Abstract
Managing the disposal of infectious animal carcasses from routine and catastrophic disease outbreaks is a global concern. Recent research suggests that burial in lined and aerated trenches provides the rapid pathogen containment provided by burial, while reducing air and water pollution potential and the length of time that land is taken out of agricultural production. Survival of pathogens in the digestate remains a concern, however. A potential answer is a ‘dual’-barrier approach in which ammonia is used as a secondary barrier treatment to reduce the risk of pathogen contamination when trench liners ultimately leak. Results of this study showed that the minimum inhibitory concentration (MIC) of NH3 is 0.1 M (~1,468 NH3-N mg/L), and 0.5 M NH3 (~7,340 NH3-N mg/L) for ST4232 & MRSA43300, respectively at 24 h and pH = 9±0.1 and inactivation was increased by increasing NH3 concentration and/or treatment time. Results for digestate treated with NH3 were consistent with the MICs, and both pathogens were completely inactivated within 24 h.
Introduction
Outbreaks of infectious animal diseases
It is estimated that in the U.S. 1.4 billion metric tons of routine livestock mortalities and 0.16 billion metric tons of routine poultry losses were disposed in 2000 [1]. Emergency disposal rates can be several orders of magnitude greater during natural disasters or intentional or accidental introduction of infectious animal disease [2]. Bacterial pathogens contribute to animal disease outbreaks [3–5] and human health concerns [6–8]. There is a growing body of evidence that livestock animals can be reservoirs of infectious foodborne diseases that pose risks to susceptible human populations [9–16]. Therefore, effective disposal of potentially infectious animal mortalities is a key component of a successful response to a disease outbreak and routine livestock production management [17–19].
Carcass disposal methods
Carcass disposal methods include burial, composting, incineration, commercial landfills, rendering, and alkaline hydrolysis [20]. Burial, composting, incineration, and rendering are commonly used for disposal of relatively small numbers of mortalities that occur routinely during production. However, in emergency situations, such as disease outbreaks, fire, flooding, and hurricanes, disposal is greatly complicated by the need to deal with large numbers of mortalities within a short time-frame to address biosecurity, transportation logistics, public perception, and environmental concerns. In such situations, the preferred methods for disposal of animal mortalities are an on-farm burial and on-farm composting. Both minimize biosecurity and environmental risks by rapidly sequestering infected and decaying mortalities at their source instead of transporting them to off-site disposal facilities [2,20]. Of the two, burial is more common than composting as it is much faster and does not require sourcing of large quantities of cover material or disposal of post-treatment residues [1, 21]. However, leachate from burial sites can cause chemical and microbial contamination of groundwater due to poor site selection or improper construction [22]. In some instances, emergency burial has also resulted in complaints about odor. Long-term loss of agricultural land use [23] is also a concern since carcass decay in burial sites is slow and undigested whole carcasses can be found at burial sites years after emergency disposal [21].
Burial-aerobic digestion hybrid concept for in-trench, on-farm carcass disposal
Following a widespread outbreak of Foot-and-Mouth disease in 2010, the Korean government (Rural Development Administration, National Institute of Animal Science) sponsored studies of several enhanced carcass disposal methods designed to overcome serious public concerns regarding odor, groundwater pollution potential; and loss of productive land due to slow carcass decomposition within burial plots. One of the proposed methods combines on-farm burial in trenches lined with impermeable fabric, with in-trench aeration to accelerate decomposition and reduce the contamination potential of liquid digestion products.
Phase 1 of the evaluation of this strategy [24] involved lab-scale studies evaluating: the ability of aerobic digestion (AeD) to accelerate carcass decay; and of the potential to use volatile organic compounds (VOCs) released during aerobic digestion as a biosecure way to assess the degree of carcass decomposition without removing digestate or solids from the trench. Results of these preliminary studies showed a reduction of biochemical oxygen demand (BOD, 99.9%), volatile suspended solids (VSS, 99.2%), and total suspended solids (TSS, 99.1%) resulting in digestate meeting the U.S. Environmental Protection Agency (EPA) wastewater disposal criteria. Also, a significant reduction (>6-log) of model Salmonella and Staphylococcus after week 1 and week 4, respectively were observed [24], thus, exemplifying the efficacy of the primary barrier treatment. Furthermore, carcass decomposition using AeD was nearly complete (95%) in about 13 weeks while decomposition of similarly sized carcasses using anaerobic digestion was negligible. However, concerns remain regarding pathogen release should the trench liner leak or if it becomes necessary to pump digestate from the trench and dispose of it elsewhere. Thus, the motivation to test a secondary barrier treatment, in conjunction with the burial-AeD hybrid concept, to further reduce the risk of pathogen re-emergence.
Evidence of AeD performance for treatment of animal and poultry carcasses is still fairly limited. Aerobic digestion was first studied in the UK as a novel technology option for temporary storing and pretreating of sheep carcasses prior to final disposal [25, 26]. It was reported that bacterial counts (Salmonella enterica (S. enterica, serotype Senftenberg and Poona), Enterococcus faecalis (E. faecalis), Campylobacter jejuni (C. jejuni), Campylobacter coli (C. coli), and Escherichia coli (E. coli) O157) in sheep carcass components, including muscle, bone, fat, pelt, blood, stomach contents, wool, and liver, decreased significantly (>5-log values from the original starting concentration) during ~3 month retention in an AeD process [26]. However, E. faecalis remained detectable until the end of 3 months of the trial [26]. These bacteria reported by Gwyther et al. [26] are also important foodborne bacteria monitored by the U.S. Food & Drug Administration [27].
Pathogen survival potential suggests the need for a secondary disinfection strategy for carcass disposal
Salmonella spp. and Staphylococcus spp. are common bacteria found in animals which may also be pathogens and potential zoonotic agents with the capacity to adapt and survive in a wide variety of different foods and environments [28, 29]. They are representative of a broad category of foodborne pathogens related to infections of humans and animals [30, 31] and are often present in poultry both externally (surface of the body) and intestinally (gastrointestinal system) [32]. Some researchers have suggested that pathogenic bacteria and other microorganisms in manure residues pose potential risks to human and animal health, and the environment [33–35]. Similarly, the biosecurity risk associated with the use of untreated digestate residues as fertilizer for farmland is difficult to assess, but this risk cannot be neglected [36]. Therefore, there is a need to investigate a secondary barrier approach (e.g.) disinfection of digestate residues with appropriate chemicals for burial-AeD treatment of animal mortalities. Salmonella spp. and Staphylococcus spp. represent different bacterial groups (gram-positive and gram negative). Therefore, the effect of disinfection can be somewhat assessed across gram-positive and gram-negative organisms.
Evidence of ammonia as an inactivating agent
While there are many possible (and stronger) disinfectants, ammonia has advantages for the on-farm treatment application. Ammonia is readily available at the relatively low cost in agricultural regions where it is a commonly used crop fertilizer. Widespread experience with handling and using ammonia in farming communities is also an important advantage in emergency situations. Ammonia is one of the products of aerobic and anaerobic digestion of organic nitrogen [37]. These two biological processes are the most common methods used to treat animal and human waste [38–41]. Ammonia is generated through ammonification during decomposition of organic matter rich in nitrogen [42]. Depending on the concentration, pH, and temperature, ammonium (NH4+) serves as a beneficial nutrient or ammonia (NH3) serves as a toxicant to various waterborne organisms [43–46]. Ammonia, the bactericidal form, so-called ‘un-ionized NH3’, or ‘free NH3’, increases in water solution with increasing pH level and starts to dominate at a pH > pKa or ~9 [47].
