A greA mutant strain had more than a 100-fold increase in transcription errors relative to wild-type or a greB mutant. However, overexpression of GreB in ΔgreA cells reduced the misincorporation errors to wild-type levels, demonstrating that GreB at high concentration could substitute for GreA in RNA proofreading activity in vivo.
Keywords: RNA polymerase, transcription errors, Gre proteins, Cre/lox fidelity reporter
THE potential impact of rare transcription errors on normal cell physiology and disease has been the subject of debate for several decades [for review see Strathern et al. (2012)]. The transient nature of messenger RNA (mRNA) and a relatively high (10−3–10−4) rate of translation misreading make detection of transcription errors extremely difficult (Edelmann and Gallant 1977; Rosenberger and Foskett 1981; Bouadloun et al. 1983; Kramer and Farabaugh 2007). To circumvent this problem, several genetic reporter assays were developed in bacteria and yeast based on a nonsense codon introduced into the open reading frame (ORF) of “fidelity reporters” such as the lacZ or luciferase (luc) genes (Rosenberger and Hilton 1983; Blank et al. 1986; Taddei et al. 1997; Shaw et al. 2002; Koyama et al. 2003; Roghanian et al. 2015). Transcription errors, transiently eliminating the nonsense mutation, would restore the full-length ORF of such a reporter, resulting in the production of a functional protein, the activity of which could be quantitatively determined. Although being broadly used, this approach suffers from an intrinsic problem of distinguishing transcription errors from a substantially more frequent ribosome misreading of a nonsense codon (Shaw et al. 2002; Kramer and Farabaugh 2007). The mRNA carrying an internal stop codon is also subjected to a nonsense-mediated decay in eukaryotes (Lykke-Andersen and Jensen 2015) and rho-dependent termination in prokaryotes (Gussin et al. 1987), further complicating the use of nonsense codons to detect transcription errors.
The high-resolution RNA sequencing (RNA-seq) method was initially developed for mammalian cells (Li et al. 2011) and then applied to study transcription misincorporation errors in Escherichia coli (Imashimizu et al. 2013) and Caenorhabditis elegans (Gout et al. 2013). The mammalian studies showed unexpectedly high levels of errors in mRNA (Li et al. 2011), many of which were later reported to be artifacts of RNA-seq (Hayden 2012; Pickrell et al. 2012), highlighting a technical problem in the detection of rare transcription errors due to the presence of higher levels (10−3/10−4/bp) of errors introduced by RNA-seq and PCR methods (Pickrell et al. 2012). More recently, a modified RNA-seq protocol was implemented that allowed separation of transcription errors from those made during reverse transcription and PCR during the processing of RNA samples. This more reliable method found ∼4 × 10−6 transcription misincorporations per base, with a highly-biased spectrum of error types (Gout et al. 2013).
Nascent elongating transcript sequencing in E. coli [NET-seq (Churchman and Weissman 2011)] has also been applied for detection (Imashimizu et al. 2015) and, more recently, for bioinformatics analysis (James et al. 2016) of transcription errors. This approach is based on the assumption that transcription errors at the 3′-end of nascent RNAs generally inhibit transcription elongation, leading to enrichment of nascent RNAs with transcription errors near the 3′-RNA end that had been isolated as part of in vivo paused RNA polymerase (RNAP) complexes (Kireeva et al. 2008; Sydow et al. 2009; Walmacq et al. 2009; Irvin et al. 2014). This method allows the identification of several different types of errors across the E. coli transcriptome, with the most frequent being G-to-A or C-to-U errors (Imashimizu et al. 2015). Despite these technical improvements, detection of transcription errors in vivo represents a very challenging and laborious task.
This work describes a coupled reporter assay to detect transcription errors in E. coli based on a Cre recombinase that is catalytically inactive due a missense mutation (Irvin et al. 2014). Restoration of active Cre depends upon a transcription misincorporation error. The transient mRNA is then translated into a functional Cre tetramer, whose activity in turn can be monitored by a Cre-dependent DNA recombination event in a galK-loxP-INV reporter that converts a gal mutant gene to gal+, giving rise to Gal+ colonies on selective or indicator agar. Due to the high sensitivity of the assay, we successfully monitored the intrinsic level of transcription errors in wild-type cells and, for the first time, identified GreA protein as a major transcription proofreading factor in E. coli.
Materials and Methods
Strains and plasmids
All strains are listed in Supplemental Material, Table S1 in File S1. The strains are derivatives of MG1655 except for recombinogenic strains used for recombineering that are derivatives of W3110. Oligonucleotides used for strain construction and PCRs are listed in Table S2 in File S1. Standard procedures for cell growth, P1 transduction, and media preparation were employed.
Strain construction by recombineering
The greA<>spc and greB<>amp knockouts were made by double-stranded DNA recombineering as described (Bubunenko et al. 2007). Briefly, the spc and amp resistance drug cassettes were constructed to precisely replace gre ORFs with the respective spc or amp ORFs. The drug cassettes were PCR amplified using the greA-spcF and -spcR and greB-ampF and -ampR sets of primers (see Table S2 in File S1), purified with a PCR Purification Kit (QIAGEN, Valencia, CA), and ∼3 ng electroporated into recombinogenic DY330 cells that were induced for Red functions for 15 min at 42° at 0.5 A600 optical units (o.u.) After electroporation, the were grown in LB at 32° for 3 hr, plated on L agar with ampicillin (Amp) 30 μg/ml or spectinomycin (Spc) 50 μg/ml, and incubated at 34° for 2 days to reveal recombinant cell colonies. The gene knockouts were identified by PCR amplification with “checking primers” that flank the respective chromosome regions and were verified by sequencing.
Insertion of cre into the ara operon was done by a counterselection procedure (Sawitzke et al. 2013b). The strain XT191, carrying the cat-sacB amp cassette located in the araD gene of the arabinose operon, expresses Red recombination functions and is used for counterselection against the sacB gene conferring sucrose sensitivity (Li et al. 2013). The cat-sacB amp region was replaced by the PCR product of cre+ flanked by DNA homology arms to the region upstream and downstream of the PBAD ara region (primers creF and creR, Table S2 in File S1) by selecting for sucrose resistance (SucR) on LB agar plates containing 6% sucrose and no NaCl. Finally, a cat cassette was made with PCR primers catF and catR and inserted downstream from cre replacing the nonessential yabQ (see Figure S4 in File S1). The entire PBAD -cre yabQ<>cat segment could then be moved between different strains by P1 transduction. The primers used to amplify the cat cassette with homology arms flanking the yabQ region are shown in Table S2 in File S1. The knockout was identified by PCR amplification with “checking primers” that flank yabQ.
