Abstract
AMP-activated protein kinase (AMPK) is an important energy-sensing protein in skeletal muscle. Mammalian target of rapamycin (mTOR) mediates translation initiation and protein synthesis through ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). AMPK activation reduces muscle protein synthesis by down-regulating mTOR signaling, whereas insulin mediates mTOR signaling via Akt activation. We hypothesized that AMPK-mediated inhibitory effects on mTOR signaling depend on catalytic α2 and regulatory γ3 subunits. Extensor digitorum longus muscle from AMPK α2 knockout (KO), AMPK γ3 KO, and respective wild-type (WT) littermates (C57BL/6) were incubated in the presence of 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside (AICAR), insulin, or AICAR plus insulin. Phosphorylation of AMPK, Akt, and mTOR-associated signaling proteins were assessed. Insulin increased Akt Ser473 phosphorylation (P < 0.01), irrespective of genotype or presence of AICAR. AICAR increased phosphorylation of AMPK Thr172 (P < 0.01) in WT but not KO mice. Insulin stimulation increased phosphorylation of S6K1 (Thr389), ribosomal protein S6 (Ser235/236), and 4E-BP1 (Thr37/46) (P < 0.01) in WT, AMPK α2 KO, and AMPK γ3 KO mice. However, in WT mice, preincubation with AICAR completely inhibited insulin-induced phosphorylation of mTOR targets, suggesting mTOR signaling is blocked by prior AMPK activation. The AICAR-induced inhibition was partly rescued in extensor digitorum longus muscle from either α2 or γ3 AMPK KO mice, indicating functional α2 and γ3 subunits of AMPK are required for the reduction in mTOR signaling. AICAR alone was without effect on basal phosphorylation of S6K1 (Thr389), ribosomal protein S6 (Ser235/236), and 4E-BP1 (Thr37/46). In conclusion, functional α2 and γ3 AMPK subunits are required for AICAR-induced inhibitory effects on mTOR signaling.
THE BALANCE BETWEEN nutrient overload and shortage is a constant challenge to energy homeostasis in living organisms. In mammalian cells, energy homeostasis is tightly regulated by growth factors, hormones, and nutrients that evoke evolutionarily conserved signaling pathways (1). The AMP-activated protein kinase (AMPK) is a ubiquitously expressed serine/threonine protein kinase that is a master regulator of energy homeostasis (2, 3). AMPK is activated during states of energy stress such as hypoxia, glucose starvation, or physical exercise and restores the energy-depleted status by concomitantly inhibiting anabolic and stimulating catabolic pathways (3, 4, 5). Protein synthesis, a major consumer of ATP in mammalian cells, is inhibited upon AMPK activation (1).
The mammalian target of rapamycin (mTOR) signaling pathway plays a key role in the regulation of protein synthesis (6). Insulin stimulates protein synthesis via activation of the canonical phosphatidylinositol 3-kinase/Akt pathway. Akt phosphorylates and inactivates the tuberous sclerosis complex (TSC2), which increases mTOR kinase activity. mTOR exists in two protein complexes; mTORC1 consists of mTOR, the G-protein β-like protein (GβL/LST8) and raptor, and is responsible for cell growth, whereas mTORC2 contains mTOR, GβL, and rictor and is important in cytoskeletal organization. Ribosomal S6 kinase 1 (S6K1) and eukaryotic initiation factor (eIF)-4E binding protein 1 (4E-BP1) are well-characterized substrates of mTOR. The phosphorylation state of these substrates reflects the activity of the mTOR-raptor branch of the pathway. Phosphorylation of 4E-BP1 inhibits binding of 4E-BP1 with mRNA cap binding protein eIF4E, thereby allowing the association of eIF4E with eIF4G to initiate translation (6, 7, 8). Upon activation, S6K1 phosphorylates ribosomal protein S6 (rpS6), a component of the 40S ribosomal subunit complex, and increases ribosomal biogenesis through translation of a subclass of mRNAs containing a short oligopyrimidine sequence (9), although this mechanism has been challenged (10).
Skeletal muscle mTOR signaling is up-regulated in response to resistance exercise, growth factor stimulation, and high-protein diet, which promotes adaptive changes in skeletal muscle mass that correlate with increased mTOR activity (11, 12). AMPK is a negative regulator of mTOR signaling and therefore may play a role in the regulation of skeletal muscle hypertrophy. AMPK is a heterotrimeric complex, consisting of the catalytic α-subunit and the regulatory β- and γ-subunits. AMPK α- and β-subunits are each encoded by two distinctive genes (α1, α2 and β1, β2), whereas the γ-subunit is encoded by three genes (γ1, γ2, and γ3). Twelve different combinations of AMPK subunits form haloenzymes with tissue-specific distribution and subcellular localization. Binding of AMP to γ-subunits allosterically activates AMPK, which promotes phosphorylation of threonine residue (Thr172) within the activation domain of the α-subunit by the upstream tumor suppressor LKB1 kinase (2, 4, 13, 14). The adenosine analog 5-aminoimidazole-4-carboxamide-1-β-d-ribonucleoside (AICAR), which is metabolized to 5-aminoimidazole-4 carboxamide ribonucleotide, can mimic the effect of AMP to activate AMPK (15).