Ammonia emitted from animal waste has been reported to inactivate several common bacterial pathogens [48] including Salmonella Typhimurium (ST), E. coli O157:H7, and Listeria monocytogenes (L. monocytogenes) in manure [49]. It also has been proposed that ammonization could be used as a disinfection process of community sewage sludge [50]. Fumigation with ammonia has been applied to inactivate E. coli O157:H7 and ST in alfalfa seeds and mung beans [51]. Injections of gaseous NH3 has been used to kill pathogens, included E. coli O157:H7, L. monocytogenes, and S. enterica, on boneless lean beef trimmings [52]. Ammonia also has been used to disinfect zoonotic bacteria such as S. Newport, C. jejuni, E. coli O157:H7, L. monocytogenes, and Yersinia enterocolitica (Y. enterocolitica) in animal feed [53]. To date, however, there is no research on ammonia disinfection of digestate residues remaining after AeD of infected animal carcasses containing pathogenic bacteria.
Study objectives
The objectives of this study were: (1) to determine the minimum inhibitory concentrations (MICs) of NH3 for Salmonella Typhimurium and Staphylococcus aureus in a laboratory setting and (2) to evaluate the efficacy of the previously determined NH3 MIC to inactivate these pathogens in a chemically- and microbially- complex digestate matrix of aerobically digested poultry carcasses inoculated with these two pathogens. Marker strains were used to improve their detection and quantification in a microbially complex digestate matrix. Our working hypothesis is that the secondary barrier treatment with NH3 will significantly reduce the level of infectious bacteria (1) during aerobic digestion (early-phase AeD) and (2) after aerobic digestion is complete (late-phase AeD). A success of secondary barrier treatment in early-phase AeD can provide useful information if time and resources can be potentially saved by applying NH3 treatment earlier. Treatment with NH3 in the early-phase AeD is a critical challenge to the NH3 treatment concept. If it worked under those complex conditions, it was likely to work under less difficult conditions. Post-digestion treatment of digestate with NH3 could conceivably become a useful addition to the burial-AeD disposal method, thereby making it more biosecure.
Materials and methods
The rationale was to test the secondary barrier approach concept in which NH3 is used as a secondary barrier treatment for further development of feasible solutions for biosecure emergency disposal of infectious carcasses. The MIC (Objective 1) was a laboratory test that was designed to reflect practical field conditions. Results from Objective 1 were then applied (time and dose) to a chemically- and microbially-complex digestate matrix (Objective 2).
Overall experimental design including primary and secondary barrier treatments
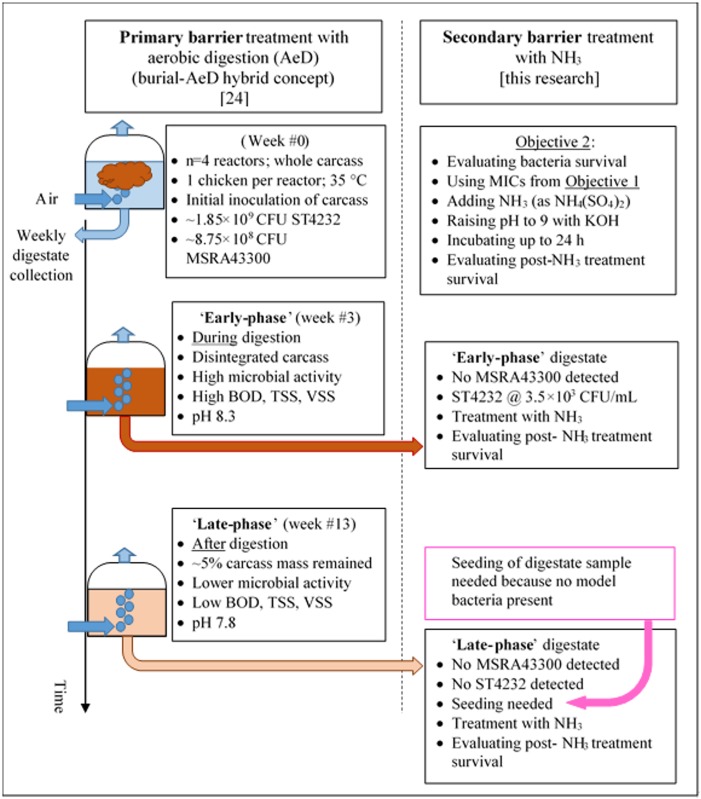
Poultry was used as a model carcass source. White Leghorn (Gallus gallus domesticus) were raised at the ISU Poultry Research and Teaching facility and were euthanizing under IACUC log #4-03-5425-G. Weekly collection of digestate samples from each (n = 4) reactor was subjected to incubation and bacterial enumeration, measuring pH, BOD, TSS, VSS, dissolved oxygen with standard methods described in detail elsewhere [24]. Fig 1 summarizes key methodology details differentiating the primary and secondary barrier treatment research.
Fig 1. Overall experimental design including primary and secondary barrier treatments.
Model bacterial strains
In this current study, two available marker strains were used with selective growth media that suppresses non-study microorganisms in the liquid digestate samples. For this practical reason, nalidixic acid-resistant ST4232 was obtained from the U.S. Department of Agriculture—Agricultural Research Service (USDA-ARS, Ames, Iowa), and MRSA43300 was obtained from the Dept. of Veterinary Diagnostic and Production Animal Medicine (VDPAM), Iowa State University (ISU). The handling of bacterial strains was approved by the IBC 11-I-0030-A/H project ‘Development of environmentally friendly livestock mortality disposal systems using aerobic digestion.'
To prepare for inoculation (of either the phosphate buffered saline (PBS) or digestate), each selected bacterial species was cultivated separately overnight on different blood agar plates (TSA with 5% sheep blood) (Remel, Lenexa, KS). Salmonella and Staphylococcus suspensions were prepared by thoroughly mixing 3 to 5 colonies of each selected bacteria in separated 15 mL sterile screw-capped glass tubes containing 5 mL of PBS solution (Becton Dickinson, Franklin Lakes, NJ). McFarland turbidity standard No. 0.5 (Becton Dickinson, Franklin Lakes, NJ) was used as a reference to adjust the turbidity of bacterial suspensions. The concentration of each bacterial suspension was estimated to be approximately 1 × 108 CFU/mL with final concentrations determined using standard plate counts.
Chrome agar (Bio-Rad Laboratories Inc., Hercules, CA) and XLT4-nalidixic acid agar (prepared and provided by VDPAM, ISU) were used for ST4232 and MRSA43300 enumeration. The number of model bacteria in each sample was obtained by spreading 100 μL of 10-fold serial dilutions (100 to 10−8) on selective media, and then incubating at 35°C for 48 h. Total colony counts were enumerated using a Q-Count Automated Colony Counter System (Spiral Biotech Advanced Instruments Inc., Norwood, MA). Bacterial colonies from XLT4-nalidixic acid agar or chrome agar were selected and identification confirmed using matrix—assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) Biotyper System (Bruker Corp., Fremont, CA).
