Abstract
Currently, gene disruption by homologous recombination in embryonic stem cells is only feasible in mice. To circumvent this problem, we silenced mineralocorticoid receptor (MR) expression by RNA interference in knockdown rats generated through lentiviral transgenesis. Analysis of the F1 progeny at 3 wk of age revealed strongly decreased MR levels. This was specific for the targeted gene and related to the abundance of the short interfering RNA. Reminiscent of MR knockout mice, the transgenic rats showed a reduced body weight, elevated serum aldosterone levels, increased plasma renin activity, and altered expression of MR target genes. Some of these effects correlated with the degree to which MR mRNA expression was reduced. Whereas disruption of the MR by gene targeting in mice leads to postnatal death, our strategy also allowed obtaining adult knockdown rats with defects in hormone and electrolyte homeostasis resembling pseudohypoaldosteronism. In conclusion, this is the first example of a human disease model based on RNA interference in rats.
THE DEVELOPMENT OF knockout mice revolutionized research in many fields of molecular biology and medicine. This technique is based on homologous recombination in embryonic stem cells and allows specifically disrupting genes in vivo (1). However, application of this method is limited to mice because all attempts to generate rat embryonic stem cells failed (2). Nuclear cloning may represent a solution to this problem because it is also feasible in rats (3). However, successful transfer of nuclei from homologously recombined cells into oocytes has not yet been reported. The generation of knockdown rats by RNA interference (RNAi) is currently the most promising alternative. Gene silencing by lentiviral delivery of short hairpin RNAs (shRNAs) in transgenic mice was successfully demonstrated in a number of cases (4, 5, 6). More recently, the generation of knockdown rats confirmed that the method can also be applied to other species (7). We now show that this strategy is particularly useful for generating human disease models because it reflects the heterogeneity both in terms of gene silencing and physiological alterations. Thus, RNA interference in transgenic rats is a promising tool with which to study human pathophysiology.
The mineralocorticoid receptor (MR) is involved in controlling the salt-water balance, neuronal excitability, and heart function. In kidney and colon, the adrenocortical hormone aldosterone induces sodium reabsorption and thereby regulates extracellular fluid volume and blood pressure (8). Loss-of-function mutations of the MR cause pseudohypoaldosteronism type I (PHA I) in humans, which is characterized by increased plasma renin activity (PRA) and an altered Na+/K+ balance. Similar physiological deficits are seen in newborn MR knockout mice, but their early lethality precluded more detailed analyses (9). In contrast, mice selectively lacking the MR in the kidney only show a mild phenotype, presumably due to an incomplete deletion in aldosterone target cells (10, 11). To generate an alternative model of PHA I, we silenced the MR in transgenic rats by lentiviral expression of a specific shRNA (12). At 3 wk of age, MR levels were strongly decreased, the body weight reduced, circulating aldosterone and PRA increased, and expression of MR target genes altered. Most importantly, we also obtained a number of adult MR knockdown rats exhibiting typical defects in endocrine and electrolyte homeostasis. We suggest that this technique is an attractive option to generate rats with diminished gene expression that may serve as models for human diseases.
RESULTS
A Lentivirus Encoding a MR-Specific shRNA
To silence MR expression in the rat, we identified a suitable shRNA sequence and cloned it into the lentiviral vector pDR. This vector encodes the Discosoma sp. red fluorescent protein (DsRed) under the control of the cytomegalovirus promoter and additionally contains the U6 promoter to drive shRNA expression (12). The resulting construct, designated pDR-siMR, was used to generate lentiviral particles (Fig. 1A). To assess the efficacy of gene silencing in vitro, HeLa cells expressing the rat MR were transduced either with pDR-siMR or pDR control lentiviral particles. Flow cytometric analysis of DsRed expression showed that transduction efficiency was greater than 95% (data not shown). Importantly, MR expression was strongly diminished in pDR-siMR transduced cells, both on the mRNA as well as the protein level (Fig. 1B). Northern blot analysis confirmed synthesis of the MR-specific short interfering RNA (siRNA) in HeLa cells transduced with pDR-siMR (Fig. 1C). Thus, our lentiviral construct allows for efficient silencing of the MR.
Fig. 1.