The existence of 12 possible AMPK heterotrimers complicates the understanding of the physiological role of the AMPK system. Tissue-specific relative expression patterns of AMPK subunits in different species may explain the relative contribution of different subunits of AMPK to specific metabolic responses (16, 17, 18, 19, 20). The predominant AMPK heterotrimeric complex expressed in glycolytic skeletal muscle contains the α2/β2/γ3 subunits (20). Functional α2 and γ3 AMPK subunits are required for AICAR-induced glucose transport in glycolytic skeletal muscle (21, 22). Although AMPK subunit-specific effects on mTOR signaling have been reported for different cell lines (23, 24), effects in skeletal muscle are unclear. We determined the role of AMPK in mTOR signaling using knockout (KO) mouse models in which either the α2 (25) or the γ3 (21) subunit has been ablated. We examined the effect of insulin on downstream targets of mTOR and the inhibitory effect of AMPK on insulin-induced mTOR signaling. We also determined whether AICAR-induced inhibition of insulin-mediated mTOR signaling is intact in α2-AMPK KO and γ3-AMPK KO mice. Our results suggest that functional α2 and γ3 subunits of AMPK are required for the AMPK-mediated inhibitory effect on insulin-induced mTOR signaling.
RESULTS
AICAR-Induced AMPK Phosphorylation Requires Functional α2 and γ3 Subunits
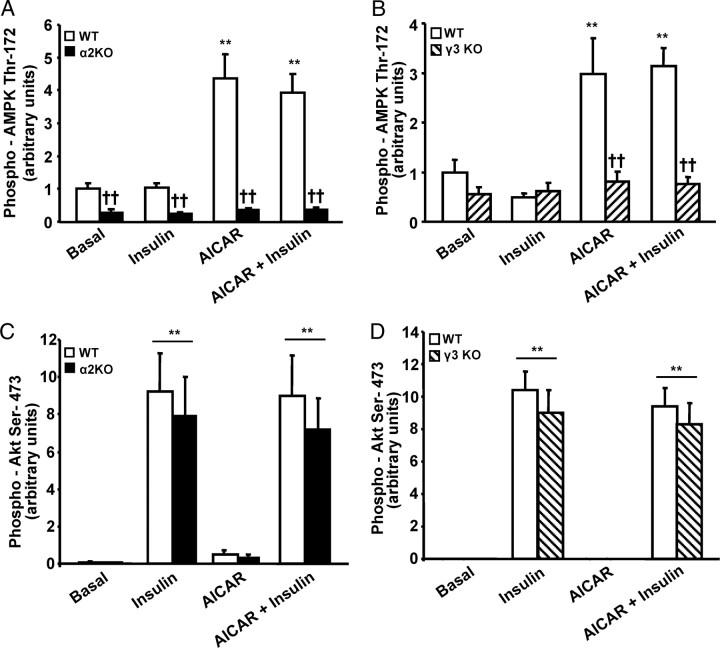
Insulin (120 nm) and AICAR (2 mm) were used as pharmacological activators (6) and repressors (26) of mTOR signaling, respectively. To study the inhibitory effect of AMPK on mTOR signaling, extensor digitorum longus (EDL) muscles from α2 AMPK KO, γ3 AMPK KO, and their corresponding wild-type (WT) littermates (C57BL/6) were incubated in the presence or absence of AICAR (60 min). Immunoblot analysis with a phospho-AMPK Thr172 antibody revealed that AICAR increased AMPK phosphorylation in EDL muscles from WT animals, and this effect was unaltered by insulin. However AICAR-induced phosphorylation of AMPK was completely blunted in EDL muscle from α2 AMPK KO and γ3 AMPK KO mice (Figs. 1 and 2, A and B). Thus, AICAR-induced AMPK phosphorylation requires functional α2 and γ3 AMPK subunits. Irrespective of genotype, insulin alone or in combination with AICAR was without effect on AMPK phosphorylation, consistent with our previous studies (21, 27).
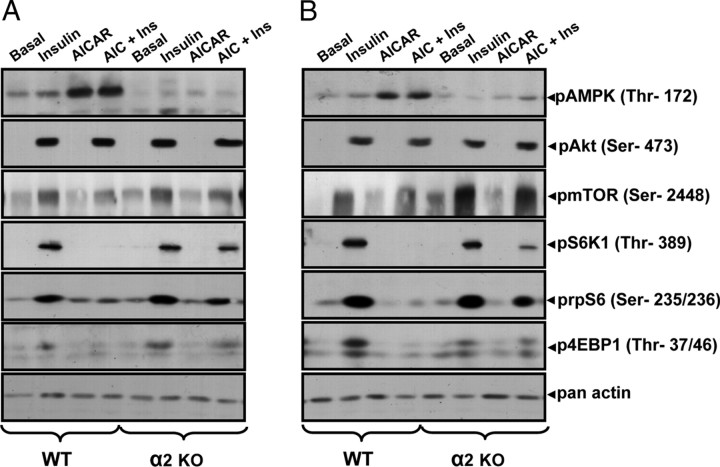
Fig. 1.