The luciferase gene luc was inserted under PBAD control in MG1655 by also replacing cat-sacB using the same homology arms for recombineering as used to insert the cre+ gene under PBAD control (primers lucF and lucR). This, like for cre, results in the AUG of luc being positioned at the araB gene start codon (Figure S4E in File S1). See Figure S4, Figure S5, and Table S2 in File S1 for details of primers used and construction. The greA<>spec and greB<>amp knockouts were each crossed into the MG1655 PBAD–luc construct CC1234 to generate, by P1 transduction, CC1235 and CC1236, respectively. These three strains were compared for their luciferase activity before and after induction with arabinose.
The TGT mutation in codon 324 of cre was created by oligo-recombineering (Sawitzke et al. 2013a). The 70-mer oligo containing the single-base change in codon TAT324 to generate TGT or TGC was electroporated into W3110 cells containing araD<>cre and pSIM18 carrying the RED recombinogenic functions at 0.35 A600 o.u. The cells were grown at 32° for 2 hr in LB, nonselectively plated on L agar, and incubated at 32° for 2 days. The TGT/TGC mutations were identified by Mismatch Amplification Mutation Assay PCR with the respective antisense primers (see Table S2 in File S1) and verified by sequencing (Thomason et al. 2014).
Construction of the galK-loxP-INV reporter system
The construction of the galK-loxP-INV reporter system is shown in Figure S1 in File S1. The galK gene is highly conserved from E. coli to humans. Alignment of all GalK protein homologs revealed regions of the protein that are not conserved. We focused on one of these regions. At a site located between amino acid residues 239 and 240 of the native E. coli protein, we inserted a 36-bp DNA segment that contained a 34-bp loxP sequence (see below and Figure S2 in File S1). Despite the 12-codon insertion, cells from this recombination formed Gal+ colonies on minimal galactose agar (Figure S1B in File S1).
Next, we deleted the region from just downstream of the loxP site to beyond the galM gene, and replaced it with a cat-sacB cassette rendering the cells galK−, chloramphenicol-resistant, and sucrose-sensitive (Figure S1C in File S1). We then used PCR to amplify DNA from the construct shown in Figure S1B in File S1, so as to include loxP and the distal galK segment through to the end of galM. The PCR primers used for amplification carried homologies on each 5′-end designed to insert the loxP galK galM DNA segment in an inverted orientation by homologous recombination. This segment replaced the cat-sacB cassette by a recombination that generated SucR and chloramphenicol-sensitive (CmS) cells. The loxP* on the left contains an engineered single-base pair change that does not affect Cre-mediated recombination efficiency, but reduces homologous recombination between these two loxP sites. The two loxP sites, indicated by carets, are in inverted orientation relative to each other (Figure S1D in File S1). Lastly, the tetA gene from the Tn10 transposon was inserted just beyond the upstream loxP* site in galK so that translation of galK was prematurely terminated beyond the loxP* site at a translational stop codon, UGA, engineered into the construct (Figure S1E in File S1). Upon addition of active Cre protein, the DNA between the two loxP sites is inverted, rendering cells Gal+. The cells remain Tet resistant because the tetA gene in this inverted orientation relative to the original construct is juxtaposed next to a fortuitous promoter generated by the fusion of loxP and the gpmA segment (Figure S1F in File S1). The regions shown in Figure S1, E and F in File S1 have been sequenced and those sequences are shown in Figure S2 and Figure S3 in File S1.
The cre/galK-loxP-INV transcription fidelity reporter assay
The creTGT or creTGC constructs under PBAD control were introduced into strain CC942, which is MG1655 with the galK-loxP-INV, by P1 transduction selecting for CmR and screening on MacConkey Galactose (MacGal) agar for the Gal− phenotype. The respective recombinant strains CC945 and CC947 were purified two times on LB plates with Na citrate and appropriate antibiotic and stored in glycerol at −80°. For qualitative analysis, a single colony from glycerol stock was patched, streaked, or spotted on MacGal agar or on minimal M63Gal agar/agarose, and incubated at 37° for 2–3 days on MacGal or for 2–4 and up to 6 days on M63Gal agar and agarose plates, respectively. The typical results are shown in Figure 2A. For quantitative analysis, to determine the apparent frequency of the G→A transcription error, 2 ml LB was inoculated with a single colony and incubated at 37° for 20–25 hr. Nine cell cultures, each from an independent cell colony, were taken for each tested strain. Cell cultures were concentrated by centrifugation for 2 min in a microfuge, washed once with M63 medium, and resuspended to their original volume in M63. Cells were plated in duplicate at different dilutions on M63Gal agarose or MacGal agar to reveal Gal+ colonies, and on L agar to count viable cells. MacGal plates were incubated for 2 days, whereas M63Gal agarose plates were incubated for 5–6 days at 37°.
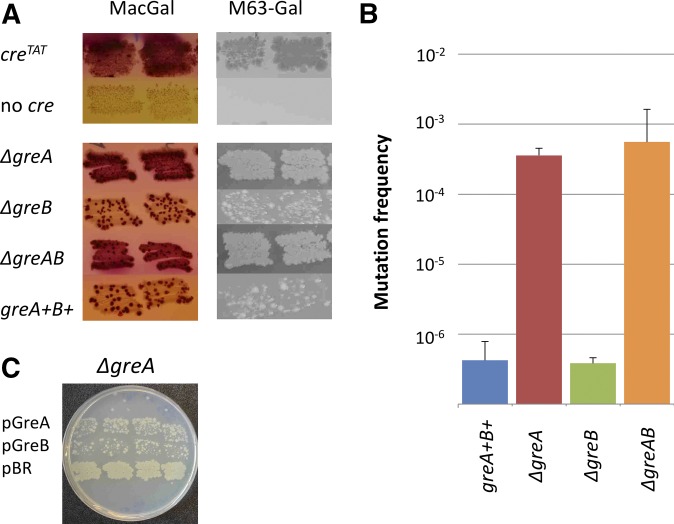
Figure 2.
GreA is a major transcription proofreading factor identified by the cre/galK-loxP-INV genetic reporter assay. (A) Cells patched on MacConkey Galactose (MacGal) plates on M63Gal agar/agarose plates all show similar results that the G→A transcription error, identified by the density of Gal+ colonies, dramatically increases if GreA (ΔgreA cells) or both GreA and GreB (ΔgreAB cells) are deleted from the wild-type cells (greA+B+ cells). Deletion of only GreB (ΔgreB cells) has no effect. (B) Quantitation of the effect of Gre factors on the apparent frequency of G→A transcription error measured by the plating efficiency assay on M63Gal agarose. Each bar represents a value of Gal+ counts from nine independent cell cultures plated in duplicate with the error bars shown. (C) Patches of ΔgreA cells on M63Gal agar show that overexpression of GreB (pGreB) or GreA (pGreA) almost equally suppresses the frequency of G→A transcription error in GreA-lacking cells as compared to the same cells containing pBR322 control plasmid.
