Abstract
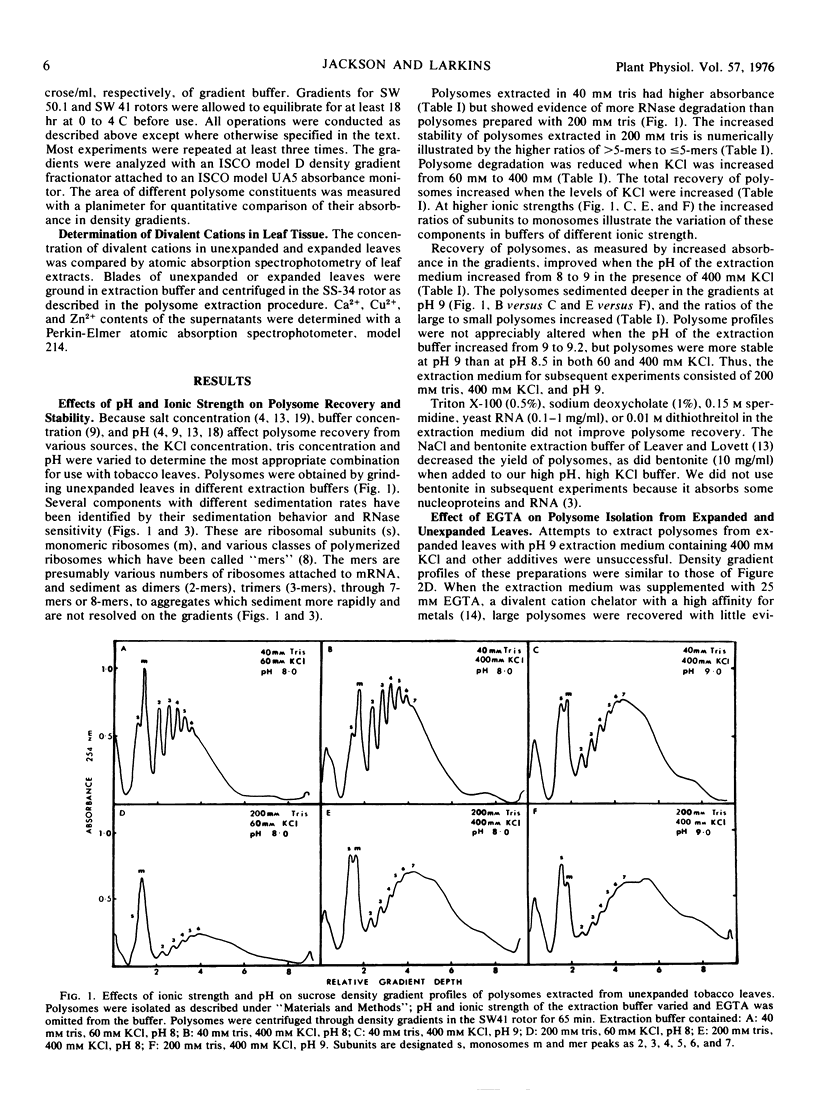
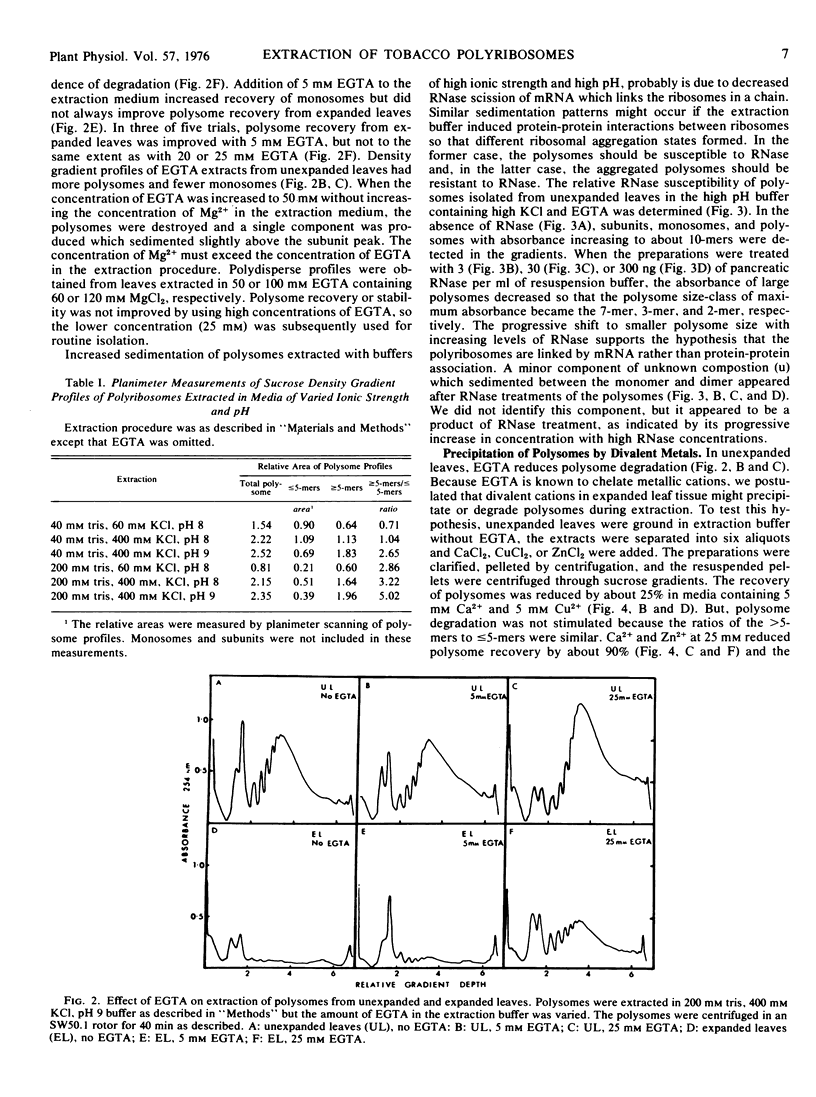
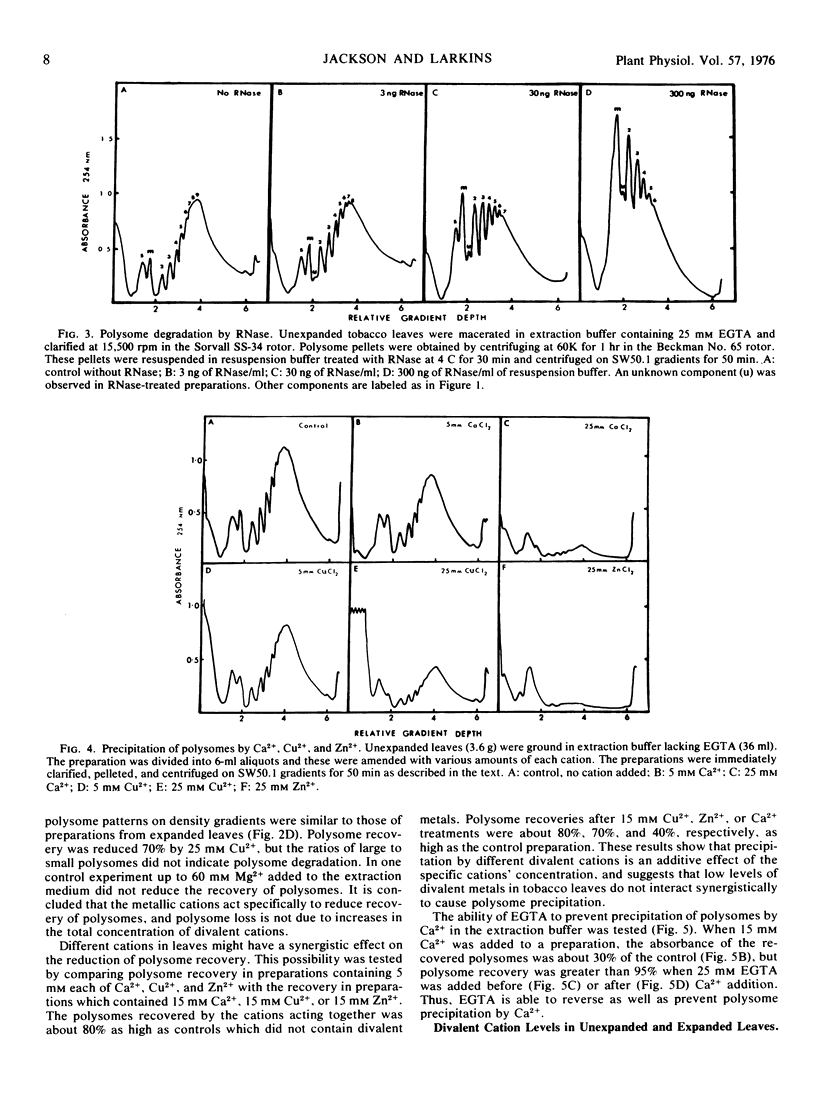
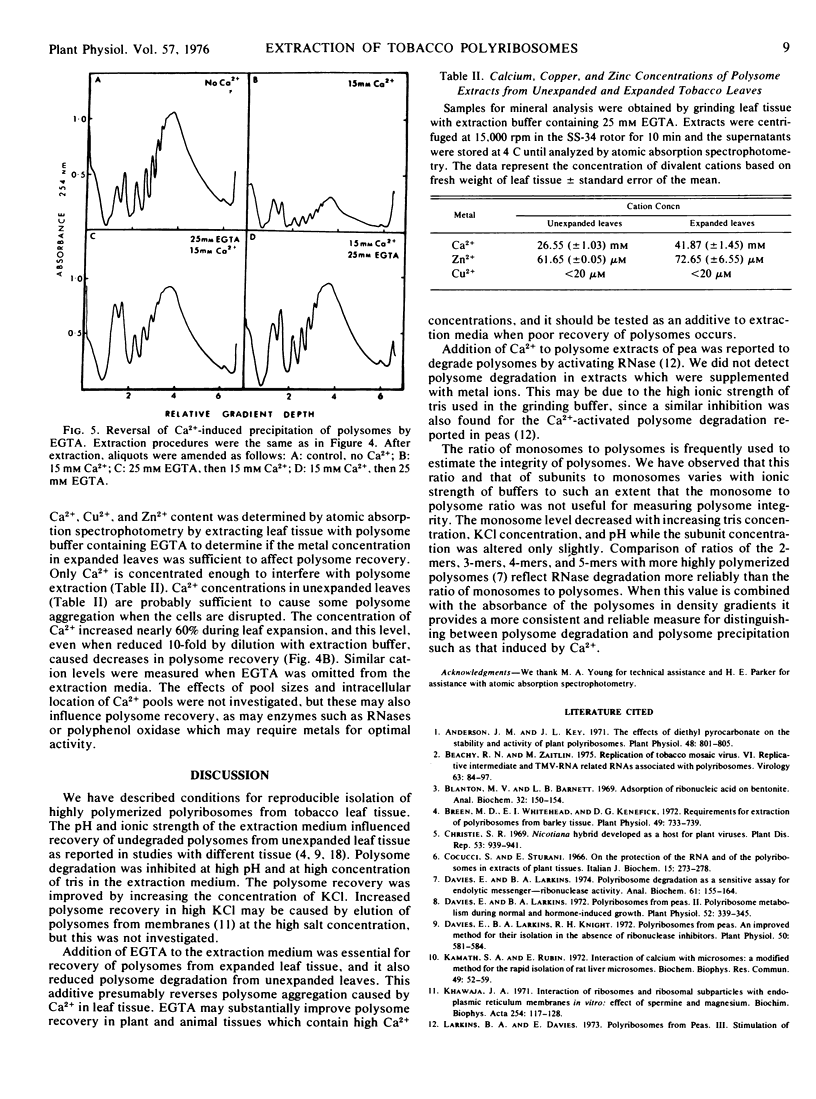
A procedure was developed for extracting polysomes from tobacco (Nicotiana sp) leaves. Unexpanded leaves ground in a medium consisting of 200 mm tris-HCl, pH 9, 400 mm KCl, 200 mm sucrose, and 35 mm MgCl2 yielded larger amounts of polysomes with less degradation than polysomes from leaves extracted with buffers of lower ionic strength or pH. Extraction of polysomes from expanded leaves required the inclusion of ethyleneglycol-bis(2-aminoethyl ether)tetraacetic acid (EGTA, a divalent cation chelator with a high affinity for Ca2+, Cu2+, and Zn2+). EGTA also improved isolation of polysomes from unexpanded leaves. Addition of 25 mm Ca2+, Cu2+, or Zn2+ to extracts from young leaves precipitated polysomes, and density gradient profiles of polysome preparations from the cation treatments mimicked profiles from expanded leaves which were extracted without EGTA. Polysome precipitation by Ca2+ was prevented by EGTA. Endogenous Ca2+ was present in unexpanded leaves in sufficient concentrations (25 mm) to cause some precipitation of polysomes during