Representative Immunoblot Showing Effect of Insulin, AICAR, or AICAR plus Insulin on Phosphorylation of AMPK Thr172, pAkt Ser473, pmTOR Ser2448, pS6K1 Thr389, p-rpS6 Ser235/236, and p4E-BP1 Thr37/46 in EDL Muscle from C57BL/6 (WT), α2 AMPK KO, and γ3 AMPK KO Mice
Representative immunoblot of lysate prepared from EDL muscle incubated in the absence (basal) or presence of insulin (30 min) or AICAR (60 min) or AICAR plus insulin (30 min AICAR preincubation followed by 30 min AICAR plus insulin). Phosphorylation of AMPK Thr172, pAkt Ser473, pmTOR Ser2448, p-rpS6 Ser235/236, and p4E-BP1 Thr37/46 in C57BL/6 (WT) vs. α2 AMPK KO mice (A) or C57BL/6 (WT) vs. γ3 AMPK KO mice (B). Forty micrograms of protein were loaded on each well, and equal loading was confirmed by pan-actin antibody.
Fig. 2.
AMPK and Insulin Signaling in EDL Muscle from α2 and γ3 AMPK KO Mice and Corresponding WT Littermates
Phosphorylation of AMPK Thr172 (A and B) was determined in EDL muscle obtained from α2 AMPK KO (black bars), γ3 AMPK KO (striped bars), and corresponding WT (white bars) littermates. Results are mean ± sem; n = 8 for each genotype. ††, Genotype effect (P < 0.01) within treatment; **, treatment effect (P < 0.01) within WT. Akt Ser473 phosphorylation (C and D) was determined in α2 AMPK KO, γ3 AMPK KO, and corresponding WT (white bars) littermates. Results are mean ± sem; n = 8 for each genotype. **, Effect (P < 0.01) of treatment.
Akt Signaling Is Unaltered in α2 AMPK KO and γ3 AMPK KO Mice
EDL muscle from α2 AMPK KO, γ3 AMPK KO, and WT mice were incubated in the absence or presence of 120 mm insulin (30 min). Insulin increased Akt Ser473 phosphorylation independent of either genotype or exposure to AICAR. In all mouse models, AICAR was without effect on Akt Ser473 phosphorylation (Figs. 1 and 2, C and D). These results support our earlier findings (27) that insulin signaling to Akt does not require the α2 or γ3 AMPK subunit.
AICAR-Induced Inhibition of mTOR Signaling Requires Functional AMPK α2 and γ3 Subunits
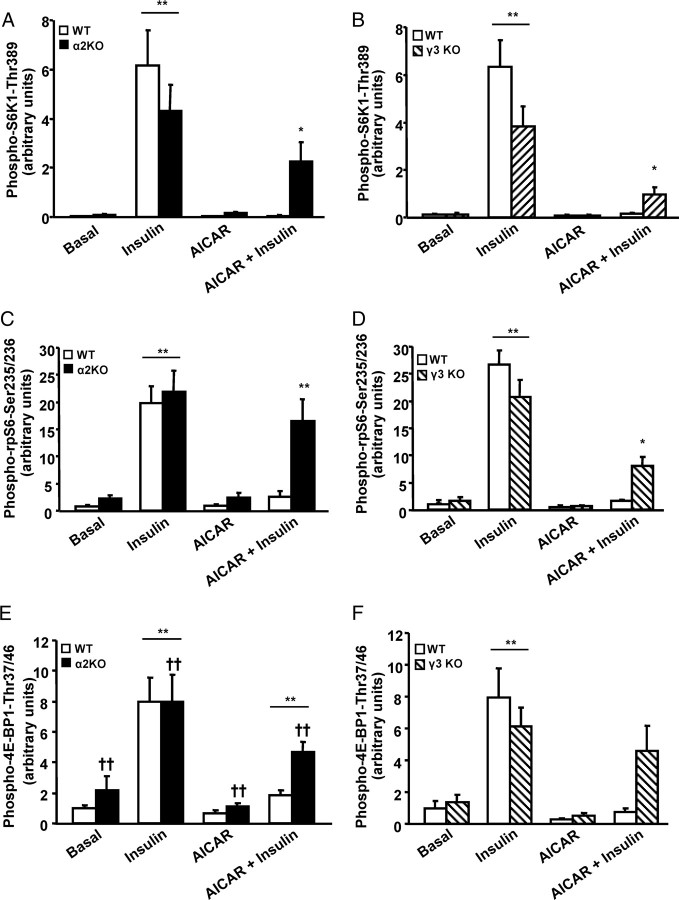
Insulin stimulation induced a profound increase in phosphorylation of S6K1 Thr389, rpS6 Ser235/236, and 4E-BP1 Thr37/46 in WT, α2 AMPK KO, and γ3 AMPK KO. However, in EDL muscle from WT mice, preincubation with AICAR completely inhibited insulin-induced phosphorylation of all mTOR targets, suggesting that mTOR signaling is blocked by AMPK activation. This inhibition was partly rescued in EDL muscle from α2 AMPK KO and γ3 AMPK KO mice, indicating that functional α2 and γ3 subunits of AMPK are required for the AMPK-mediated inhibition of mTOR signaling. AICAR alone was without effect on the basal phosphorylation of S6K1 Thr389, rpS6 Ser235/236, or 4E-BP1(Thr37/46) (Figs. 1 and 3, A–F). Similar patterns of phosphorylation were also noted for 4E-BP1 Thr37/46, although this did not achieve statistical significance. Expression of S6K1, rpS6, and 4E-BP1 was similar between genotypes and unaltered by the in vitro treatments (data not shown).