Determination of the minimum inhibitory concentrations of NH3
Objective #1: Determination of the MICs of ammonia for inactivation of ST4232 and MRSA43300. Several lessons were learned while conducting preliminary treatment tests at mid-to-low pH (4.4 to 4.6) in sterile saline solution to observe the effects of NH3 dose (NH3 source was NH4Cl) and time (24, 48, and 72 h) on Salmonella. Due to low pH, there was no NH3, i.e., an NH3-N fraction of the total ammoniacal nitrogen (TAN) ranging from 0 to 0.001 at 12.5 to 15.9°C. (NH3-N is just an expression for measurement of NH3 in water solution.) More importantly, there was no effect on Salmonella growth at low pH (S1 Fig).
Due to the lack of effect at low initial pH = 4.4 to 4.6 and relatively low temperatures, new experiments were conducted at a pH 9.0±0.1, close to the pKa of TAN, to about half of the NH3-N fraction of TAN at 35°C [54]. Temperature itself can boost the NH3-N fraction of TAN [54]. Three replicates of treatment and control were used. (NH4)2SO4 was added to achieve a desired, nominal [NH3] of 0.05 M, 0.1 M, 0.3 M, and 0.5 M. More accurate concentrations and the bactericidal NH3 molar fractions are reported in Table 1. TAN and [NH3-N] were confirmed using Standard Methods [55] #4500-NH3-N. No (NH4)2SO4 was added into the controls. While there is a consensus that NH3 is bactericidal, there is not much known about the potentially toxic role of sulfate. To the contrary, sulfate-reducing bacteria are thought to be beneficial to environmental cleanup for metals and hydrocarbons. The pH of the experimental liquid was adjusted with potassium hydroxide (KOH) to 9.0 ± 0.1. The caps of tubes were screwed tight to protect the experimental liquid and to prevent loss of NH3 gas to the atmosphere. All experimental tubes were incubated at 35°C during four different treatment times (0.5 h, 4 h, 8h, and 24 h). Surviving numbers of the two model bacteria were determined using the enumeration procedure described above.
Table 1. Summary of ammonia treatment concentrations.
| Nominal* [NH3] | 0.05 M | 0.1 M | 0.3 M | 0.5 M |
| Actual** [NH3] (M) | 0.0524 | 0.105 | 0.313 | 0.524 |
| pH (+/- 0.1) | 9 | 9 | 9 | 9 |
| T (°C) | 35 | 35 | 35 | 35 |
| pKa | 8.949 | 8.949 | 8.949 | 8.949 |
| Molar fraction of NH3-N (-) | 0.529 | 0.529 | 0.529 | 0.529 |
| NH3-N (mg/L) | 734 | 1,468 | 4,404 | 7,340 |
| NH4-N (mg/L) | 653 | 1,307 | 3,921 | 6,535 |
| TAN [NH3-N + NH4-N] (mg/L) | 1,387 | 2,775 | 8,325 | 13,875 |
| TAN (M) | 0.0991 | 0.1982 | 0.5946 | 0.9911 |
*Nominal concentrations in this manuscript are based on rounded-off single significant figure [NH3].
**Actual [NH3] (M) rounded-off to 3 significant figures.
The following formula was used to calculate ammonia concentrations: f = 1/[10^(pKa-pH) + 1]; where: pKa = 0.0901821 + (2729.92/T); f = mole fraction of NH3-N; T = temperature (K) [54].
NH3 treatment after the 1st barrier-treated digestate
The resulting MIC informed experimental design for Objective #2, i.e., ‘secondary barrier’ treatment of digestate at two stages of the digestion process, i.e. the ‘early-’ and ‘late-phase’ case scenarios, respectively. The ‘early-phase’ scenario represented a high microbial activity during aerobic digestion (week #3, high BOD, highest TSS & VSS levels, and the first visual evidence of the whole carcass breakup and disintegration) and the ‘late-phase’ scenario when aerobic digestion is complete (week #13, represented by lower microbial activity post the 99% reduction of BOD, TSS, and VSS, respectively) [24].
Testing secondary barrier treatment in the ‘early-phase’ AeD scenario
The ‘early-phase’ AeD scenario represented an opportunity to test the secondary barrier approach at a period of high microbial activity (initial first few weeks) while the carcass digestion was still in progress. Visual observations of process confirmed that the carcasses disintegrated spilling internal organs into the digestate [24] at about week #3. In this trial, a 5-mL aliquot of the 3rd week digestate was withdrawn directly from each (n = 4) aerobic reactor, dispensed into 15 mL tubes and cultured for surviving ST4232 and MRSA43300. These model bacteria were initially inoculated into chicken carcasses at week #0 (1.85 × 109 CFU/carcass (ST4232) and 8.75 × 108 CFU/carcass (MSRA)) and monitored weekly for concurrent (proof-of-concept, primary barrier treatment only) study [24]. At week #3, MRSA was not recovered; however, ST4232 was recovered and enumerated (3.5 × 103 CFU/mL). Concurrently, an amount of (NH4)2SO4 was added into each treatment tube to obtain NH3 = 0.1 M (i.e. the MIC concentration consistent with the results from testing Objective #1 for ST4232). No (NH4)2SO4 was added to the control tubes. Then, the pH of digestate samples was adjusted with KOH to 9.0±0.1, capped tight, and incubated at 35°C during four different treatment times (0.5 h, 4 h, 8h, and 24 h). Four replicates with digestate from separate reactors were treated with NH3. The bacterial counts were determined using a similar procedure as described earlier.
Testing secondary barrier treatment in the ‘late-phase’ AeD scenario
The ‘late-phase’ AeD scenario represented an opportunity to test the secondary barrier approach at a period of lower microbial activity at the end of digestion (week #13) when only digested feather and bone fragments were left in the digestate [24]. At week #13, neither ST4232 nor MRSA43300 were recovered. Thus, both model bacteria were seeded into digestate samples before NH3 treatment. To make 100 mL of the mixture with 1 × 106 CFU/mL ST4232 and MRSA43300 concentrations, an amount of 99 mL of digestate sample was withdrawn from each reactor and mixed with 1 mL of each pure selected bacterial suspension (approximately 1 × 108 CFU/mL). Inoculated digestate samples were treated with (NH4)2SO4 at two different NH3 concentrations (0.1 M, and 0.5 M, i.e. MIC concentrations consistent with results for Objective #1) with 4 treatment times (0.5 h, 4 h, 8 h, and 24 h). No (NH4)2SO4 was added into the controls. The pH adjustment to 9.0±0.1, tube capping, incubation temperature, and enumeration procedure was identical as described earlier.
Statistical analyses
Pathogen concentrations were analyzed for the effects of ammonia using the one-way analysis of variance (ANOVA) method. The significance of differences in pathogen levels between control and treated samples was tested using the Tukey-Kramer HSD (honestly significant difference) test at p ≤ 0.05. Microbiological data were transformed into log10 to avoid analyzing data with zero values. All computations were carried out using SigmaPlot v11.0.0.77 (Systat Software Inc., San Jose, CA).