Overexpression of GreA and GreB transcription factors
pGreA and pGreB (Koulich et al. 1997) were kind gifts from Sergei Borukhov. Both plasmids are pTrc88A-based with gre genes expressed from the Ptrc hybrid promoter. The plasmids were electroporated into the NB1001 ΔgreA cells and, after 2 hr of incubation at 37°, the cell cultures were plated on L agar with Amp 100 μg/ml. The creTGT-cat reporter was introduced into the pGre-containing cells by P1 transduction and the resulting cells were patched on M63Gal agar with Amp 50 μg/ml and then incubated at 37° for 3 days. Cells transformed with the related pBR322 vector were used as a control.
Luciferase assays
Luciferase activity was measured by using the reagents and methods described in the Promega Luciferase Assay System according to the company’s directions (Promega, Madison, WI). Cells were grown in LB to an OD650 of 0.4. Culture aliquots (0.1 ml) were centrifuged for 2 min at 21,000 × g, and pellets were resuspended in 0.4 ml of Cell Culture Lysis Reagent with 2.5 mg/ml BSA and 1.25 mg/ml lysozyme. Twenty microliters of cell lysate were mixed with 0.1 ml of the luciferin substrate, incubated for 120 sec, and read in and EG&G Berthold Lumat LB 9507 single sample luminometer for 10 sec. The relative light unit was normalized to A650.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All strains and reagents are available upon request.
Results
Cre/lox reporter assay for detection of transcription errors in bacterial cells
The Cre/Lox reporter assay described in this work was designed on the basis of our previous assay to study transcription fidelity in yeast cells (Irvin et al. 2014). The assay involved two sequential events that allowed the conversion of rare, transient transcription errors at a unique codon position of the cre gene to be detected as Cre-mediated recombination (Figure 1A). It was previously shown that the tyrosine residue in the active site of Cre was required in all the subunits of the active tetramer and that no other residue can substitute (Wierzbicki et al. 1987). In the bacterial system described here, Cre mediates recombination elsewhere in the genome causing Gal− cells to become Gal+. The two hallmarks of the assay are: (i) a catalytically inactive Cre recombinase caused by a missense mutation replacing the codon for the active site tyrosine (Y324), and (ii) a Cre recombination substrate, i.e., two inverted LoxP sites flanking an inverted galK segment of the galactose operon such that Cre-mediated recombination between the inverted LoxP sites restores an intact galK gene. The Gal+ phenotype can be detected as cells able to form colonies on minimal M63 galactose or as red colonies on indicator MacGal agar (Figure 1B). The same recombination event also activates transcription of an inverted tetA gene located in the inversion site causing the cells to also become resistant to 12.5 μg/ml tetracycline.
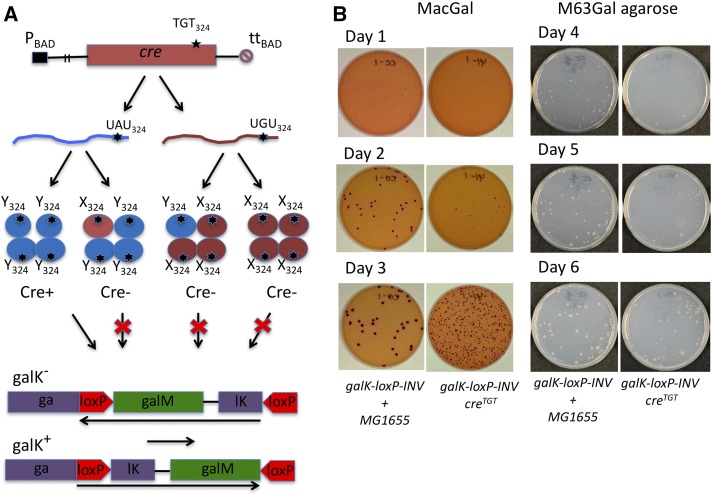
Figure 1.
The principles of the creTGT/galK-loxP-INV assay designed for detection of G→A transcription errors in E. coli. (A) Flowchart of the assay. A cre gene with a TAT to TGT mutation in codon 324 was placed into the ara operon of the E. coli chromosome to ensure low-level expression of the totally inactive Cre Y324C protein from the PBAD promoter. Infrequent G→A transcription misincorporation events restore the wild-type Tyr324 residue in Cre yielding transient Cre recombination activity. (B) Plating efficiency assay used to quantitate preexisting Gal+ as a measure of transcription misincorporation errors. The apparent frequency of Gal−→Gal+ events is quantitated as a number of red colonies on indicator MacConkey Galactose (MacGal) plates or Gal+ prototrophic colonies on selective M63Gal agarose plates. The time window for detection of preexisting Gal+ is determined using a reference culture (shown as galK-loxP-INV + MG1655), which is composed of the defined small number of Gal+ wild-type MG1655 cells (∼60–80 cells) and the Gal− galK-loxP-INV cells (104–106) containing no Cre reporter and, therefore, yielding no Gal+ colonies. In this setup, MG1655 cells produce red Gal+ colonies on an outgrowth of the Gal− galK-loxP-INV cells (the first column). Time when these colonies appear provides an important information on a time window for counting the Gal+ colonies derived from Cre-mediated recombination in galK-loxP-INV/creTGT cells (shown in the second column). A number of the Gal+ colonies significantly increased after 3-day plating of galK-loxP-INV/creTGT cells, but not the reference galK-loxP-INV + MG1655 cells, which was expected because the Cre-mediated recombination continued in galK-loxP-INV/creTGT cells after they have already been plated on MacGal. In the example representing herein the quantitation of the G→A transcription error in the wild-type background (galK-loxP-INV/creTGT cells), it takes 2 days to detect preexisting Gal+ colonies on MacGal agar plates (the middle row) and up to 6 days to detect the colonies on the M63Gal agarose plates (the bottom row).