extraction, and this cation increased by 60% in expanded leaves. Cu2+ and Zn2+ were not present in amounts sufficient to cause polysome precipitation. The results show that recovery of polyribosomes may be reduced by divalent cations in leaf tissue, and this can be overcome by chelation of these ions with EGTA.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Key J. L. The effects of diethyl pyrocarbonate on the stability and activity of plant polyribosomes. Plant Physiol. 1971 Dec;48(6):801–805. doi: 10.1104/pp.48.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R. N., Zaitlin M. Replication of tobacco mosiac virus, VI Replicative intermediate and TMV-RNA-related RNAs associated with polyribosomes. Virology. 1975 Jan;63(1):84–97. doi: 10.1016/0042-6822(75)90373-6. [DOI] [PubMed] [Google Scholar]
- Blanton M. V., Barnett L. B. Adsorption of ribonucleic acid on Bentonite. Anal Biochem. 1969 Oct 15;32(1):150–154. doi: 10.1016/0003-2697(69)90115-8. [DOI] [PubMed] [Google Scholar]
- Breen M. D., Whitehead E. I., Kenefick D. G. Requirement for extraction of polyribosomes from barley tissue. Plant Physiol. 1972 May;49(5):733–739. doi: 10.1104/pp.49.5.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosome degradation as a sensitive assay for endolytic messenger-ribonuclease activity. Anal Biochem. 1974 Sep;61(1):155–164. doi: 10.1016/0003-2697(74)90342-x. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A. Polyribosomes from Peas: II. Polyribosome Metabolism during Normal and Hormone-induced Growth. Plant Physiol. 1973 Oct;52(4):339–345. doi: 10.1104/pp.52.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath S. A., Rubin E. Interaction of calcium with microsomes: a modified method for the rapid isolation of rat liver microsomes. Biochem Biophys Res Commun. 1972 Oct 6;49(1):52–59. doi: 10.1016/0006-291x(72)90008-3. [DOI] [PubMed] [Google Scholar]
- Khawaja J. A. Interaction of ribosomes and ribosomal subparticles with endoplasmic reticulum membranes in vitro: effect of spermine and magnesium. Biochim Biophys Acta. 1971 Nov 29;254(1):117–128. doi: 10.1016/0005-2787(71)90118-3. [DOI] [PubMed] [Google Scholar]
- Leaver C. J., Lovett J. S. An analysis of protein and RNA synthesis during encystment and outgrowth (germination) of Blastocladiella zoospores. Cell Differ. 1974 Sep;3(3):165–192. doi: 10.1016/0045-6039(74)90028-1. [DOI] [PubMed] [Google Scholar]