Results and discussion
Minimum inhibitory concentration of NH3
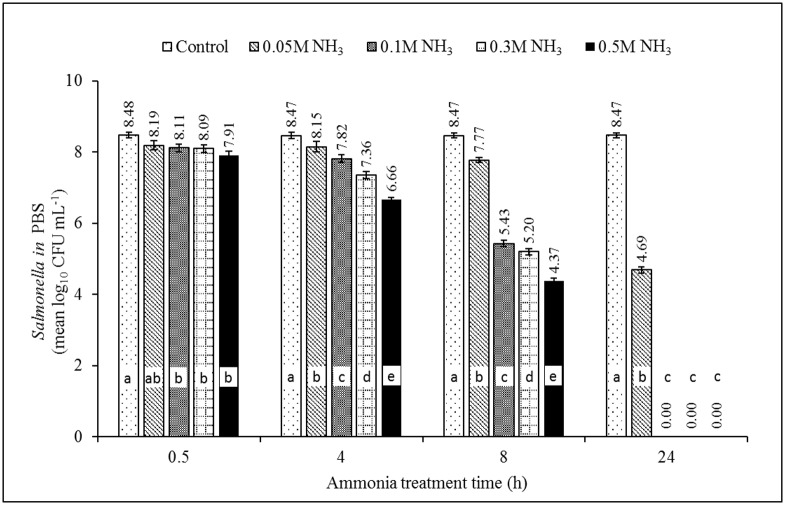
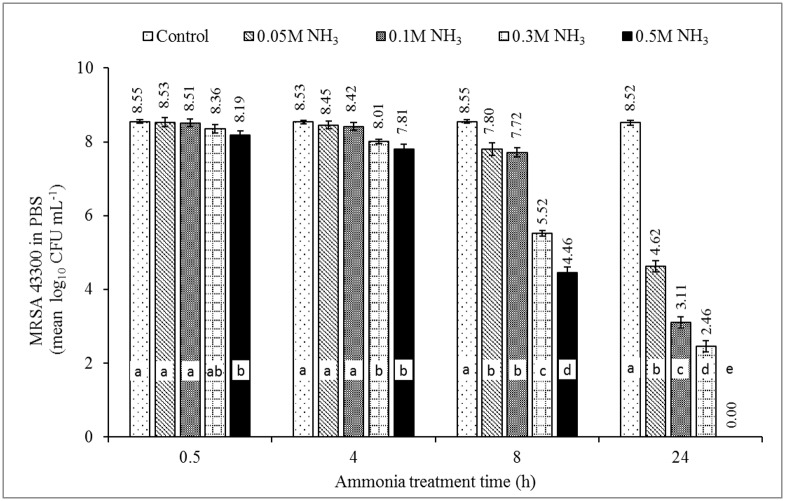
The effect of NH3 on survival of ST4232 and MRSA43300 at pH = 9.0±0.1 (NH3-N = 0.052 M, 0.105 M, 0.315 M, and 0.524 M) is shown in Figs 2 and 3, respectively. Growth was inhibited more effectively with greater dose and longer contact time. The statistically significant difference of the dose was observed as early as 4 h for ST4232 and at 24 h for MRSA43300. Growth ceased to occur at 24 h incubation, i.e., permitting the MIC to be estimated as defined in [56]. A visual example of plate count for Salmonella with treatment time is presented in S2 Fig. The MICs of ammonia were 0.105 M NH3-N (~1,468 mg/L of NH3-N), and 0.524 M NH3-N (~7,340 mg/L of NH3-N) for ST4232, and MRSA 43300, respectively (Figs 2 & 3, Table 2). The MICs are similar to those reported by Leejeerajumnean et al. [46] in culture media, i.e., 0.3 M for both ST and SA albeit for different strains, longer time (48 h), and higher incubation temperature (37°C). Lower MICs were reported in the same study for other important foodborne pathogens, i.e., 0.025 M (E. feacalis, Listeria innocua, E. coli) and 0.050 M (Pseudomonas aeruginosa) [46]. The MICs for various species of Bacillus ranged from 0.025 to 0.5 M [46].
Fig 2. The effect of ammonia on inactivation of Salmonella Typhimurium χ4232 in phosphate buffer solution (PBS).
Note: pH = 9.0±0.1, T = 35°C. NH3 concentrations of 0.052 M, 0.105 M, 0.315 M, and 0.524 M are equivalent to 734, 1,468, 4,404, and 7,340 NH3-N mg/L, respectively (estimated mole fraction of NH3-N to TAN = 0.529). Source of NH3 was (NH4)2SO4. Different letters within each treatment time indicate significant difference (p<0.05), n = 3.
Fig 3. The effect of ammonia on inactivation of methicillin resistant Staphylococcus aureus MRSA ATCC 43300 in phosphate buffer solution (PBS).
Note: pH = 9.0±0.1, T = 35°C. NH3 concentrations of 0.052 M, 0.105 M, 0.315 M, and 0.524 M are equivalent to 734, 1,468, 4,404, and 7,340 NH3-N mg/L, respectively (estimated mole fraction of NH3-N to TAN = 0.529). Source of NH3 was (NH4)2SO4. Different letters in each treatment time indicate significant difference (p<0.05), n = 3.
Table 2. Minimum inhibitory concentrations (MICs) of NH3 (un-ionized ammonia) (24 h treatment) for pure ST4232 and MRSA43300 in PBS at pH = 9.0±0.1 and T = 35°C.
| Bacteria strains | MIC [NH3-N] (M) |
MIC [NH3-N] (mg/L) |
|---|---|---|
| Salmonella Typhimurium χ4232 | 0.105 M | 1,468 |
| methicillin resistant Staphylococcus aureus (MRSA) ATCC 43300 | 0.524 M | 7,340 |
NH3 treatment after the 1st barrier-treated digestate
Testing secondary barrier treatment in the ‘early-phase’ AeD scenario
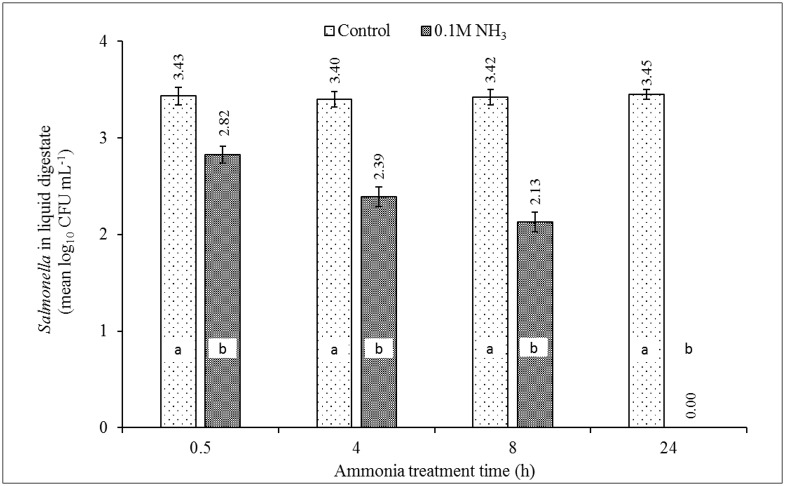
The surviving level of ST4232 at week #3 of aerobic digestion was 3.5 × 103 CFU/mL while MRSA43300 was not detected [24]. The experimental results showed that the reduction in a number of surviving ST4232 from the initial (start of week 1) inoculation in digestate were 0.6, 1.0, 1.3, and 3.5 log10 CFU/mL after 0.5, 4, 8, and 24 h of treatment with 0.1 M NH3 respectively (Fig 4). The statistically significant difference for the dose was observed for all treatment times. The results were consistent with Objective (1), i.e., complete inactivation of ST4232 after 24 h of 0.105 M NH3 (1,468 NH3-N mg/L). Again, the results showed that ST4232 in the control samples were not inactivated at the high concentration of OH- (pH = 9), i.e., pH did not contribute to growth inhibition (Fig 4).