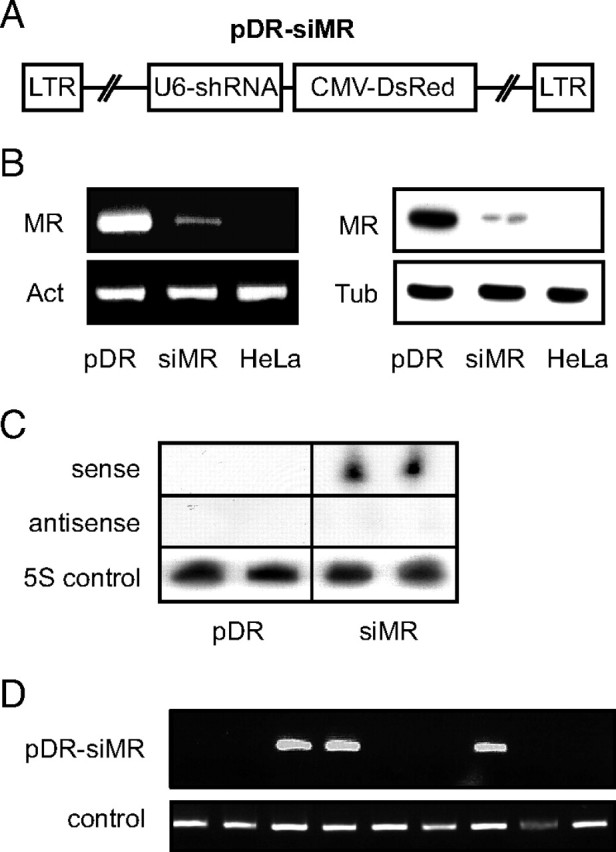
An shRNA-Encoding Lentivirus Allows for Efficient Silencing of the MR
A, Schematic representation of pDR-siMR used to express the MR-specific shRNA and the DsRed protein. B, Analysis of MR-expressing HeLa cells transduced with pDR (first lane) or pDR-siMR (second lane); normal HeLa cells served as a control (third lane). MR mRNA levels were studied by PCR (left panel) and MR protein levels by Western blot (right panel). β-Actin mRNA (Act) and β-tubulin (Tub) protein expression were used for normalization. C, HeLa cells were transduced with pDR or pDR-siMR, and two individual samples each were analyzed by Northern blot for the expression of the processed shRNA. A sense and an antisense oligonucleotide corresponding to the 19-nucleotide MR-specific siRNA as well as an oligonucleotide corresponding to the 5S rRNA were used for hybridization. D, PCR analysis of genomic DNA derived from the nine founder rats for the presence of the pDR-siMR provirus and the Notch1 gene as a control. CMV, Cytomegalovirus; LTR, long terminal repeat.
Generation of MR Knockdown Rats
Transgenic rats can be obtained by injecting lentiviruses into the perivittelin space of fertilized oocytes (13, 14). To generate MR knockdown rats, we infected zygotes from Crl:CD rats with a highly concentrated pDR-siMR lentivirus preparation and transferred them to the oviduct of pseudopregnant foster mothers. PCR analysis confirmed that three of the nine pups born had stably integrated the provirus into the genome (Fig. 1D). Two of the founder rats, designated short interfering MR (siMR), were female and one was male. Despite successful transgene integration, DsRed expression was quite variable in different cell types, consistent with the known expression pattern of the cytomegalovirus promoter (15). Specifically, we could demonstrate red fluorescence in pancreas and kidney of young transgenic rats but not in peripheral blood leukocytes (data not shown; see also Fig. 4A). Consequently, we were unable to assess the degree of mosaicism of the founder rats by flow cytometric analysis as in previous experiments (13).
Fig. 4.