The creY324C mutation changes the UAU codon to UGU and thus requires a G→A transcription error to create an mRNA encoding functional Cre (Irvin et al. 2014). The G→A error has been shown to be the major class of transcription misreading in E. coli (Imashimizu et al. 2013; James et al. 2016) and yeasts (Kireeva et al. 2008). When the mRNA is translated, a now functional Cre catalyzes recombination via a Lox-mediated DNA flipping event (Nagy 2000) at a galK-loxP-INV reporter gene. In this manner, a transient transcription error in the Cre mRNA is converted into a stable genetic change as a restored galK gene, the activity of which is assessed using Gal+ selection or indicator agar. Importantly, the Cre protein acts as a tetramer strictly requiring Tyr324 to be present in all four subunits (Figure 1) (Hartung and Kisters-Woike 1998; Ennifar et al. 2003; Lee and Sadowski 2003; Ghosh et al. 2007; Gibb et al. 2010). This property of Cre ensures that an active tetramer can arise from suppression of the codon 324 mutation at the transcription, rather than the translation, level. Indeed, translational suppression would require ribosomes to misincorporate tyrosine for cysteine at the UGU codon four times in the same cell to have a chance of creating an active Cre tetramer, whereas only a single transcription error would be sufficient to produce an active Cre tetramer by multi-round translation of a single UAU-containing mRNA transcript. The estimated ribosomal error rate in E. coli is ∼10−3 per codon (Kramer and Farabaugh 2007), which makes four independent translation errors (10−12) happening at the UGU codon in the same cell and in a short period of time extremely unlikely. On the other hand, transcription errors, though rarer than translation errors, occur with rates of 10−5–10−7/nt in bacteria and eukaryotes (Rosenberger and Hilton 1983; Blank et al. 1986; Ninio 1991; Gout et al. 2013). We used this unique property of the Cre recombinase to reduce the impact of translation errors on the assay.
Construction of galK-loxP-INV reporter
The construction of the galK-loxP-INV reporter system is shown in Figure S1 in File S1. The galK gene is highly conserved from E. coli to humans. Alignment of several homologs indicated four regions of the protein with reduced conservation, variable spacing, and located in loops on the protein surface. Using recombineering technology (Court et al. 2002), we first inserted DNA at these four positions to put the galK gene out of frame. We then restored the reading frame by substituting a 36-bp in-frame sequence containing the 34-bp loxP site, and selected for Gal+ cells. In all four cases, the 36-bp insertions resulted in a Gal+ phenotype. For pragmatic reasons, we focused on a site located between amino acid residues 239 and 240 of the native E. coli sequence (Figure S1B in File S1). Next, we deleted the region from just downstream of the loxP site to beyond the galM gene, and replaced it with a cat sacB cassette (Sawitzke et al. 2013b), rendering the cells Gal−, chloramphenicol-resistant, and sucrose-sensitive (Figure S1C in File S1). Inversion of the downstream portion of galK including galM and a second loxP site was inserted by recombineering and selecting for sucrose resistance. (Figure S1D in File S1). This loxP site contained a single-base pair change that does not affect cre-mediated recombination efficiency, but reduces unwanted homologous recombination between loxP sites. Lastly, the tetA gene from Tn10 was inserted just beyond the upstream loxP site in galK to cause translation of the galK mRNA to terminate beyond the loxP site at a translational stop codon UGA engineered into the construct (Figure 1C). In this configuration, the cells have a Gal− phenotype. Upon providing active Cre protein, recombination between the two loxP sites inverts the DNA to generate an intact galK gene and restoring a Gal+ phenotype (Figure S1F in File S1).
Construction of the cre gene under pBAD promoter control
The P1 Cre recombinase gene cre was PCR amplified with an optimized ribosome-binding site (Shine-Dalgarno, SD) upstream and inserted into the E. coli arabinose operon, replacing the native araBAD genes and their native SD by recombineering (Sawitzke et al. 2013b). This single copy of cre is under the control of the ara promoter region and AraC repressor, which provides very low-level transcription of Cre in the noninduced state used here. Any rare Cre transcripts are translated efficiently by the optimized SD (Guzman et al. 1995) ensuring that multiple peptides are made from one transcript. By keeping the level of transcription low, we hoped to minimize subunit mixing of Cre translated from functional and mutant transcripts. A cat gene conferring chloramphenicol resistance was placed downstream of the cre gene in the construct as a selectable marker to move the cre construct to other strains, and to also enable further cre gene modifications. These constructions are outlined in Figure S4 in File S1.
Characterization of the coupled Cre and galK-loxP-INV reporter system
The Cre and galK-loxP-INV reporter system was constructed by P1 transduction of creTGT or creTGC into the galK-loxP-INV reporter strain selecting for the linked CmR marker (yabQ<>cat). These CmR transductants were screened to be defective for utilization of either arabinose or galactose on MacConkey arabinose or MacGal agar plates, respectively. We note that a fraction of the transduced CmR cells had a Gal+ phenotype, as indicated by red colonies on MacGal plates. This was caused by the presence of the P1 phage during the transduction. P1 carries the wild-type cre gene in its native genome, allowing expression of Cre and flipping of the LoxP sites in some of the cells being transduced. The cells remaining Gal− retained the inverted galK region as defined by PCR analysis.
Optimization of the Cre/galK-loxP-INV reporter assay
We tested various growth conditions and levels of the creTGT expression to increase the sensitivity of the assay. Two different creTGT/galK-loxP-INV clones kept at −80° were plated on LB medium at 37°, and four independent colonies of each clone were taken for these experiments.
For qualitative analysis, we employed cell patching and streaking techniques from single colonies on MacGal and M63Gal plates incubated at 30–42°. When the Gal− creTGT/galK-loxP-INV cells were patched/streaked on MacGal indicator plates, infrequent Gal+ red papillae appeared on top of a dense lawn of white Gal− cells. On minimal M63Gal plates, the rare Gal+ colonies were formed because only Gal+ cells were able to grow on a minimal medium with galactose. Thus, both types of the selection plates effectively detected LoxP recombinants. Three and five days of incubation at 37° on either MacGal or M63Gal plates, respectively, were optimal to reliably detect the Gal+ colonies. These conditions were chosen as a standard for the patching and streaking assays throughout the work. Importantly, when the cre gene was not present with the galK-loxP-INV reporter (strain CC942), Gal+ colonies were not detected even when > 109 cells were spread on the agar plates. This demonstrated that the galK-loxP-INV reporter was stable and did not undergo detectable recombination in the absence of Cre protein.