Fig 4. The effect of MIC of ammonia (0.105 M NH3; the equivalent of 1,468 NH3-N mg/L) on inactivation of ST4232 in digestate of week #3 of poultry carcass aerobic digestion.
Source of NH3 was (NH4)2SO4. The control contained only residual TAN (0.039 M) in the digestate collected at week #3 [24]. Week #3 represents an early-phase of decomposition characterized by high BOD, highest TSS & VSS levels, and the first visual evidence of the whole carcass breakup and disintegration. Data represent the means log10 of measured Salmonella concentrations of 4 aerobic reactors ± SD. Different letters in each treatment time indicate significant difference (p<0.05), n = 3.
Testing secondary barrier treatment in the ‘late-phase’ AeD scenario
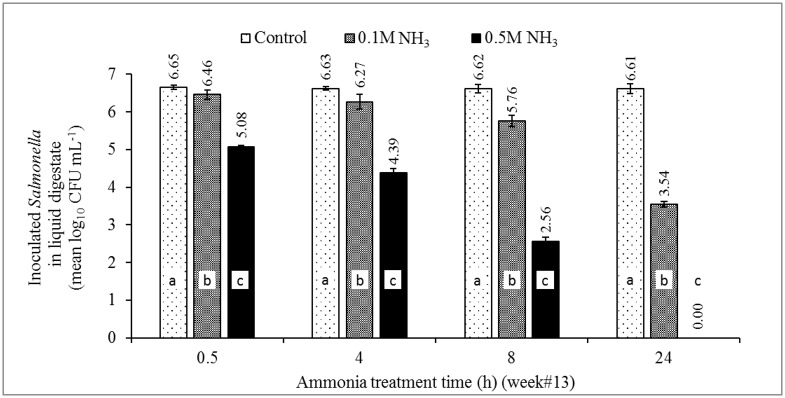
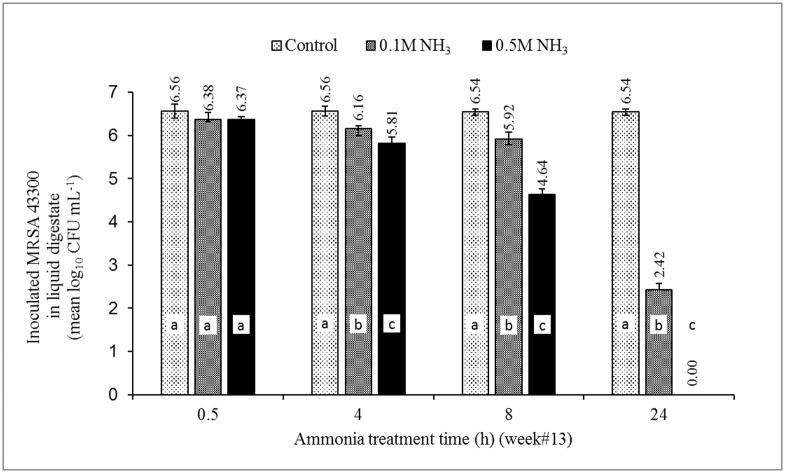
Compared to initial concentrations of inoculated bacteria, the reduction in numbers of ST4232 in digestate with NH3 concentrations of 0.105 M and 0.524 M ranged from 0.19–3.07, and 1.57–6.61 log10 CFU/mL, respectively (Fig 5). Statistically significant differences to the dose were observed for all treatment times. Likewise, the reduction in numbers of MRSA43300 ranged from 0.18–4.12, and 0.19–6.54 log10 CFU/mL with NH3 concentrations of 0.105 and 0.524 M, respectively (Fig 6). The statistically significant difference to the dose was observed for treatment times ≥ 4 h.
Fig 5. The effect of MIC of ammonia (0.105 and 0.524 M NH3; the equivalent of 1,468 and 7,340 NH3-N mg/L) on the survival of newly seeded ST4232 in digestate of week #13 poultry carcass aerobic digestion.
Source of NH3 was (NH4)2SO4. The control contained only residual TAN (0.00057 M) in the digestate collected at week #13 [24]. Week #13 represents a late-phase of a complete aerobic digestion represented by lower microbial activity post the 99% reduction of BOD, TSS, and VSS. Data represents the mean log10 of measured Salmonella concentrations of 4 aerobic reactors ± SD. Different letters in each treatment time indicate significant difference (p<0.05), n = 3.
Fig 6. The effect of MIC of ammonia (0.105 and 0.524 M NH3; the equivalent of 1,468 and 7,340 NH3-N mg/L) on the survival of newly seeded MRSA43300 in digestate of week #13 poultry carcass aerobic decomposition.
Source of NH3 was (NH4)2SO4. Week #13 represents a late-phase of a complete aerobic digestion represented by lower microbial activity post the 99% reduction of BOD, TSS, and VSS. Data represents the mean log10 of measured concentrations of MRSA43300 of 4 aerobic reactors ± SD. Different letters in each treatment time indicate significant difference (p<0.05), n = 3.
The required dose of ammonia to inactivate ST4232 increased from 0.105 to 0.524 M NH3 compared to Objective 1 (MIC) and Objective 2 (treatment of digestate, week 3 early-phase). This is also illustrated in S3 Fig comparing survival of ST4232 after NH3 treatment. ST4232 was most easily killed in PBS, then week #3 digestate and then week #13 digestate. Possibly either microbial population or matrix differences (early- vs. late-phase) contributed to the decreased effect of ammonia by either (1) utilization of ammonia or (2) binding of the active form of ammonia. This difference in ammonia dose for ST4232 may be a consideration when using the ammonia treatment option in a secondary barrier approach. There was very little apparent difference between surviving MSRA43300 post-NH3 treatment in PBS and week #13 digestate (S4 Fig).
Mechanism of pathogen inactivation by NH3
It was reported that ammonia could be toxic to a variety microorganisms ranging from bacteria and viruses to mammals [57–61]. According to Sprott (1984), when NH3 enters the bacterial cells, it drives some protons off the cell. To maintain its internal pH, the cell takes up some protons from the outside of the cell. At the same time, it ‘sacrifices’ the potassium ion (K+) via efflux from the cell, and the cell dies due to the lack of this essential nutrient [62]. In this current study, elevated NH3 levels at pH 9.0 ± 0.1 were the prerequisite for significant reductions of the two model bacteria, ST4232 and MRSA43300. High pH = 9 and the resulting high molecular fractions of NH3 to TAN used in this study are believed to provide efficient means of bacterial inactivation. The TAN, by itself, does not appear to be the reason for pathogen inactivation. A sufficient source of NH3 (e.g., (NH4)2SO4) must be accompanied by elevated pH (e.g., by the addition of KOH) to sustain an NH3 fraction of TAN as shown in S1 Table. Increased temperature can also boost the NH3 fraction (S1 Table). S1 Table provides practical information on how to achieve NH3 concentrations required to meet MICs for model strains of ST4232 and MRSA4330 under varied conditions of pH (4 to 12) and temperature (mesophilic temperature = 35°C, room temperature = 20°C).