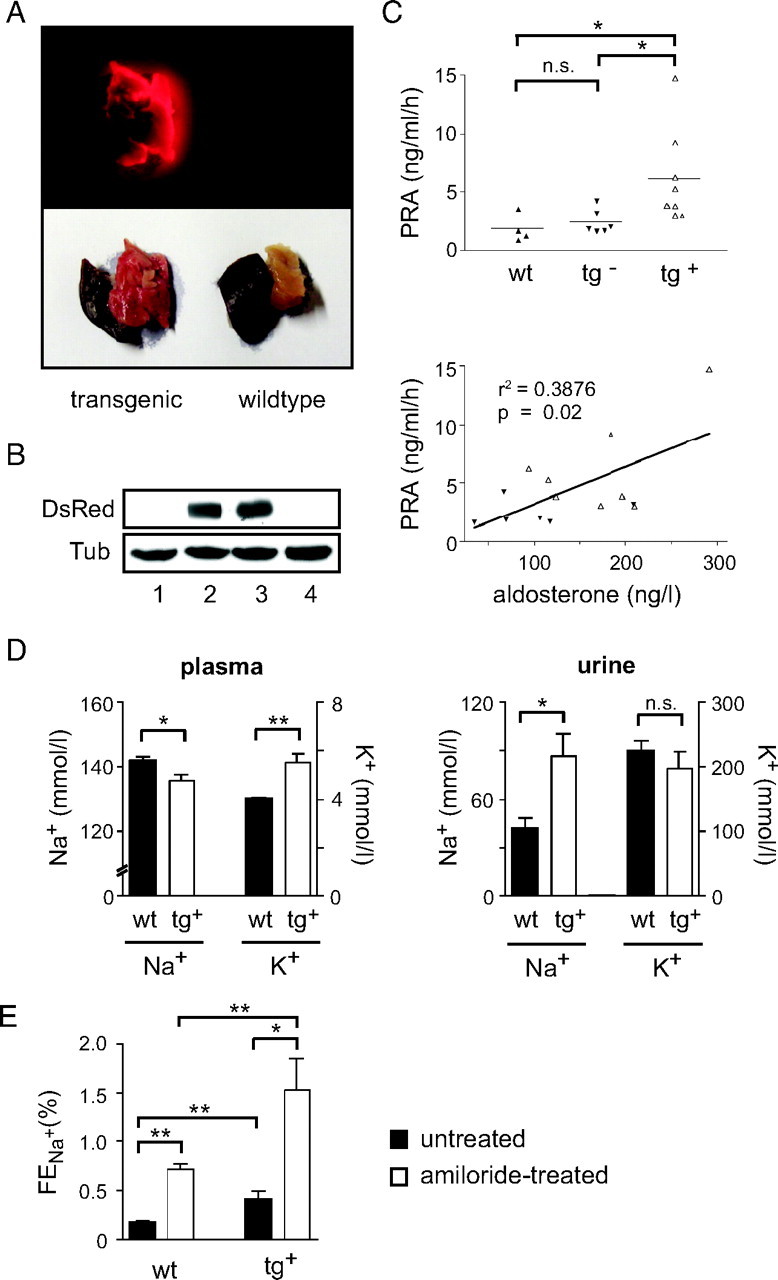
Analysis of Adult siMR Transgenic Rats
A, Inspection of tissue biopsies from adult transgenic and wild-type rats by fluorescent and normal light (liver; P, pancreas). B, Western blot analysis of DsRed protein expression in the kidney of four adult siMR transgenic rats. β-Tubulin (Tub) served as a loading control. C, Analysis of PRA in adult wild-type and transgenic rats expressing (tg+) or lacking (tg−) DsRed protein (upper panel). Correlation between PRA and serum aldosterone levels in adult transgenic rats (lower panel). Solid symbols represent DsRed− animals, and open symbols represent DsRed+ rats in both panels. D, Plasma and urine concentrations of Na+ (left y-axis) and K+ (right y-axis) were determined in adult wild-type and DsRed-expressing transgenic rats (n = 3 for wild type and n = 6–8 for transgenic). E, The fractional excretion of sodium (FENa+) was determined in adult wild-type and DsRed-expressing transgenic rats before and 3 h after amiloride injection (n = 6 for wild type and n = 4 for transgenic). Statistical analysis in all panels was performed by Student’s t test or linear regression. *, P < 0.05; **, P < 0.01; n.s., not significant (n.s., P > 0.05). r2, correlation coefficient; tg, transgenic; wt, wild type.
Efficient Silencing of MR Expression in siMR Rats
To assess the efficiency of MR silencing, we analyzed composite F1 offspring of the three siMR founder rats. In particular, we wished to determine whether different levels of gene silencing could be achieved by intercrossing the founder rats, each carrying one individual provirus integration (data not shown). To this end, the male founder rat was mated with the two female founders, resulting in offspring carrying five different provirus combinations. Subsequently, we compared MR expression between 3-wk-old wild-type and transgenic animals based on the presence of the provirus in the genome but irrespective of its copy number and integration site.
First, we investigated MR mRNA levels in kidney and hippocampus by quantitative PCR. Expression of the highly related glucocorticoid receptor (GR) served as a control. Analysis of 33 transgenic rats revealed a reduction of MR mRNA expression by 75% on average, whereas GR levels were unaltered (Fig. 2A). Interestingly, we observed a great diversity of MR levels ranging from a reduction by less than half to an almost complete loss. As previously reported, the copy number and integration site of the provirus strongly impact on the level of transgene expression (13), thereby explaining the variability in gene silencing.
Fig. 2.