Quantifying the apparent frequency of Gal−→Gal+ events
We used two approaches to detect Gal+ cells: (1) MacGal plates on which Gal+ colonies are red while Gal− colonies are white or (2) minimal plates in which galactose is a carbon source. Both approaches have features that require special attention to detail. On MacGal plates, all the Gal+ and Gal− cells can grow into colonies and can continue to produce Gal+ recombinants during that growth. Preexisting Gal+ cells in LB were expected to form colonies more rapidly and be larger than their counterparts that became Gal+ only after a growth period on the agar plate. We observed the appearance of two different Gal+ populations when plating the creTGT/galK-loxP-INV reporter strain on MacGal plates: (1) red colonies appearing between 24 and 48 hr of growth and (2) red colonies of variable sizes appearing after 48-hr incubation. The colonies coming up after 48-hr likely occur due to Cre-mediated recombination on the plate after these cells were put down. We have adopted a time window (24–48 hr) to identify red colonies derived by Cre-mediated recombination to gal+ during the assay, from those occurring after the experiment. This was validated by control experiments with mixed plating of MG1655 Gal+ cells in the presence of a lawn of Gal− cells from a gal deletion mutant. Similarly, we observed substantial growth of Gal− cells on M63Gal agar plates due to an unknown carbon source in the Difco agar. On these plates, we could even detect residual growth of a gal deletion mutant. Because the creTGT/galK-loxP-INV reporter strain could grow slowly on M63Gal agar, it generated an ever-increasing number of Cre-generated Gal+ colonies with increased time of incubation. However, plates made with agarose instead of the Difco agar did not show this growth, and Gal+ colonies derived during the experimental assay and before plating were readily detected at ∼5–6 days and their number did not dramatically increase with time. Based on this finding, we have used M63Gal agarose and scored Gal+ colonies after 5-days incubation at 37° throughout this work to determine the frequency of Gal+ colonies. On the other hand, the MacGal plates were very useful for rapid and preliminary screening in E. coli strains with mutants affecting transcription fidelity and for wild-type cells growing under various conditions and stresses.
The MacGal method reproducibly yielded a ∼10−6 frequency of preexisting Gal+ cells for the creTGT/galK-loxP reporter strain when grown overnight in LB at 37°. A fluctuation test performed on M63 agarose yielded a similar Cre-mediated galK+ recombination frequency (∼10−6/cell) (see Materials and Methods for details). The scheme and the timeline for screening the Gal+ colonies and the typical appearance of the colonies on MacGal and M63Gal agarose plates are shown in Figure 1B.
Role of GreA and GreB in correcting transcription errors in E. coli
A role of GreA and GreB proteins in proofreading of transcription errors is widely postulated [Fish and Kane 2002; Borukhov et al. 2005; Zenkin and Yuzenkova 2015; and demonstrated in vitro for GreA (Erie et al. 1993)]. Recently, we gained evidence for an in vivo role of Gre factors by NET-seq, which detects transcription errors localized at the 3′-end of the nascent RNA (Imashimizu et al. 2015). We found that G→A transcription errors were substantially enriched at the 3′ RNA ends in the E. coli cells lacking both GreA and GreB. The creTGT/galK-loxP-INV reporter is designed to detect these G→A errors. Using our assay, we attempted to assess the role of Gre factors in modulating the G→A-type errors in E. coli. The ΔgreAB mutant used in the RNA-seq study was reconstructed for this experiment by introducing the greA<>spc and greB<>amp knockouts in the galK-loxP-INV background followed by the introduction of the creTGT reporter. The ΔgreAB and greA+B+ strains were patched on MacGal and M63Gal plates to observe the formation of Gal+. As shown by cell patching (Figure 2A), ΔgreAB significantly increased the accumulation of Gal+ revertants, as compared to the greA+B+/creTGT cells. Quantification of error frequencies in the ΔgreAB cells (Figure 2B) using M63Gal agarose plates solidly supported the patch analysis (Figure 2B). The number of Gal+ revertants in ΔgreAB cells was ∼2 orders of magnitude higher than in the gre+ cells. Thus, the creTGT/galK-loxP-INV reporter assay can easily detect transcription errors revealed in the absence of Gre factors.
GreA, but not GreB, acts as an RNA proofreading factor
The relative impact of GreA vs. GreB factor on proofreading of transcription errors in vitro and in vivo has never been approached experimentally. To examine their impact on fidelity, we constructed single greA<>spc and greB<>amp knockouts in the galK-loxP-INV background. As with the ΔgreAB strain, these strains did not form Gal+ colonies either on MacGal or M63Gal plates in the absence of a cre gene. When the creTGT allele was present, the ΔgreA but not the ΔgreB strain showed an increased accumulation of Gal+ revertants as compared to the greA+B+/creTGT strain (Figure 2A). These data demonstrated a strong effect of GreA and no apparent effect of GreB on transcription fidelity of RNAP in correcting a G-to-A mistake in transcription.
Quantification of error frequencies in the ΔgreA cells (Figure 2B) using M63Gal agarose plates confirmed the results from the cell patching analysis (Figure 2B). Similarly to the ΔgreAB cells, the number of Gal+ revertants in the ΔgreA cells was ∼2 orders of magnitude higher than in the gre+ cells, but was virtually unaffected in the ΔgreB cells. Clearly, a lack of GreB did not change the G→A error frequency either in the greA+ or in the ΔgreA cells. Thus, the quantitative analysis revealed a critical role of GreA but not GreB in controlling the transcription errors in E. coli cells under growth conditions in rich media.
The lack of a GreB effect on transcription fidelity was surprising, especially in contrast to the substantial effect of its counterpart GreA (Figure 2, A and B). The two Gre factors have always been considered as having similar roles that would enhance fidelity of RNAP transcription (Fish and Kane 2002; Borukhov et al. 2005; Zenkin and Yuzenkova 2015). Both stimulate the intrinsic RNA cleavage activity of RNAP when it is stalled in the backtracked state (Borukhov et al. 1993; Toulme et al. 2000). Both also interact with the same site on the RNA polymerase (Laptenko et al. 2003; Opalka et al. 2003; Sosunova et al. 2003), and both can be efficient in proofreading of G→A errors, as demonstrated by our in vitro misincorporation experiments (Figure S6 in File S1). Therefore, we reasoned that the apparent lack of an impact of GreB on transcription fidelity in vivo might be due either to its lower affinity for the RNAP elongation complex than that of GreA, or to a relatively lower concentration of GreB in the cell (Rutherford et al. 2007; Lamour et al. 2008; Vinella et al. 2012), or both.
We tested this by overexpressing the GreB protein in the ΔgreA background. The experiment was done with the ΔgreA creTGT/galK-loxP-INV strain transformed with plasmids that expressed either GreA or GreB protein from a leaky Ptrc promoter (Hsu et al. 1995) without IPTG induction. The pBR322 vector was taken as a control. Gal+ revertants were scored by patching on M63Gal. The results showed (Figure 2C) that, in comparison to the ΔgreA cells containing pBR322, GreB overexpressed from the plasmid in ΔgreA cells reduced the intensity of Gal+ patches almost to the level of cells overexpressing GreA. Thus, transcription fidelity in the ΔgreA mutant can be complemented by either GreA or GreB overexpression with efficient correction of G→A transcription errors, at least in the sequence context of our cre reporter.