Potential use of NH3 treatment for other pathogens of interest
The effect of NH3 as a secondary barrier treatment for inactivation of two model bacteria representing gram-positive (MSRA43300) and gram-negative (ST4232) pathogens was studied. The results (Objective 1) were in agreement with earlier observations that the gram-negative bacteria are more susceptible to ammonia at higher pH than gram-positive bacteria [52]. The results from Objective 2 are of particular utility for further development of the burial-AeD hybrid concept and the use of NH3 as a secondary barrier treatment. Gwyther et al. [26] reported that bacterial counts of S. enterica, E. faecalis, C. jejuni, C. coli, and E. coli O157 in sheep carcass components were decreased significantly (>5-log 10). However, E. faecalis (gram-positive) remained detectable until the end of 3 months of the trial [26]. The results of this study suggest that higher NH3 dose is generally needed to inactivate gram-positive bacteria. This observation is in general agreement with reports on ammonia effects on bacterial population in manures, feed and meat [48–53].
The results can be also informative to consider the potential usefulness of NH3 in the context of improving biosecurity in livestock and poultry production systems. Himathongkham et al. [48] reported similar survival rates for S. typhimurium and E.coli (0157:H7) (both gram-negative bacteria) in stored cow manure and slurry with controlled and elevated pH. Himathongkham et al. [49] reported similar significant reduction for S. typhimurium, E.coli (0157:H7), and ~half as effective reduction of L. monocytogenes (a gram-positive bacteria) in chicken manure gassed with NH3. Niebuhr and Dickson reported on the impact of pH enhancement with ammonia gas to pH ~ 9.6 on populations of Salmonella, L. monocytogenes, and E. coli O157:H7 in boneless lean beef trimmings [52]. Ammoniation reduced E.coli (0157:H7) by ~3-log10 CFU/g and Salmonellae by ~4.5-log10 CFU/g, and only ~0.5-log10 CFU/g of L. monocytogenes [52]. Effectiveness of NH3 treatment of inoculated animal feed was also studied by Tajkarimi et al. [53]. C. jejuni, E.coli (0157:H7), Y. enterocolitica and L. monocytogenes were consistently reduced at or above 5-log10 [53].
Conclusions
The MICs (Objective 1) for ST4232 and MRSA43300 were 0.1 M NH3 (~1,468 mg/L of NH3-N) and 0.5 M NH3 (~7,340 mg/L of NH3-N) concentrations, respectively. Inactivation was increased by increasing NH3 concentration and/or treatment time. Although the chemistry and microbiology of digestate are complex, the effectiveness of NH3 treatment of digestate (Objective 2) was consistent with the MICs determined in sterile saline solution except ST4232 in the late-phase AeD scenario where the MIC was 5x greater. Both pathogens, however, were completely inactivated after 24 h. A sufficient source of NH3 (e.g., (NH4)2SO4) must be accompanied by elevated pH (e.g., by the addition of KOH) to sustain an NH3 fraction of TAN. Increased temperature can also boost the NH3 fraction. High pH (≥9) and the resulting high molecular fraction of NH3 are believed to provide efficient means of bacterial inactivation. Further work is warranted to determine what other important pathogen species important in livestock production systems could be practically inactivated with NH3. Results of this proof-of-concept study show that the secondary barrier approach can reduce the risks of pathogen contamination of shallow groundwater pollution when the temporary liner employed in the burial-AeD hybrid carcass disposal concept ultimately ruptures or if digestate is pumped out of the lined trench. If found to be true in the field, post-digestion treatment of digestate with NH3 could conceivably become a useful addition to the in-trench burial-AeD disposal method, thereby making it more biosecure.
Supporting information
Note: pH = 5.8 (Control, T = 21.3°C), 4.6 (3.7 M TAN, T = 15.9°C), 4.5 (5.6 M TAN, T = 13.6°C), and 4.4 (7.5 M TAN, T = 12.5°C). NH3-N fractions of TAN were extremely low and ranged from 0 to 0.001. Different letters in each treatment time indicate significant difference (p<0.05), n = 3.
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
Acknowledgments
This research was funded by Republic of Korea Rural Development Administration through a grant from the National Institute of Animal Science (Project Title: Development of environmentally friendly livestock mortality disposal system using aerobic digestion. Project no.: PJ90713006, PI Prof. Thomas Glanville). The authors gratefully acknowledge Brad Bearson at the USDA-ARS, National Laboratory for Agriculture and the Environment, Ames, IA for providing Salmonella enterica ser. Typhimurium strain BSX 8 (χ4232). The authors also would like to thank ISU, VDPAM for providing methicillin-resistant Staphylococcus aureus–ATCC 43300 strain and conducting the microbiological analysis. Special thanks to Joann Kinyon and Susan Cramer for their support with microbiological analyses. Special thanks to Dr. Susan Lamont and the staff of the ISU Poultry Research and Teaching facility for their assistance with raising and euthanizing poultry (IACUC log #4-03-5425-G).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by Republic of Korea Rural Development Administration through a grant from the National Institute of Animal Science (Project title: Development of environmentally friendly livestock mortality disposal system using aerobic digestion. Project no.: PJ90713006). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sparks Companies Inc. 2002. Livestock Mortalities: Methods of Disposal and Their Potential Costs. Report for the National Renderers Association, Alexandria, VA. http://assets.nationalrenderers.org/mortalities_final.pdf.