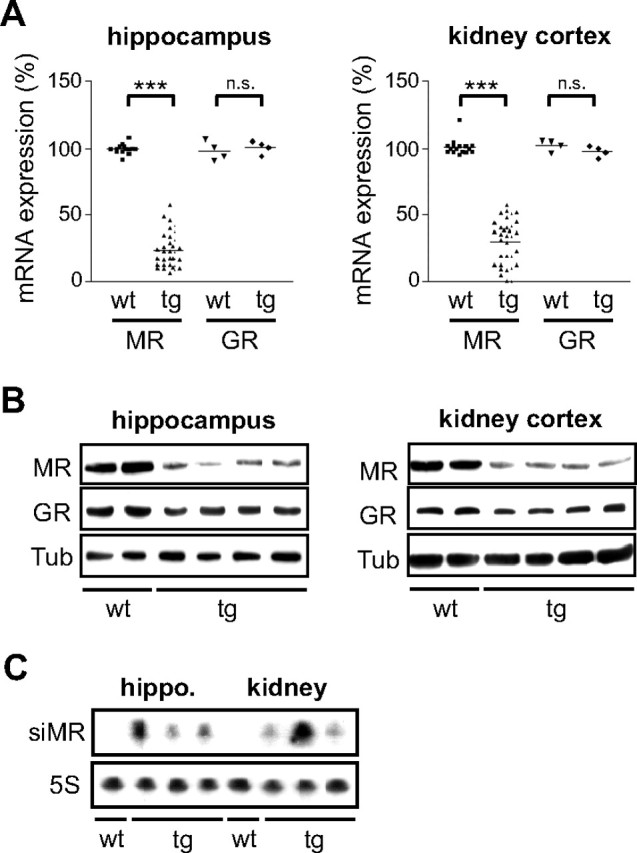
Silencing of the MR in Transgenic Rats
A, Analysis of GR and MR mRNA levels in kidney and hippocampus of 3-wk-old wild-type and transgenic rats by quantitative PCR. Each symbol represents one individual animal. Values are normalized to β-actin expression. The average expression in wild-type animals was set to 100%. Statistical analysis was performed by Student’s t test. ***, P < 0.001; n.s., not significant (n.s., P > 0.05). B, Analysis of GR and MR levels by Western blot using protein lysates of some of the animals depicted in panel A (20 μg per lane). β-Tubulin (Tub) served as loading control. C, Analysis of MR-specific siRNA expression in hippocampus and kidney of four selected animals from panel A by Northern blot. Expression of the 5S rRNA served as control. tg, Transgenic; wt, wild type.
To confirm our results on the protein level we performed a Western blot analysis. Reduced levels of MR protein were detected in the kidney and hippocampus, accompanied by unaltered expression of the GR (Fig. 2B). Finally, we could show that the siRNA was successfully synthesized from the integrated provirus, although there was considerable variability in the levels of the siRNA in individual animals and tissues (Fig. 2C). We conclude that 1) specific and efficient silencing of the MR is achieved in knockdown rats, and 2) that the effect on gene expression reflects the heterogeneity found in many human diseases.
Endocrine Homeostasis Is Disrupted in Young MR Knockdown Rats
Previous analyses have shown that MR knockout mice die shortly after birth due to a severe disturbance of the salt-water balance (9). Their phenotype is characterized by growth retardation, elevated serum aldosterone levels, and increased PRA, two hormones involved in the regulation of sodium reabsorption in the kidney. To investigate whether MR knockdown rats show similar physiological alterations, we first analyzed them at approximately 3 wk of age. Strikingly, some of the pups were lost around 2 wk after birth. We suspect that similar to homozygous MR knockout mice, the silencing was too efficient in these transgenic rats to be compatible with life. However, most of the offspring survived and could be analyzed. First, we observed a significantly lower body weight in the transgenic rats as compared with wild-type littermates (Fig. 3A). Although there was a considerable degree of heterogeneity among the offspring, body weight reduction correlated with the degree of MR mRNA reduction (Fig. 3B). This suggests that the phenotype of the MR knockdown rats is a direct consequence of gene silencing by RNAi. In addition, the 3-wk-old MR knockdown rats had elevated PRA and serum aldosterone levels (Fig. 3C). Again, there was a significant inverse correlation between the degree to which MR expression was diminished and the observed increase in aldosterone (Fig. 3D). We conclude that endocrine homeostasis directly depends on the MR dosage.
Fig. 3.