- McGown E., Richardson A., Henderson L. M., Swan P. B. Anomalies in polysome profiles caused by contamination of the gradients with Cu 2+ or Zn 2+ . Biochim Biophys Acta. 1971 Sep 30;247(1):165–169. doi: 10.1016/0005-2787(71)90820-3. [DOI] [PubMed] [Google Scholar]
- PETERMANN M. L., PAVLOVEC A. STUDIES ON RIBONUCLEIC ACID FROM RAT LIVER RIBOSOMES. J Biol Chem. 1963 Nov;238:3717–3724. [PubMed] [Google Scholar]
- Ramagopal S., Hsiao T. C. Polyribosomes from maize leaves. Isolation at high pH and amino acid incorporation. Biochim Biophys Acta. 1973 Mar 28;299(3):460–467. doi: 10.1016/0005-2787(73)90270-0. [DOI] [PubMed] [Google Scholar]
- Sargent M. L. Use of liquid nitrogen and high ionic strength for the isolation of functional polyribosomes from Neurospora crassa. Biochim Biophys Acta. 1973 Oct 12;324(2):267–274. doi: 10.1016/0005-2787(73)90143-3. [DOI] [PubMed] [Google Scholar]
- Watts R. L., Mathias A. P. The use of bentonite in the isolation of plant polyribosomes. Biochim Biophys Acta. 1967;145(3):828–831. doi: 10.1016/0005-2787(67)90142-6. [DOI] [PubMed] [Google Scholar]
- Weeks D. P., Marcus A. Polyribosome isolation in the presence of diethyl pyrocarbonate. Plant Physiol. 1969 Sep;44(9):1291–1294. doi: 10.1104/pp.44.9.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]