- 2.Nutsch A, Kastner J. Carcass Disposal Options, In: Voeller JG (ed), Wiley Handbook of Science and Technology for Homeland Security. John Wiley & Sons, Inc., 2010. Hoboken, NJ: p. 1959. [Google Scholar]
- 3.Kawakami VM, Bottichio L, Angelo K, Linton N, Kissler B, Basler C, et al. Notes from the Field. Outbreak of multidrug-resistant Salmonella infections linked to pork—Washington, 2015. MMWR Morb Mortal Wkly Rep. 2016;65: 379–381. 10.15585/mmwr.mm6514a4. [DOI] [PubMed] [Google Scholar]
- 4.Anderson TC, Marsden-Haug N, Morris JF, Culpepper W, Bessette N, Adams JK, et al. (2016), Multistate outbreak of human Salmonella Typhimurium infections linked to pet hedgehogs—United States, 2011–2013. Zoonoses Public Health. 10.1111/zph.12310. [DOI] [PubMed] [Google Scholar]
- 5.Huong VT, Thanh LV, Phu VD, Trinh DT, Inui K, Tung N, et al. Temporal and spatial association of Streptococcus suis infection in humans and porcine reproductive and respiratory syndrome outbreaks in pigs in northern Vietnam. Epidemiology and Infection. 2016;144(1): 35–44. 10.1017/S0950268815000990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen ML. Changing patterns of infectious disease. Nature. 2000:406:762–767. 10.1038/35021206. [DOI] [PubMed] [Google Scholar]
- 7.Taylor LH, Latham SM, Woolhouse MEJ. Risk factors for human disease emergence. Philosophical Transactions of the Royal Society B. 2001;356: 983–989. 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angus KW. Cryptosporidiosis in man, domestic animals and birds: a review. J R Soc Med. 1983;76(1): 62–70. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1438565/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatcher SM, Myers KW, Heaney CD, Larsen J, Hall D, Miller MB, et al. Occurrence of methicillin-resistant Staphylococcus aureus in surface waters near industrial hog operation spray fields. Sci Total Environ. 2016;565: 1028–1036. 10.1016/j.scitotenv.2016.05.083. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed AN, Abdel-Latef GK, Abdel-Azeem NM, El-Dakhly KM. Ecological study on antimicrobial-resistant zoonotic bacteria transmitted by flies in cattle farms. Parasitol Res. 2016;115(10): 3889–3896. 10.1007/s00436-016-5154-7. [DOI] [PubMed] [Google Scholar]
- 11.Dahms C., Hübner NO, Wilke F, Kramer A. Mini-review: Epidemiology and zoonotic potential of multiresistant bacteria and Clostridium difficile in livestock and food. GMS Hyg Infect Control. 2014;30:9(3):Doc21 10.3205/dgkh000241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulz J, Friese A, Klees S, Tenhagen BA, Fetsch A, Rösler U, et al. Longitudinal study of the contamination of air and of soil surfaces in the vicinity of pig barns by livestock-associated methicillin-resistant Staphylococcus aureus. Appl Environ Microbiol. 2012;78(16): 5666–5671. 10.1128/AEM.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer J, Hille K, Ruddat I, Mellmann A, Köck R, Kreienbrock L. Simultaneous occurrence of MRSA and ESBL-producing Enterobacteriaceae on pig farms and in nasal and stool samples from farmers. Vet Microbiol. 2016; forthcoming. 10.1016/j.vetmic.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 14.Guerra B, Fischer J, Helmuth R. An emerging public health problem: acquired carbapenemase-producing microorganisms are present in food-producing animals, their environment, companion animals and wild birds. Vet Microbiol. 2014;171(3–4): 290–297. 10.1016/j.vetmic.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Brooks JP, Adeli A, McLaughlin MR. Microbial ecology, bacterial pathogens, and antibiotic resistant genes in swine manure wastewater as influenced by three swine management systems. Water Res. 2014;57: 96–103. 10.1016/j.watres.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Michitsch R, Jamieson R, Gordon R, Stratton G, Lake C. Bacterial pathogen indicator transport from livestock mortality biopiles. J Environ Qual. 2015;44(5): 1355–1365. 10.2134/jeq2015.01.0034. [DOI] [PubMed] [Google Scholar]
- 17.Fairbrother JM, Nadeau E, Gyles CL. Escherichia coli in postweaning diarrhea in pigs: an update on bacterial types, pathogenesis, and prevention strategies. Anim Health Res Rev. 2005;6(1): 17–39. 10.1079/AHR2005105. [DOI] [PubMed] [Google Scholar]
- 18.Mohler VL, Izzo MM, House JK. Salmonella in calves. Vet Clin North Am Food Anim Pract. 2009;25(1):37–54. 10.1016/j.cvfa.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 19.Fasina FO, Bwala DG, Madoroba E. Investigation of multidrug-resistant fatal colisepticaemia in weanling pigs. Onderstepoort J Vet Res. 2015;82(1): 986 10.4102/ojvr.v82i1.986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Animal and Plant Health Inspection Service. 2005. National animal health emergency management system guidelines—Operational guidelines: Disposal. Veterinary Services, Animal and Plant Health Inspection Service, U.S. Department of Agriculture. https://www.aphis.usda.gov/emergency_response/tools/on-site/htdocs/images/nahems_disposal.pdf
- 21.Council for Agricultural Science and Technology (CAST). 2008. Poultry Carcass Disposal Options for Routine and Catastrophic Mortality. Issue Paper 40. CAST, Ames, Iowa. http://www.cast-science.org/download.cfm?PublicationID=2941&File=f030eee6c1a96d4919406e1f396879496e70.
- 22.Kim H-s, Kim K. Microbial and chemical contamination of groundwater around livestock mortality burial sites in Korea—a review. Geosciences Journal. 2012;16: 479–489. 10.1007/s12303-012-0036-1. [DOI] [Google Scholar]
- 23.Brglez B, Hahn J. Methods for Disposal of Poultry Carcasses, p. 333–352, Avian Influenza. Blackwell Publishing Ltd; 2009. [Google Scholar]
- 24.Koziel JA, Ahn HK. Glanville TD, Frana TS, van Leeuwen J (Hans), Nguyen LT. Lab-scale evaluation of burial-aerobic digestion hybrid concept for treatment for emergency disposal of infectious animal carcasses. Waste Manag. 2017; forthcoming. [DOI] [PubMed] [Google Scholar]
- 25.Williams AP, Edwards-Jones G, Jones DL. In-vessel bioreduction provides an effective storage and pre-treatment method for livestock carcasses prior to final disposal. Bioresour Technol. 2009;100: 4032–4040. 10.1016/j.biortech.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 26.Gwyther CL, Jones DL, Golyshin PN, Edwards-Jones G, Williams AP. Fate of pathogens in a simulated bioreduction system for livestock carcasses. Waste Manag. 2012;32: 933–938. 10.1016/j.wasman.2011.10.031. [DOI] [PubMed] [Google Scholar]
- 27.National Antimicrobial Resistance Monitoring System. U.S. Food & Drug Administration. http://www.fda.gov/AnimalVeterinary/SafetyHealth/AntimicrobialResistance/NationalAntimicrobialResistanceMonitoringSystem/ucm418883.htm.