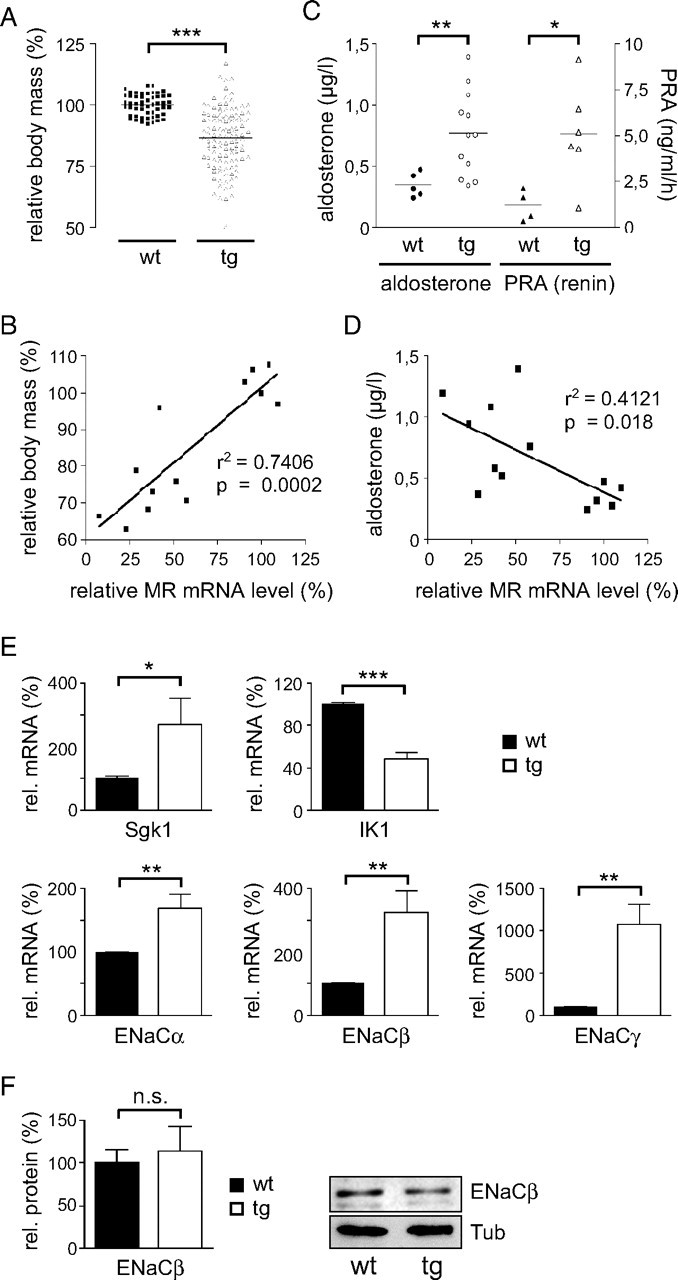
Pathophysiology of Young siMR Transgenic Rats
A, Relative body mass of the siMR transgenic rats at 3 wk of age compared with wild-type controls. The average weight of the wt rats was set to 100%. B, Correlation of the reduced relative body mass with the diminished relative MR mRNA expression. C, Analysis of serum aldosterone levels (left y-axis) and plasma renin activity (PRA, right y-axis) in 3-wk-old wild-type and transgenic rats by RIA. D, Inverse correlation of the increased serum aldosterone levels with the diminished relative MR mRNA expression. Each symbol in panels A–D represents one individual animal. E, Expression analysis of five bona fide MR target genes in the kidney of 3-wk-old wild-type and transgenic rats by quantitative PCR. Values are normalized to β-actin expression. The average expression in wild-type animals was set to 100% (n = 4–6 for wild type and n = 8–18 for transgenic). F, Analysis of ENaCβ protein levels in the kidney of wild-type and transgenic rats by Western blot (20 μg per lane). Values are normalized to β-Tubulin (Tub). The relative amount of ENaCβ protein was quantified by densitometry (left panel, n = 4). A representative Western blot analysis is depicted in the right panel. Statistical analysis in all panels was performed by Student’s t test or linear regression. *, P < 0.05; **, P < 0.01; ***, P < 0.001; n.s., not significant (n.s., P > 0.05). r2, Correlation coefficient; Tg, transgenic; wt, wild type.
The MR controls the salt-water balance, at least in part, by transcriptional regulation of target genes. Therefore, we studied expression of Sgk1 as well as the three subunits of the epithelial sodium channel ENaC in the kidney. Surprisingly, the mRNA levels of all four bona fide MR target genes were up-regulated in siMR transgenic rats, whereas expression of the intermediate-conductance potassium channel IK1/Kcnn4, which is regulated by aldosterone through a nongenomic mechanism (16), was decreased in the knockdown rats (Fig. 3E). Importantly, ENaC protein levels were not altered in siMR transgenic rats as exemplified for the β-subunit (Fig. 3F). This suggests that different regulatory mechanisms operate on the transcriptional as compared with the posttranscriptional level.
Analysis of Adult MR Knockdown Rats
One advantage of the RNAi technology and the concomitant incomplete gene silencing is the ability to circumvent an early lethal phenotype often encountered in conventional knockout mice (5). Because MR-deficient mice die shortly after birth (9), we were curious whether adult MR knockdown rats could be obtained that had a sufficient degree of gene silencing to result in an appreciable physiological phenotype.