The Gre factors also play a role in the global regulation of gene expression (Rutherford et al. 2007; Stepanova et al. 2007; Vinella et al. 2012). To rule out that the effect of Gre factors on Gal+ accumulation in the assay is caused by their regulation of cre expression from the arabinose promoter, luc gene fusions were constructed by precisely replacing the cre ORF in the ara operon with the luc ORF in the strains with ΔgreA greB+, greA+ΔgreB, and greA+greB+ backgrounds. All three strains were inducible by arabinose from ∼2 × 105 light units to 2 × 108 light units. Less than a twofold variation in luciferase activity was seen among the three strains, indicating that the effect of Gre in the assay is attributable to modulating transcription errors, but not the level of cre expression from the arabinose promoter. This is consistent with the ara operon genes not having been reported as targets for Gre factors (Stepanova et al. 2007; Vinella et al. 2012).
Discussion
In the present work, we describe a genetic assay to detect transcription misincorporation of G by A in E. coli. The genetic assay (Figure 1) consists of two main modules residing in the bacterial chromosome: (1) a gene for Cre recombinase with a single-nucleotide change (A→G) in the second position of the TAT324 codon that inserts cysteine for the essential tyrosine residue at position 324, and (2) a substrate for Cre recombinase, the inverted portion of galK flanked by a pair of loxP sites oriented so as to confer a Gal− phenotype until inverted by Cre-mediated recombination. A rare transcription misincorporation event converting the mutated codon back to UAU in the Cre mRNA results in the production of active Cre tetramers via multiple rounds of translation of the unique Cre+ mRNA molecule. The recombination activity of the Cre tetramer at the loxP sites in galK-loxP-INV restores the galK gene, yielding Gal+ colonies on minimal Gal-selective or MacGal indicator agar. In this manner, misincorporation in the Cre mRNA is detected by a secondary event, i.e., a genetic change restoring the galK gene and the Gal+ phenotype to the cell. The virtual absence of Cre-independent recombination events allows detection of transcription errors with apparent frequencies as low as 10−8 per cell.
The TGT324 cre reporter mutant was designed for detection of the G→A transition errors that had been previously found to be the most frequent type of transcription misincorporation error both in yeast and bacterial cells (Kireeva et al. 2008; Imashimizu et al. 2013). Our reporter system has been used to optimize some of the conditions for the assay and to analyze the natural level of the G→A error at this position in wild-type E. coli, and to determine the role of Gre proofreading factors in control of error frequency. GreA and GreB were long suspected to have a role in proofreading of transcription misincorporation errors in vivo (Erie et al. 1993). Both factors (Erie et al. 1993), as well as their eukaryotic analog TFIIS (Thomas et al. 1998; Sydow et al. 2009; Irvin et al. 2014; Schweikhard et al. 2014), reduce errors in vitro by stimulating intrinsic RNA cleavage by RNAP in elongation complexes that are arrested by backtracking. Errors at the 3′-end of nascent RNA isolated from stalled elongation complexes in vitro and in vivo (Imashimizu et al. 2013) have been shown to promote RNAP backtracking. Gre-mediated proofreading activity has been directly demonstrated in vitro for GreA (Erie et al. 1993) and more recently in vivo over the entire transcriptome by RNA-seq and NET-seq analyses of transcription errors when comparing wild-type vs. a ΔgreAB double mutant (Imashimizu et al. 2013, 2015), and by bioinformatics analysis (James et al. 2016).
In the present work, G→A errors were found to occur with a relatively low 10−6 frequency in wild-type E. coli using our reporter assay. The error frequency was dramatically increased in the ΔgreAB mutant, consistent with our previously published data on genome-wide NET-seq analysis comparing transcription errors in wild-type and ΔgreAB cells (Imashimizu et al. 2015). Here, we show that GreA has the major impact on transcription fidelity because a similar increase of magnitude in G→A misincorporation occurred in either a ΔgreA single or ΔgreAB double knockouts, whereas wild-type and ΔgreB mutant cells were virtually indistinguishable in error frequency.
Our in vivo results for GreB were surprising since GreB has always been assigned a more versatile and potent function from in vitro studies (Borukhov et al. 2005). For example, GreB is more efficient at catalyzing RNA cleavage for in vitro transcription assays, causing cleavage from the RNA 3′-end of up to 18-nt long RNA fragments, whereas GreA cleaves off only 2–4-nt long fragments (Borukhov et al. 1993). However, an overall examination of the characteristics of the two Gre proteins revealed that, while both Gre proteins bind to a coincident site within the secondary pore of RNAP to exert their effect (Laptenko et al. 2003; Opalka et al. 2003; Sosunova et al. 2003), GreB is five times less abundant in cells (Rutherford et al. 2007; Lamour et al. 2008). These properties might disfavor GreB binding to RNAP. Indeed, we observed that overexpression of GreB in the ΔgreA cells reduced G→A errors to wild-type levels, demonstrating that GreB at high concentration could substitute for GreA in RNA proofreading activity in vivo. Thus, one cannot exclude the possibility that GreB may act as a proofreading factor in wild-type cells under certain growth conditions or on other sequence contexts, or perhaps with rare RNAP elongation conformations that are not accessible by GreA. It has been shown in vitro that when RNAP backtracks at distances > 2–3-bp, RNA catalysis by GreA is inhibited while that of GreB is stimulated (Borukhov et al. 1993). GreB may primarily use RNA proofreading activity for transcriptional emergencies that are distinct from transcription fidelity, e.g., the rescue of RNAP from a prolonged pausing after backtracking (James et al. 2016). For these reasons, the limited proofreading capabilities of GreB under normal E. coli growth and its role in cell physiology will require further examination.
In conclusion, the assay that we have developed on the basis of the Cre/Lox reporter system has shown great promise for detecting transcription errors and for uncovering new details of Gre-mediated transcription proofreading in living cells. Importantly, the results of its application are consistent with our whole transcriptome analysis of misincorporation errors in E. coli (Imashimizu et al. 2013, 2015). The reporter assay will be useful in furthering our understanding of the role and mechanisms of transcription fidelity in bacterial cells, as well as for identifying RNAP mutants with altered error rates. Other proteins, e.g., DksA and RNK, in addition to GreA and GreB also bind to the secondary channel of RNAP and are known to affect RNAP elongation (Fish and Kane 2002; Borukhov et al. 2005; Zenkin and Yuzenkova 2015) and transcription misincorporation (Roghanian et al. 2015; Satory et al. 2015). An evaluation of the impact of these proteins on transcription fidelity in E. coli with the Cre/Lox reporter is currently underway.