- 28.Bendinelli M, Honeyman A, Friedman H. Staphylococcus aureus Infection and Disease. Kluwer Acedemic / Plenum Publishers, New York; 2002. [Google Scholar]
- 29.Bell C, Kyriakides A. Salmonella: A practical approach to the organism and its control in foods Blackwell Science, London, United Kingdom: 2002. [Google Scholar]
- 30.Cabello F, Hormaeche C, Mastroeni P, Bonina L (ed). Biology of Salmonella. NATO Advanced Studies Institute / Plenum Press, New York; 1993. [Google Scholar]
- 31.Persoons D, Persoons S, Van Hoorebeke K, Hermans P, Butaye A, de Kruif F, et al. Methicillin-resistant Staphylococcus aureus in poultry. Emerging Infectious Diseases 2009;15: 452–453. 10.3201/eid1503.080696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolder NM. Decontamination of meat and poultry carcasses. Trends Food Sci Technol. 1997;8: 221–227. 10.1016/S0924-2244(97)01040-6. [DOI] [Google Scholar]
- 33.Guan TY, Holley RA. Pathogen survival in swine manure environments and transmission of human enteric illness-a review. J Environ Qual. 2003;32: 383–392. 10.2134/jeq2003.0383. [DOI] [PubMed] [Google Scholar]
- 34.Casey JA, Curriero FC, Cosgrove SE, Nachman KE, Schwartz BS. High-density livestock operations, crop field application of manure, and risk of community-associated methicillin-resistant Staphylococcus aureus infection in Pennsylvania. JAMA Intern Med. 2013;173(21): 1980–1990. 10.1001/jamainternmed.2013.10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrel M, Schweizer ML, Sarrazin MV, Smith TC, Perencevich EN. Residential proximity to large numbers of swine in feeding operations is associated with increased risk of methicillin-resistant Staphylococcus aureus colonization at time of hospital admission in rural Iowa Veterans’, Infect Control Hosp Epidemiol. 2014;35(2): 190–192. 10.1086/674860. [DOI] [PubMed] [Google Scholar]
- 36.Sahlström L. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresour Technol. 2003;87: 161–166. 10.1016/S0960-8524(02)00168-2 [DOI] [PubMed] [Google Scholar]
- 37.Reddy KR, Patrick WH Jr. Effect of alternate aerobic and anaerobic conditions on redox potential, organic matter decomposition and nitrogen loss in a flooded soil. Soil Biol Biochem. 1975;7: 87–94. 10.1016/0038-0717(75)90004-8. [DOI] [Google Scholar]
- 38.Hansen KH, Angelidaki I, Ahring BK. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998;32: 5–12. 10.1016/S0043-1354(97)00201-7. [DOI] [Google Scholar]
- 39.Mohaibes M, Heinonen-Tanski H. Aerobic thermophilic treatment of farm slurry and food wastes. Bioresour Technol. 2004;95: 245–254. 10.1016/j.biortech.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Ganidi N, Tyrrel S, Cartmell E. Anaerobic digestion foaming causes—A review. Bioresour Technol. 2009;100: 5546–5554. 10.1016/j.biortech.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 41.Ugwuanyi JO, Harvey LM, McNeil B. Application of thermophilic aerobic digestion in protein enrichment of high strength agricultural waste slurry for animal feed supplementation. J Chem Technol Biotechnol. 2006;81: 1641–1651. 10.1002/jctb.1574. [DOI] [Google Scholar]
- 42.Metcalf, Eddy. Wastewater Engineering: Treatment and Reuse, Fourth ed Tata McGraw-Hill Publishing Company Ltd, New Delhi; 2005. [Google Scholar]
- 43.Müller T, Walter B, Wirtz A, Burkovski A. Ammonium Toxicity in Bacteria. Curr Microbiol. 2006;52: 400–406. 10.1007/s00284-005-0370-x. [DOI] [PubMed] [Google Scholar]
- 44.Ip YK, Chew SF, Randall DJ. 2001. Ammonia toxicity, tolerance, and excretion, p 109–148, Fish Physiology, Vol. 20 Academic Press. [Google Scholar]
- 45.Tenuta M, Lazarovits G. Ammonia and nitrous acid from nitrogenous amendments kill the Microsclerotia of Verticillium dahliae. Phytopathology. 2002;92: 255–264. 10.1094/PHYTO.2002.92.3.255. [DOI] [PubMed] [Google Scholar]
- 46.Leejeerajumnean A, Ames JM, Owens JD. Effect of ammonia on the growth of Bacillus species and some other bacteria. Lett Appl Microbiol. 2000;30: 385–389. 10.1046/j.1472-765x.2000.00734.x. [DOI] [PubMed] [Google Scholar]
- 47.Warren KS. Ammonia toxicity and pH. Nature. 1962;195: 47–49. 10.1038/195047a0. [DOI] [PubMed] [Google Scholar]
- 48.Himathongkham S, Bahari S, Riemann H, Cliver D. Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol Lett. 2006;178: 251–257. 10.1111/j.1574-6968.1999.tb08684.x. [DOI] [PubMed] [Google Scholar]
- 49.Himathongkham S, Riemann H. Destruction of Salmonella typhimurium, Escherichia coli O157:H7 and Listeria monocytogenes in chicken manure by drying and/or gassing with ammonia. FEMS Microbiol Lett. 2006;171: 179–182. 10.1111/j.1574-6968.1999.tb13430.x. [DOI] [PubMed] [Google Scholar]
- 50.Mendez JM, Jimenez B, Maya C. Disinfection kinetics of pathogens in physicochemical sludge treated with ammonia. Water Sci Technol. 2004;50: 67–74. http://wst.iwaponline.com/content/50/9/67.abstract. [PubMed] [Google Scholar]
- 51.Himathongkham S, Nuanualsuwan S, Riemann H, Cliver DO. Reduction of Escherichia coli O157:H7 and Salmonella typhimurium in artificially contaminated alfalfa seeds and mung beans by fumigation with ammonia. J Food Prot. 2001;64: 1817–1819. 10.4315/0362-028X-64.11.1817. [DOI] [PubMed] [Google Scholar]
- 52.Niebuhr SE, Dickson JS. Impact of pH enhancement on populations of Salmonella, Listeria monocytogenes, and Escherichia coli O157:H7 in boneless lean beef trimmings. J Food Prot. 2003;66: 874–877. 10.4315/0362-028X-66.5.874. [DOI] [PubMed] [Google Scholar]
- 53.Tajkarimi M, Riemann HP, Hajmeer MN, Gomez EL, Razavilar V, Cliver DO. Ammonia disinfection of animal feeds—Laboratory study. Int J Food Microbiol. 2008;122: 23–28. 10.1016/j.ijfoodmicro.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 54.Emerson K, Russo RC, Lund RE, Thurston VR. 1975. Aqueous ammonia equilibrium calculations: effect of pH and temperature. J Fish Res Board Can. 1975;32: 2379–2383. 10.1139/f75-274. [DOI] [Google Scholar]
- 55.American Public Health Association. 2005. Standard methods for examination of water and wastewater. 21st ed, (Eds.) Eaton A, Clesceri L, Rice E, Greenberg A. American Public Health Association (APHA) Washington, DC. [Google Scholar]
- 56.Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001;48: 5–16. 10.1093/jac/48.suppl_1.5. [DOI] [PubMed] [Google Scholar]
- 57.Ward RL, Ashley CS. Identification of the virucidal agent in wastewater sludge. Appl Environ Microbiol. 1977;33: 860–864. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC170780/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Albertson OE. Ammonia nitrogen and the anaerobic environment. J Water Pollut Control Fed. 1961;33: 978–995. Stable URL: http://www.jstor.org/stable/i25034473. [Google Scholar]
- 59.Taylor BL. How do bacteria find the optimal concentration of oxygen? Trends Biochem Sci. 1983;8:438–441. http://jb.asm.org/content/179/17/5598.full.pdf. [Google Scholar]
- 60.Borgmann U, Borgmann AI. Control of ammonia toxicity to Hyalella azteca by sodium, potassium and pH. Environ Pollut. 1997; 95: 325–331. 10.1016/S0269-7491(96)00138-8. [DOI] [PubMed] [Google Scholar]
- 61.Kleiner D, Traglauer A, Domm S. Does ammonia production by Klebsiella contribute to pathogenesis? Bull Inst Pasteur. 1998;96: 257–265. 10.1016/S0020-2452(99)80006-2. [DOI] [Google Scholar]
- 62.Sprott GD, Shaw KM, Jarrell KF. Ammonia/potassium exchange in methanogenic bacteria. J Biol Chem. 1984;259: 12602–12608. http://www.jbc.org/content/259/20/12602.full.pdf. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Note: pH = 5.8 (Control, T = 21.3°C), 4.6 (3.7 M TAN, T = 15.9°C), 4.5 (5.6 M TAN, T = 13.6°C), and 4.4 (7.5 M TAN, T = 12.5°C). NH3-N fractions of TAN were extremely low and ranged from 0 to 0.001. Different letters in each treatment time indicate significant difference (p<0.05), n = 3.
(TIF)
(TIF)
(TIF)
(TIF)
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.