Fig. 3.
AICAR-Induced Inhibition of mTOR Signaling Requires Functional α2 and γ3 Subunits of AMPK
Phosphorylation of S6K1 Thr389 (A and B), rpS6 Ser235/236 (C and D), and 4E-BP1 Thr37/46 (E and F) in EDL muscle from α2 AMPK KO (black bars), γ3 AMPK KO (striped bars), and corresponding WT (white bars) littermates was determined. There is no interaction effect in F. Results are mean ± sem; n = 8 for each genotype. ††, Main effect of genotype (P < 0.01); */**, treatment effect within genotype (P < 0.05/0.01).
DISCUSSION
AMPK suppresses mTOR signaling and protein synthesis (24, 26, 28) via phosphorylation and activation of TSC2 (Thr1227 and Ser1345) (29) and/or phosphorylation and inactivation of mTOR on Thr2446 (30). Using two animal models in which the predominant catalytic and regulatory AMPK isoforms expressed in glycolytic skeletal muscle have been genetically manipulated, we provide direct evidence linking AMPK subunits to mTOR signaling. AICAR-induced activation of AMPK in EDL muscle of α2 AMPK KO and γ3 AMPK KO mice was markedly impaired, indicating α2 and γ3 subunits are critical for the activation of AMPK.
After insulin/growth factor-mediated activation, Akt stimulates mTOR signaling via phosphorylation and inactivation of TSC2 (Thr1462) (31) and/or phosphorylation of mTOR (Ser2448) (30). Insulin signaling was unaltered in α2 AMPK KO and γ3 AMPK KO mice, and AICAR was without effect on phosphorylation of Akt in the absence of insulin. Our results provided evidence against cross talk between AMPK and Akt signaling pathways under basal or insulin-stimulated conditions.
To determine whether AMPK and mTOR signaling pathways intersect, skeletal muscle was exposed to a combination of insulin and the AMPK activator, AICAR and S6K1 and 4E-BP1 were studied. Phosphorylation of eEF2K and its substrate eEF2 (eukaryotic elongation factors) were unaltered in skeletal muscle (data not shown). In contrast, AICAR repressed insulin-induced phosphorylation of mTOR (Ser2448) in wild-type mice, whereas this effect was partially blocked in α2 AMPK KO and γ3 AMPK KO mice (Fig. 1).
mTOR regulates protein synthesis by two common mechanisms; first, it activates S6K1, which is a positive regulator of protein translation, and second, it phosphorylates and inhibits 4E-BP1 activity, a negative regulator of the protein initiation factor eIF4E (7, 8). Activity of S6K1 is regulated by multiple-site phosphorylation. Thr389 phosphorylation is rapamycin sensitive via the mTOR/raptor complex and appears to be a critical rate-limiting step for mTOR activation (25, 32). The rpS6 protein is the first identified substrate of S6K1, and its phosphorylation is directly associated with activation of S6K as well as regulation of cell size (33). Insulin phosphorylates S6K1 Thr389 and rpS6 Ser235/236 in WT, α2 AMPK KO and γ3 AMPK KO mice, providing evidence that S6K1 is activated and correlated with Akt phosphorylation (Figs. 3, A–D, and 2, C and D). Therefore, activation of the Akt-S6K1 pathway is independent of AMPK α2 and γ3 subunits in skeletal muscle. However, AICAR blocked insulin-mediated phosphorylation of S6K1 and rpS6 in wild-type mice (Figs. 1 and 3), suggesting opposing effects of Akt and AMPK on S6K1 activity. In α2 AMPK KO and γ3 AMPK KO mice, the inhibitory effect of AICAR was blocked, clearly indicating AMPK-mediated inhibitory signals are integrated through α2 and γ3 subunits. Interestingly, activity of the mTOR pathway is increased in obese insulin-resistant rodents, whereas depletion of S6K1 protects against diet-induced insulin resistance (34). S6K-deficient skeletal muscle is associated with an activation of AMPK, and AMPK inhibition restores cell growth and sensitivity to nutrient signals (35). Our results support the hypothesis that cross talk at the level of AMPK and mTOR are critical for cellular energy homeostasis in health and disease.