MR knockdown rats were identified by PCR based on the presence of the provirus in the genome and killed at an age of 3–6 months. As a measure for an actively transcribed transgene, we analyzed DsRed expression. Interestingly, red fluorescence in the pancreas could be detected only in about half of the adult MR knockdown rats (Fig. 4A), which was also true for the analysis of the DsRed protein in kidney by Western blot (Fig. 4B). To determine whether the siMR transgenic rats show any discernable endocrine phenotype and whether DsRed expression correlates with PRA and serum aldosterone levels, we divided them into two groups based on the traceability of red fluorescence. PRA was significantly elevated in rats expressing DsRed whereas the levels in the nonfluorescent rats were in the range of the wild-type controls (Fig. 4C). Moreover, there was a correlation between serum aldosterone levels and PRA, confirming that endocrine homeostasis was only affected in DsRed-positive rats (Fig. 4C). Thus, the provirus appears to be inactive in some of the transgenic animals while being functional in the rest.
Because the MR controls sodium reabsorption in epithelial tissues, we investigated electrolyte homeostasis in the kidney. In transgenic rats, Na+ plasma levels were decreased whereas K+ levels were increased (Fig. 4D). Conversely, the concentration of sodium in urine was strongly elevated whereas potassium was not significantly affected (Fig. 4D). Thus, adult MR knockdown rats indeed have a disturbed salt-water balance.
Finally, we examined ENaC activity in DsRed-positive siMR transgenic rats by investigating the effect of amiloride, a specific blocker of ENaC, on renal sodium reabsorption. To this end, the fractional excretion of sodium (FENa+) was determined before and 3 h after sc injection of amelioride. In line with the electrolyte measurements, FENa+ in knockdown rats was approximately twice as high as compared with controls under basal conditions (Fig. 4E). As expected, amiloride increased FENa+ in wild-type rats. Importantly, the effect of amiloride in siMR transgenic rats was comparable (Fig. 4E), indicating that renal ENaC activity was largely intact. In summary, our findings suggest that lentiviral RNAi allows establishment of adult MR knockdown rats with a phenotype reminiscent of MR-deficient mice and PHA I patients.
DISCUSSION
Until recently, the analysis of gene ablation in vivo was limited to mice. However, a number of biomedical questions, e.g. related to cardiovascular function, autoimmune diseases, or transplantation are better addressed using rats (17, 18, 19). Surgical procedures can be performed more easily, and disease models sometimes more closely reflect the situation encountered in humans. In view of these facts, it is important to develop new tools that allow for gene silencing in rats. Currently, the delivery of specific shRNAs via transgenesis is the most promising strategy to achieve this. The feasibility of such an approach has been demonstrated in mice and most recently also in rats (4, 5, 7). We now provide evidence that silencing of a physiologically relevant gene in the rat leads to the graded and specific loss of gene expression and mimics the physiological consequences as well as the heterogeneity seen in human patients.
In our study we have identified a number of advantages as well as disadvantages of the knockdown technology using lentiviruses for the delivery of siRNAs. First, we found that the vector used in this study is able to specifically reduce gene expression. However, because the DsRed protein was not expressed in peripheral blood leukocytes, we were unable to judge the degree of mosaicism and to noninvasively control for the expression of the transgene. To overcome this problem, a different promoter should be used in future constructs. For that reason, we have recently generated shRNA-expressing transgenic rats with various lentiviral constructs utilizing the Ubiquitin C promoter and successfully demonstrated green and red fluorescence in all peripheral blood leukocytes (our unpublished data). Second, our study shows that the expression level of the siRNA and therefore the degree of gene silencing differs between individual knockdown rats and tissues. We have previously shown that both the proviral integration site in the founder rats and their copy number impact transgene expression (13). Therefore, MR levels and knockdown efficacy strongly depend on the identity and number of proviral integration sites transmitted from the founder rats to their offspring. Consequently, the level of gene expression must be separately determined for each animal. Although this complicates the analysis, it also reflects the heterogeneity seen in many genetic diseases and can be useful in mimicking human pathophysiology. Third, by comparing the phenotypic consequences of MR silencing in the transgenic rats with those published for MR knockout mice, we could demonstrate that similar effects are achieved using both approaches. This holds true for the observed growth retardation, the altered hormone levels, and the disturbed electrolyte homeostasis. We therefore believe that siMR rats are a useful model for human PHA I. Fourth, we were able to obtain a number of adult MR knockdown rats. Nevertheless, the fact that some of them have silenced the transgene hampers their analysis. Although it is impossible to assess the degree of gene inactivation in living animals, our results indicate that knockdown efficacy correlates with PRA. Therefore, transgenic rats can be selected for experimentation on the basis of their elevated hormone levels whereas expression of MR and DsRed are controlled afterward. Using this strategy, it should be feasible to perform physiological studies with adult rats. Nevertheless, we are currently working on an improved method circumventing this problem, i.e. we have evidence that silencing of the transgene is prevented when using a tetracyclin-inducible construct (our unpublished data).