Supplementary Material
Supplemental material is available online at www.genetics.org/lookup/suppl/doi:10.1534/genetics.116.198960/-/DC1.
Acknowledgments
We thank Thomas Schneider for bioinformatics support in identification of the fortuitous promoter for the tetA gene. We are also grateful to the other members of the RNA Biology Laboratory for discussion. This work was funded by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (to M.K., J.N.S., D.L.C., and D.J.J.). Funding for open access charge: The Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research.
Footnotes
Communicating editor: M. Hampsey
Literature Cited
- Blank A., Gallant J. A., Burgess R. R., Loeb L. A., 1986. An RNA polymerase mutant with reduced accuracy of chain elongation. Biochemistry 25: 5920–5928. [DOI] [PubMed] [Google Scholar]
- Borukhov S., Sagitov V., Goldfarb A., 1993. Transcript cleavage factors from E. coli. Cell 72: 459–466. [DOI] [PubMed] [Google Scholar]
- Borukhov S., Lee J., Laptenko O., 2005. Bacterial transcription elongation factors: new insights into molecular mechanism of action. Mol. Microbiol. 55: 1315–1324. [DOI] [PubMed] [Google Scholar]
- Bouadloun F., Donner D., Kurland C. G., 1983. Codon-specific missense errors in vivo. EMBO J. 2: 1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bubunenko M., Baker T., Court D. L., 2007. Essentiality of ribosomal and transcription antitermination proteins analyzed by systematic gene replacement in Escherichia coli. J. Bacteriol. 189: 2844–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchman L. S., Weissman J. S., 2011. Nascent transcript sequencing visualizes transcription at nucleotide resolution. Nature 469: 368–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Court D. L., Sawitzke J. A., Thomason L. C., 2002. Genetic engineering using homologous recombination. Annu. Rev. Genet. 36: 361–388. [DOI] [PubMed] [Google Scholar]
- Edelmann P., Gallant J., 1977. Mistranslation in E. coli. Cell 10: 131–137. [DOI] [PubMed] [Google Scholar]
- Ennifar E., Meyer J. E., Buchholz F., Stewart A. F., Suck D., 2003. Crystal structure of a wild-type Cre recombinase-loxP synapse reveals a novel spacer conformation suggesting an alternative mechanism for DNA cleavage activation. Nucleic Acids Res. 31: 5449–5460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erie D. A., Hajiseyedjavadi O., Young M. C., von Hippel P. H., 1993. Multiple RNA polymerase conformations and GreA: control of the fidelity of transcription. Science 262: 867–873. [DOI] [PubMed] [Google Scholar]
- Fish R. N., Kane C. M., 2002. Promoting elongation with transcript cleavage stimulatory factors. Biochim. Biophys. Acta 1577: 287–307. [DOI] [PubMed] [Google Scholar]
- Ghosh K., Guo F., Van Duyne G. D., 2007. Synapsis of loxP sites by Cre recombinase. J. Biol. Chem. 282: 24004–24016. [DOI] [PubMed] [Google Scholar]
- Gibb B., Gupta K., Ghosh K., Sharp R., Chen J., et al. , 2010. Requirements for catalysis in the Cre recombinase active site. Nucleic Acids Res. 38: 5817–5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout J. F., Thomas W. K., Smith Z., Okamoto K., Lynch M., 2013. Large-scale detection of in vivo transcription errors. Proc. Natl. Acad. Sci. USA 110: 18584–18589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gussin G. N., Brown S., Matz K., 1987. Translational polarity of a mutation in the initiator AUG codon of the Λ cI gene. Genetics 117: 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman L. M., Belin D., Carson M. J., Beckwith J., 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177: 4121–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung M., Kisters-Woike B., 1998. Cre mutants with altered DNA binding properties. J. Biol. Chem. 273: 22884–22891. [DOI] [PubMed] [Google Scholar]
- Hayden E. C., 2012. RNA studies under fire. Nature 484: 428. [DOI] [PubMed] [Google Scholar]
- Hsu L. M., Vo N. V., Chamberlin M. J., 1995. Escherichia coli transcript cleavage factors GreA and GreB stimulate promoter escape and gene expression in vivo and in vitro. Proc. Natl. Acad. Sci. USA 92: 11588–11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imashimizu M., Oshima T., Lubkowska L., Kashlev M., 2013. Direct assessment of transcription fidelity by high-resolution RNA sequencing. Nucleic Acids Res. 41: 9090–9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imashimizu M., Takahashi H., Oshima T., McIntosh C., Bubunenko M., et al. , 2015. Visualizing translocation dynamics and nascent transcript errors in paused RNA polymerases in vivo. Genome Biol. 16: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvin J. D., Kireeva M. L., Gotte D. R., Shafer B. K., Huang I., et al. , 2014. A genetic assay for transcription errors reveals multilayer control of RNA polymerase II fidelity. PLoS Genet. 10: e1004532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James K., Gamba P., Cockell S. J., Zenkin N., 2016. Misincorporation by RNA polymerase is a major source of transcription pausing in vivo. Nucleic Acids Res. 45: 1105–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva M. L., Nedialkov Y. A., Cremona G. H., Purtov Y. A., Lubkowska L., et al. , 2008. Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol. Cell 30: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulich D., Orlova M., Malhotra A., Sali A., Darst S. A., et al. , 1997. Domain organization of Escherichia coli transcript cleavage factors GreA and GreB. J. Biol. Chem. 272: 7201–7210. [DOI] [PubMed] [Google Scholar]
- Koyama H., Ito T., Nakanishi T., Kawamura N., Sekimizu K., 2003. Transcription elongation factor S-II maintains transcriptional fidelity and confers oxidative stress resistance. Genes Cells 8: 779–788. [DOI] [PubMed] [Google Scholar]
- Kramer E. B., Farabaugh P. J., 2007. The frequency of translational misreading errors in E. coli is largely determined by tRNA competition. RNA 13: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamour V., Rutherford S. T., Kuznedelov K., Ramagopal U. A., Gourse R. L., et al. , 2008. Crystal structure of Escherichia coli Rnk, a new RNA polymerase-interacting protein. J. Mol. Biol. 383: 367–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptenko O., Lee J., Lomakin I., Borukhov S., 2003. Transcript cleavage factors GreA and GreB act as transient catalytic components of RNA polymerase. EMBO J. 22: 6322–6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L., Sadowski P. D., 2003. Identification of Cre residues involved in synapsis, isomerization, and catalysis. J. Biol. Chem. 278: 36905–36915. [DOI] [PubMed] [Google Scholar]