4E-BP1 is subjected to series of hierarchical phosphorylation events induced by mTOR (7, 8). Phosphorylation of 4E-BP1 Thr37/46 is an initial and important step in mTOR signaling. Insulin phosphorylates 4E-BP1 in WT, α2 AMPK KO, and γ3 AMPK KO mice, with effects positively correlated with the degree of Akt phosphorylation (Fig. 3, E and F). AICAR preincubation completely blocked the insulin-mediated phosphorylation of 4E-BP1 in WT, α2 AMPK KO, and γ3 AMPK KO mice, which tightly correlates with AMPK phosphorylation. Although 4E-BP1 phosphorylation was not significantly increased, in both KO models, the phosphorylation pattern was strikingly similar to that observed for S6K1 and rpS6 phosphorylation. The role of 4E-BP1 in protein synthesis remains obscure, mainly because disruption of the 4E-BP1 gene does not alter growth and protein synthesis (36). Our results suggest that AMPK α2 and γ3 are partly required for the inhibitory effects on insulin-stimulated phosphorylation of 4E-BP1.
The inhibitory effect of AMPK on insulin-induced mTOR signaling is physiologically relevant, because the γ3 isoform is mainly found in complexes containing α2 and β2 subunits in mouse glycolytic skeletal muscle (20). However, the inhibitory effects of AMPK on insulin-induced mTOR signaling are incompletely prevented in α2 AMPK KO and γ3 AMPK KO mice (Fig. 3). The expression of other AMPK subunits is unaltered in γ3 AMPK KO mice (21). Conversely, expression of β1, β2, and γ3 is decreased and α1 is increased in α2 AMPK KO mice (37). However, the increase in α1 subunit expression in α2 AMPK KO mice does not restore total AMPK activity in α2 AMPK KO mice (37). Nevertheless, changes in AMPK subunit expression may possibly explain why the inhibitory effects of AICAR on insulin-induced mTOR signaling are almost completely prevented in α2 AMPK KO mice compared with γ3 AMPK KO mice.
Glycolytic skeletal muscle such as the EDL expresses α1 and γ1 subunits of AMPK (20), which might contribute to the AICAR-induced inhibition of mTOR signaling in α2 AMPK KO and γ3 AMPK KO mice. Compounded KO mice, whereby α2 and γ3 subunits are deleted, or β2 AMPK KO mice may show a prominent rescue effect of AICAR on insulin-induced mTOR signaling. Although the major support for repressive effects of AMPK on mTOR signaling comes from experimental approaches where AICAR has been used as a pharmacological activator of AMPK, we cannot exclude the possibility that AICAR may act independently of AMPK activation through intracellular P(i) depletion and 5-aminoimidazole-4 carboxamide ribonucleotide accumulation (38).
Skeletal muscle hypertrophy is associated with resistance exercise, increased mechanical load, Akt activation, mTOR signaling, and increased rates of protein synthesis, whereas nutrient overload induces hypertrophy in an Akt-independent manner (12). During endurance exercise, the AMP to ATP ratio progressively increases, which leads to activation of AMPK (39). Although increased AMPK activity is associated with decreased muscle hypertrophy (28), skeletal muscle-specific knockout of LKB1, the upstream activator for AMPK, is associated with an increase in cell size (40). Activation of TSC2 by AMPK is dominant over protein kinase B-mediated inactivation and leads to the inactivation of mTOR and a decrease in protein synthesis (29). The temporal relationship between Akt and AMPK might be central to the regulation of mTOR signaling and skeletal muscle mass.
The regulation of mTOR is complex because it is a point of convergence, which integrates several signals (7, 8, 41, 42). A defect in mTOR signaling is associated with several diseases including diabetes and cancer. Our results provide evidence that AMPK is a negative regulator of mTOR signaling in skeletal muscle. We reveal that AMPK complexes containing the α2 and γ3 subunits are required for the inhibitory effect of AMPK on mTOR signaling in glycolytic muscle. Thus, AMPK is an important regulator of mTOR signaling that provides a level of cross talk and feedback inhibition on downstream signaling toward mTOR.
MATERIALS AND METHODS
Animals
All experiments were approved by the Regional Animal Ethical Committee (Stockholm, Sweden) and the Danish Animal Experimental Inspectorate. Four- to five month-old female mice from three different strains were used: α2-AMPK KO (25), γ3-AMPK KO (21), and C57BL/6 mice. WT littermates were used as controls for each genetic model studied. Within each KO strain, mice of different genotypes were produced by intercross breeding (hetero-hetero for α2 and γ3). Mice were maintained on a 10-h light, 14-h dark cycle and received standard rodent chow.