MR knockdown rats exhibit some of the endocrine features also seen in PHA I patients or MR knockout mice. Suprisingly, however, mRNA levels of the bona fide MR target gene Sgk1 as well as the α-, β-, and γ-subunits of ENaC were elevated despite diminished MR protein levels. It appears that up-regulation of those genes is a consequence of increased aldosterone secretion in the presence of the continuous expression of at least low receptor levels. In this respect our model differs from MR knockout mice in which no receptor protein is left at all (9). In contrast, ENaC activity as well as protein expression was unchanged in siMR transgenic rats. This indicates that transcriptional and posttranscriptional control of ENaC by the MR differs, and that the impaired renal sodium reabsorption observed in siMR transgenic rats is, at least in part, due to the deregulation of yet unidentified amiloride-insensitive channel proteins. Interestingly, mRNA expression of the intermediate-conductance potassium channel IK1/Kcnn4, which is believed to be regulated by aldosterone through nongenomic mechanisms, was diminished (16). It is therefore tempting to speculate that genomic and nongenomic effects of the MR are differently influenced by gene silencing.
In our view, MR knockdown rats have several advantages over the already existing mouse models. Due to the larger size and their closer proximity to humans, rats in general are often preferred for the analysis of kidney and heart physiology. In addition, partial gene silencing by RNAi allows circumvention of the early postnatal lethality observed in MR-deficient mice (9). Thus, our strategy allows, for the first time, obtaining adult animals with reduced MR expression that exhibit a pathology resembling PHA I. However, we admit that the lack of tissue specificity, which can only be achieved with the Cre-loxP system, is a drawback. On the other hand, such an approach is often impeded by the lack of suitable promoters to drive Cre expression. Whereas region specificity was achieved in forebrain-specific MR knockout mice, deletion of the MR in the principal cells of the kidney resulted in an unexpectedly mild phenotype. In this approach, Cre expression driven by the aquaporin 2 promoter did not fully match the expression pattern of the MR in the kidney (10, 11). Consequently, residual MR present in the distal tubular segment of the kidney resulted in normal sodium and potassium concentrations in plasma and urine under a standard diet. To our knowledge, adult siMR transgenic rats are therefore the only loss-of-function model for the MR characterized by a disturbed salt-water balance under normal conditions.
In summary, we have identified lentiviral RNAi in transgenic rats as a powerful technology by which to generate human disease models that allows combining the advantages of this species with the power of targeted gene silencing.
MATERIALS AND METHODS
Cloning of the Lentiviral Vector pDR-siMR
We modified the lentiviral vector pLL-3.7 (12) by replacing enhanced green fluorescent protein with the DsRed fluorescent protein (BD, Heidelberg, Germany) and designated it pDR. To clone pDR-siMR, two 19-bp oligonucleotides complementary to the rat MR mRNA were annealed and inserted into the HpaI and XhoI sites of pDR (Fig. 1A; 5′-T-GACAATAGTCGGTCTGGGA-TTCAAGAGA-TCCCAGACCGACTATTGTC-TTTTTT-C-3′ and 5′-TCGAG-AAAAAA-GACAATAGCGGTCTGGGA-TCTCTTGAA-TCCCAGACCGACTATTGTC-A-3′).
Virus Production and Concentration of Lentiviruses
Lentiviral particles were produced by transient cotransfection of the four plasmids pMDL/RRE, RSV-Rev, pMD2G-VSVG, and pDR-siMR into human embryonic kidney 293 T cells using the calcium-phosphate method (12). The supernatants containing the lentivirus were passed through a 0.45-μm filter, concentrated by ultracentrifugation at 25,000 rpm for 90 min at 4 C and resuspended in 20 μl PBS. The titer was determined by transducing HeLa cells followed by flow cytometric analysis of DsRed expression.
Transgenesis
Transgenic rats were generated as described previously (13). Of the concentrated lentiviral preparation, 10–100 pl were injected into the perivitelline space of fertilized single-cell embryos obtained from superovulated Crl:CD rats (Charles River, Sulzfeld, Germany). The injected zygotes were cultured overnight and transferred into the oviduct of pseudopregnant Crl:CD females. All animal experimentation was conducted in accord with standards of human animal care and approved by the Bavarian state authorities.