- Li M., Wang I. X., Li Y., Bruzel A., Richards A. L., et al. , 2011. Widespread RNA and DNA sequence differences in the human transcriptome. Science 333: 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. T., Thomason L. C., Sawitzke J. A., Costantino N., Court D. L., 2013. Positive and negative selection using the tetA-sacB cassette: recombineering and P1 transduction in Escherichia coli. Nucleic Acids Res. 41: e204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykke-Andersen S., Jensen T. H., 2015. Nonsense-mediated mRNA decay: an intricate machinery that shapes transcriptomes. Nat. Rev. Mol. Cell Biol. 16: 665–677. [DOI] [PubMed] [Google Scholar]
- Nagy A., 2000. Cre recombinase: the universal reagent for genome tailoring. Genesis 26: 99–109. [PubMed] [Google Scholar]
- Ninio J., 1991. Connections between translation, transcription and replication error-rates. Biochimie 73: 1517–1523. [DOI] [PubMed] [Google Scholar]
- Opalka N., Chlenov M., Chacon P., Rice W. J., Wriggers W., et al. , 2003. Structure and function of the transcription elongation factor GreB bound to bacterial RNA polymerase. Cell 114: 335–345. [DOI] [PubMed] [Google Scholar]
- Pickrell J. K., Gilad Y., Pritchard J. K., 2012. Comment on “Widespread RNA and DNA sequence differences in the human transcriptome.” Science 335: 1302, author reply 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roghanian M., Zenkin N., Yuzenkova Y., 2015. Bacterial global regulators DksA/ppGpp increase fidelity of transcription. Nucleic Acids Res. 43: 1529–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberger R. F., Foskett G., 1981. An estimate of the frequency of in vivo transcriptional errors at a nonsense codon in Escherichia coli. Mol. Gen. Genet. 183: 561–563. [DOI] [PubMed] [Google Scholar]
- Rosenberger R. F., Hilton J., 1983. The frequency of transcriptional and translational errors at nonsense codons in the lacZ gene of Escherichia coli. Mol. Gen. Genet. 191: 207–212. [DOI] [PubMed] [Google Scholar]
- Rutherford S. T., Lemke J. J., Vrentas C. E., Gaal T., Ross W., et al. , 2007. Effects of DksA, GreA, and GreB on transcription initiation: insights into the mechanisms of factors that bind in the secondary channel of RNA polymerase. J. Mol. Biol. 366: 1243–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satory D., Gordon A. J. E., Wang M. Y., Halliday J. A., Golding I., et al. , 2015. DksA involvement in transcription fidelity buffers stochastic epigenetic change. Nucleic Acids Res. 43: 10190–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzke J. A., Thomason L. C., Bubunenko M., Li X., Costantino N., et al. , 2013a Recombineering: highly efficient in vivo genetic engineering using single-strand oligos. Methods Enzymol. 533: 157–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawitzke J. A., Thomason L. C., Bubunenko M., Li X., Costantino N., et al. , 2013b Recombineering: using drug cassettes to knock out genes in vivo. Methods Enzymol. 533: 79–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweikhard V., Meng C., Murakami K., Kaplan C. D., Kornberg R. D., et al. , 2014. Transcription factors TFIIF and TFIIS promote transcript elongation by RNA polymerase II by synergistic and independent mechanisms. Proc. Natl. Acad. Sci. USA 111: 6642–6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R. J., Bonawitz N. D., Reines D., 2002. Use of an in vivo reporter assay to test for transcriptional and translational fidelity in yeast. J. Biol. Chem. 277: 24420–24426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosunova E., Sosunov V., Kozlov M., Nikiforov V., Goldfarb A., et al. , 2003. Donation of catalytic residues to RNA polymerase active center by transcription factor Gre. Proc. Natl. Acad. Sci. USA 100: 15469–15474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova E., Lee J., Ozerova M., Semenova E., Datsenko K., et al. , 2007. Analysis of promoter targets for Escherichia coli transcription elongation factor GreA in vivo and in vitro. J. Bacteriol. 189: 8772–8785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathern J. N., Jin D. J., Court D. L., Kashlev M., 2012. Isolation and characterization of transcription fidelity mutants. Biochim. Biophys. Acta 1819: 694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydow J. F., Brueckner F., Cheung A. C., Damsma G. E., Dengl S., et al. , 2009. Structural basis of transcription: mismatch-specific fidelity mechanisms and paused RNA polymerase II with frayed RNA. Mol. Cell 34: 710–721. [DOI] [PubMed] [Google Scholar]
- Taddei F., Hayakawa H., Bouton M., Cirinesi A., Matic I., et al. , 1997. Counteraction by MutT protein of transcriptional errors caused by oxidative damage. Science 278: 128–130. [DOI] [PubMed] [Google Scholar]
- Thomas M. J., Platas A. A., Hawley D. K., 1998. Transcriptional fidelity and proofreading by RNA polymerase II. Cell 93: 627–637. [DOI] [PubMed] [Google Scholar]
- Thomason L. C., Sawitzke J. A., Li X., Costantino N., Court D. L., 2014. Recombineering: genetic engineering in bacteria using homologous recombination. Curr. Protoc. Mol. Biol. 106: 1.16.1–1.16.39. [DOI] [PubMed] [Google Scholar]
- Toulme F., Mosrin-Huaman C., Sparkowski J., Das A., Leng M., et al. , 2000. GreA and GreB proteins revive backtracked RNA polymerase in vivo by promoting transcript trimming. EMBO J. 19: 6853–6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinella D., Potrykus K., Murphy H., Cashel M., 2012. Effects on growth by changes of the balance between GreA, GreB, and DksA suggest mutual competition and functional redundancy in Escherichia coli. J. Bacteriol. 194: 261–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walmacq C., Kireeva M. L., Irvin J., Nedialkov Y., Lubkowska L., et al. , 2009. Rpb9 subunit controls transcription fidelity by delaying NTP sequestration in RNA polymerase II. J. Biol. Chem. 284: 19601–19612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicki A., Kendall M., Abremski K., Hoess R., 1987. A mutational analysis of the bacteriophage P1 recombinase Cre. J. Mol. Biol. 195: 785–794. [DOI] [PubMed] [Google Scholar]
- Zenkin N., Yuzenkova Y., 2015. New insights into the functions of transcription factors that bind the RNA polymerase secondary channel. Biomolecules 5: 1195–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article. All strains and reagents are available upon request.