Muscle Incubation Procedures
Mice were anesthetized via ip injection of 2.5% avertin (0.02 ml/g body weight) or pentobarbital (6 mg/100 g body weight), and EDL muscles were quickly removed and incubated in Krebs-Henseleit prebuffer at 30 C oxygenated with a gas containing 95% O2 and 5% CO2. The content of the prebuffer has been described previously (21, 25). Incubations were carried out using prebuffer, to which either AICAR (2 mm; Toronto Research Chemicals Inc., Toronto, Canada), or insulin (120 nm Actrapid; Novo Nordisk, Bagsværd, Denmark) was added. All muscles were preincubated for 30 min in prebuffer followed by four different incubation conditions: basal (control), insulin, AICAR, or AICAR plus insulin. The total incubation time for AICAR and insulin was 60 and 30 min, respectively. To check the inhibitory effect of AMPK on insulin-mTOR signaling, muscles were preincubated with AICAR (30 min) and subsequently with AICAR plus insulin (30 min). After incubation, muscles were harvested, washed in ice-cold Krebs-Henseleit buffer, blotted on filter paper, and quickly frozen with aluminum tongs precooled in liquid nitrogen and stored at −80 C.
Muscle Lysate Preparation
Muscles were homogenized in ice-cold buffer [10% glycerol, 20 mm sodium pyrophosphate, 150 mm NaCl, 50 mm HEPES (pH 7.5), 1% Nonidet P-40, 20 mm β-glycerophosphate, 10 mm sodium fluoride, 1 mm EDTA, 1 mm EGTA, 2 mm phenylmethylsulfonyl fluoride, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 2 mm sodium orthovanadate, 3 mm benzamidine (pH 7.4)] for 20 sec using a motor-driven pestle. Homogenates were rotated end-over-end for 1 h at 4 C and subjected to centrifugation (14,000 × g for 10 min) at 4 C. The supernatant was frozen in liquid nitrogen and stored at −80 C. Protein content in lysates was measured by the bicinchoninic acid method (Pierce, Rockford, IL).
Immunoblot Analysis
Phosphorylation and expression of various proteins were determined by using the following antibodies against phospho-AMPK Thr172, phospho-Akt Ser473, phospho-p70S6K Thr389, phospho-rpS6 Ser235/236, phospho-4E-BP1 Thr37/46 (Cell Signaling Technology, Beverly, MA). A pan-actin (Cell Signaling Technology) antibody was used to confirm equal loading. Muscle lysates were adjusted to equal protein concentration and boiled in Laemmli buffer and loaded on 7.5, 12, or 6–12% gradient gels and transferred to a polyvinylidene difluoride membrane (Immobilon Transfer Membrane; Millipore A/S, Bedford, MA). Membranes were blocked in TBST buffer (10 mm Tris-base, 150 mm NaCl, 0.25% Tween 20) containing 5% low-fat milk protein for 2 h at room temperature. Membranes were then incubated with primary antibodies overnight at 4 C, washed with TBST buffer followed by incubation with appropriate horseradish peroxidase-conjugated secondary antibody (Bio-Rad, Richmond, CA) for 1 h at room temperature. Immunoreactive proteins were visualized by enhanced chemiluminescence (ECL or ECL plus; Amersham, Arlington Heights, IL) and quantified by densitometry using Molecular Analyst Software (Bio-Rad), and results are expressed as relative units compared with basal samples loaded on each gel.
Statistics
Data are expressed as mean ± sem. Statistical evaluation was performed by two-way ANOVA, and Tukey’s post hoc analysis was applied to identify significant differences between groups when effects were statistical significant. All statistical analyses were performed using SigmaStat 3.5 (Systat Software Inc., Richmond, CA). P < 0.05 was considered significant.
Acknowledgments
We thank Anna Krook, Alexander Chibalin, Sonia Métayer Coustard, and Marie Björnholm for their valuable comments during the preparation of this manuscript.
Footnotes
This work was supported by grants from the Swedish Research Council, the Swedish Diabetes Association, the Foundation for Scientific Studies of Diabetology, the Swedish Centre for Sports Research, the Danish Medical Research Council, the Novo Nordisk Foundation, the Danish Diabetes Association, the Copenhagen Muscle Research Centre, the Strategic Research Foundation (INGVAR II and Center for Functional Genetics), the Agence Nationale de la Recherche, the Association Française contre les Myopathiesthe, Commission of the European Communities (Contract No. LSHM-CT-2004-005272 EXGENESIS and Contract No LSHM-CT-2004-512013 EUGENE2).
Disclosure Summary: The authors have nothing to disclose.
First Published Online February 14, 2008
Abbreviations: AICAR, 5-Aminoimidazole-4-carboxamide-1-β-d-ribonucleoside; AMPK, AMP-activated protein kinase; 4E-BP1, 4E-binding protein 1; EDL, extensor digitorum longus; eIF, eukaryotic initiation factor; GβL, G-protein β-like protein; KO, knockout; mTOR, mammalian target of rapamycin; rpS6, ribosomal protein S6; S6K1, ribosomal S6 kinase 1; TSC2, tuberous sclerosis complex.
References
- 1.Rolfe DF, Brown GC 1997. Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–758 [DOI] [PubMed] [Google Scholar]
- 2.Kahn BB, Alquier T, Carling D, Hardie DG 2005. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 1:15–25 [DOI] [PubMed] [Google Scholar]
- 3.Long YC, Zierath JR 2006. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest 116:1776–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardie DG 2004. The AMP-activated protein kinase pathway: new players upstream and downstream. J Cell Sci 117:5479–5487 [DOI] [PubMed] [Google Scholar]
- 5.Mu J, Brozinick Jr JT, Valladares O, Bucan M, Birnbaum MJ 2001. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol Cell 7:1085–1094 [DOI] [PubMed] [Google Scholar]
- 6.Hayashi AA, Proud CG 2007. The rapid activation of protein synthesis by growth hormone requires signaling through the mammalian target of rapamycin, mTOR. Am J Physiol Endocrinol Metab 292:E1647–E1655 [DOI] [PubMed]
- 7.Abraham RT 2005. TOR signaling: an odyssey from cellular stress to the cell growth machinery. Curr Biol 15:R139–R141 [DOI] [PubMed]
- 8.Harris TE, Lawrence Jr JC 2003. TOR signaling. Sci Stke 2003:re15 [DOI] [PubMed]
- 9.Jefferies HB, Fumagalli S, Dennis PB, Reinhard C, Pearson RB, Thomas G 1997. Rapamycin suppresses 5′TOP mRNA translation through inhibition of p70s6k. EMBO J 16:3693–3704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang H, Hornstein E, Stolovich M, Levy G, Livingstone M, Templeton D, Avruch J, Meyuhas O 2001. Amino acid-induced translation of TOP mRNAs is fully dependent on phosphatidylinositol 3-kinase-mediated signaling, is partially inhibited by rapamycin, and is independent of S6K1 and rpS6 phosphorylation. Mol Cell Biol 21:8671–8683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glass DJ 2003. Molecular mechanisms modulating muscle mass. Trends Mol Med 9:344–350 [DOI] [PubMed] [Google Scholar]
- 12.Glass DJ 2005. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol 37:1974–1984 [DOI] [PubMed] [Google Scholar]
- 13.Carling D 2004. The AMP-activated protein kinase cascade: a unifying system for energy control. Trends Biochem Sci 29:18–24 [DOI] [PubMed] [Google Scholar]
- 14.Kemp BE, Stapleton D, Campbell DJ, Chen ZP, Murthy S, Walter M, Gupta A, Adams JJ, Katsis F, van Denderen B, Jennings IG, Iseli T, Michell BJ, Witters LA 2003. AMP-activated protein kinase, super metabolic regulator. Biochem Soc Trans 31:162–168 [DOI] [PubMed] [Google Scholar]
- 15.Sullivan JE, Brocklehurst KJ, Marley AE, Carey F, Carling D, Beri RK 1994. Inhibition of lipolysis and lipogenesis in isolated rat adipocytes with AICAR, a cell-permeable activator of AMP-activated protein kinase. FEBS Lett 353:33–36 [DOI] [PubMed] [Google Scholar]
- 16.Birk JB, Wojtaszewski JF 2006. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol 577:1021–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA, Lynch GS, Kemp BE, Stapleton D 1999. Expression of the AMP-activated protein kinase β1 and β2 subunits in skeletal muscle. FEBS Lett 460:343–348 [DOI] [PubMed] [Google Scholar]
- 18.Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D 2000. Characterization of AMP-activated protein kinase γ-subunit isoforms and their role in AMP binding. Biochem J 346(Pt 3):659–669 [PMC free article] [PubMed]
- 19.Gao G, Fernandez CS, Stapleton D, Auster AS, Widmer J, Dyck JR, Kemp BE, Witters LA 1996. Non-catalytic β- and γ-subunit isoforms of the 5′-AMP-activated protein kinase. J Biol Chem 271:8675–8681 [DOI] [PubMed] [Google Scholar]
- 20.Mahlapuu M, Johansson C, Lindgren K, Hjalm G, Barnes BR, Krook A, Zierath JR, Andersson L, Marklund S 2004. Expression profiling of the γ-subunit isoforms of AMP-activated protein kinase suggests a major role for γ3 in white skeletal muscle. Am J Physiol Endocrinol Metab 286:E194–E200 [DOI] [PubMed]
- 21.Barnes BR, Marklund S, Steiler TL, Walter M, Hjalm G, Amarger V, Mahlapuu M, Leng Y, Johansson C, Galuska D, Lindgren K, Abrink M, Stapleton D, Zierath JR, Andersson L 2004. The 5′-AMP-activated protein kinase γ3 isoform has a key role in carbohydrate and lipid metabolism in glycolytic skeletal muscle. J Biol Chem 279:38441–38447 [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen SB, Viollet B, Andreelli F, Frosig C, Birk JB, Schjerling P, Vaulont S, Richter EA, Wojtaszewski JF 2004. Knockout of the α2 but not α1 5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranosidebut not contraction-induced glucose uptake in skeletal muscle. J Biol Chem 279:1070–1079 [DOI] [PubMed] [Google Scholar]
- 23.Blair E, Redwood C, Ashrafian H, Oliveira M, Broxholme J, Kerr B, Salmon A, Ostman-Smith I, Watkins H 2001. Mutations in the γ2 subunit of AMP-activated protein kinase cause familial hypertrophic cardiomyopathy: evidence for the central role of energy compromise in disease pathogenesis. Hum Mol Genet 10:1215–1220 [DOI] [PubMed] [Google Scholar]
- 24.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, Kemp BE, Witters LA, Mimura O, Yonezawa K 2003. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells 8:65–79 [DOI] [PubMed] [Google Scholar]
- 25.Viollet B, Andreelli F, Jorgensen SB, Perrin C, Geloen A, Flamez D, Mu J, Lenzner C, Baud O, Bennoun M, Gomas E, Nicolas G, Wojtaszewski JF, Kahn A, Carling D, Schuit FC, Birnbaum MJ, Richter EA, Burcelin R, Vaulont S 2003. The AMP-activated protein kinase α2 catalytic subunit controls whole-body insulin sensitivity. J Clin Invest 111:91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolster DR, Crozier SJ, Kimball SR, Jefferson LS 2002. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem 277:23977–23980 [DOI] [PubMed] [Google Scholar]
- 27.Treebak JT, Glund S, Deshmukh A, Klein DK, Long YC, Jensen TE, Jorgensen SB, Viollet B, Andersson L, Neumann D, Wallimann T, Richter EA, Chibalin AV, Zierath JR, Wojtaszewski JF 2006. AMPK-mediated AS160 phosphorylation in skeletal muscle is dependent on AMPK catalytic and regulatory subunits. Diabetes 55:2051–2058 [DOI] [PubMed] [Google Scholar]
- 28.Thomson DM, Gordon SE 2005. Diminished overload-induced hypertrophy in aged fast-twitch skeletal muscle is associated with AMPK hyperphosphorylation. J Appl Physiol 98:557–564 [DOI] [PubMed] [Google Scholar]
- 29.Inoki K, Zhu T, Guan KL 2003. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115:577–590 [DOI] [PubMed] [Google Scholar]
- 30.Cheng SW, Fryer LG, Carling D, Shepherd PR 2004. Thr2446 is a novel mammalian target of rapamycin (mTOR) phosphorylation site regulated by nutrient status. J Biol Chem 279:15719–15722 [DOI] [PubMed] [Google Scholar]
- 31.Manning BD, Tee AR, Logsdon MN, Blenis J, Cantley LC 2002. Identification of the tuberous sclerosis complex-2 tumor suppressor gene product tuberin as a target of the phosphoinositide 3-kinase/akt pathway. Mol Cell 10:151–162 [DOI] [PubMed] [Google Scholar]
- 32.Pullen N, Thomas G 1997. The modular phosphorylation and activation of p70s6k. FEBS Lett 410:78–82 [DOI] [PubMed] [Google Scholar]
- 33.Krieg J, Hofsteenge J, Thomas G 1988. Identification of the 40 S ribosomal protein S6 phosphorylation sites induced by cycloheximide. J Biol Chem 263:11473–11477 [PubMed] [Google Scholar]
- 34.Um SH, D’Alessio D, Thomas G 2006. Nutrient overload, insulin resistance, and ribosomal protein S6 kinase 1, S6K1. Cell Metab 3:393–402 [DOI] [PubMed] [Google Scholar]
- 35.Aguilar V AS, Sotiropoulos A, Sobering A, Athea Y, Djouadi F, Miraux S, Thiaudière E, Foretz M, Viollet B, Diolez P, Bastin J, Benit P, Rustin P, Carling D, Sandri M, Ventura-Clapier R, Pende M 2007. S6 kinase deletion suppresses muscle growth adaptations to nutrient availability by activating AMP kinase. Cell Metab 5:476–487 [DOI] [PubMed] [Google Scholar]
- 36.Blackshear PJ, Stumpo DJ, Carballo E, Lawrence Jr JC 1997. Disruption of the gene encoding the mitogen-regulated translational modulator PHAS-I in mice. J Biol Chem 272:31510–31514 [DOI] [PubMed] [Google Scholar]
- 37.Jorgensen SB, Treebak JT, Viollet B, Schjerling P, Vaulont S, Wojtaszewski JF, Richter EA 2007. Role of AMPKα2 in basal, training-, and AICAR-induced GLUT4, hexokinase II, and mitochondrial protein expression in mouse muscle. Am J Physiol Endocrinol Metab 292:E331–E339 [DOI] [PubMed]
- 38.Guigas B, Taleux N, Foretz M, Detaille D, Andreelli F, Viollet B, Hue L 2007. AMP-activated protein kinase-independent inhibition of hepatic mitochondrial oxidative phosphorylation by AICA riboside. Biochem J 404:499–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holloszy JO 2005. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol 99:338–343 [DOI] [PubMed] [Google Scholar]
- 40.Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, Alessi DR 2005. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 24:1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proud CG 2006. Regulation of protein synthesis by insulin. Biochem Soc Trans 34:213–216 [DOI] [PubMed] [Google Scholar]
- 42.Proud CG 2007. Signalling to translation: how signal transduction pathways control the protein synthetic machinery. Biochem J 403:217–234 [DOI] [PubMed] [Google Scholar]