MR-Expressing HeLa Cells
HeLa cells were transduced with FUrMRW lentiviral particles and cultured for 5 d. The transduced cells were pooled and monitored for MR expression by Western blot. The FUrMRW vector had been cloned by inserting the full-length rat MR cDNA (a kind gift from Dr. Onno Meijer, Leiden, The Netherlands) into the BamHI site of the lentiviral vector FUW (14).
PCR and Western Blot Analyses
Total RNA was isolated and transcribed into cDNA as described elsewhere (20). Quantitative RT-PCR was performed using an iCycler instrument (Bio-Rad Laboratories, München, Germany). The sequences of the gene-specific primers are available upon request. Transgenic rats were genotyped by conventional PCR using genomic tail DNA digested with Proteinase K in TNES buffer (10 mm Tris, pH 7.5; 0.4 m NaCl, 0.1 m EDTA; 0.6% SDS; 100 μg/ml Proteinase K). Amplification was done at 64 C for 30 cycles using two primers specific for the lentiviral vector: 5′-AGGAAACTCACCCTAACTGTA A-3′ and 5′-CGGCCGCTTAAGCTTGGA AC-3′.
Protein extracts from cells and tissues were prepared in radioimmune precipitation assay lysis buffer, separated on a SDS-PAGE gel, transferred to a polyvinylidine difluoride membrane, and detected using the following antibodies: anti-MR (H-300; Santa Cruz Biotechnology, Heidelberg, Germany), anti-GR (M-20; Santa Cruz), anti-β-tubulin (clone TUB2.1, Sigma Aldrich, Taufkirchen, Germany) and anti-βENaC (H-190; Santa Cruz). Quantification of band intensities was achieved using the GelPro Analyzer 4.5 software (Media Cybernetics, Silver Spring, MD).
Northern Blot Analysis of siRNA Expression
Total RNA (15 mg) was electrophoresed on a 10% Tris-borate, EDTA-urea polyacrylamide gel, transferred onto a nylon membrane, and hybridized to oligonucleotide probes labeled by [32P]γ-ATP. The three probes corresponded to the 19-nucleotide siRNA sequence specific for the MR (in sense or antisense orientation) and a sequence complementary to the 5S-rRNA (used as a control). The sequences of the oligos were as follows: 5′-GACAATAGTCGGTCTGGGA-3′ (siMR, sense); 5′-TCCCAGACCGACTATTGTC-3′ (siMR, antisense); 5′-CCCGATCCTGCTTAGCTTCCG-3′ (5S rRNA).
Hormone and Electrolyte Measurements
PRA and serum aldosterone levels were determined by RIA using commercially available kits (RENCTK, DiaSorin, Dietzenbach, Germany and DPC Biermann, Bad Nauheim, Germany). The intraassay and interassay coefficients of variation were below 8% and 12%, respectively. PRA was measured on the basis of angiotensin I formation. Plasma and urine concentrations of Na+ and K+ were measured with standard ion-selective electrodes using samples collected in the morning. Creatinine levels were determined using a standard enzymatic test (Cobas Integra, Roche Diagnostics, Mannheim, Germany). The values obtained are in concordance with those published by The National BioResource Project for the Rat in Japan (www.anim.med.kyoto-u.ac.jp).
ENaC Activity Measurement
Plasma and urine samples were collected before and 3 h after amiloride treatment. To this end, 5 mg/kg amiloride hydrochloride (Sigma), dissolved in distilled water at a concentration of 50 mg/ml, were administered sc. The fractional excretion of sodium (FENA+) was determined based on Na+ and creatinine levels in plasma and urine using the following equation: FENA+ = (Na+U × CreaP)/(Na+P × CreaU).
Acknowledgments
We thank Christian Bauer, Katrin Voss, and Amina Bassibas for expert technical assistance as well as Dr. Gudrun Balling and Kirsten Brunner for performing the electrolyte and creatinine measurements.
NURSA Molecule Pages:
Nuclear Receptors: GR | MR.
Footnotes
This work was supported by grants from Deutsche Forschungsgemeinschaft (No. 1631/1-3 to H.M.R. and No. FOR124 to H.M.R. and M.F.), VolkswagenStiftung (to H.M.R.), and Deutsche Krebshilfe (No. 107111 to M.F.).
Disclosure Statement: The authors of this manuscript have nothing to declare.
First Published Online March 12, 2008
H.-Y.L. and J.v.d.B. contributed equally to this work and should both be considered as first authors.
Abbreviations: DsRed, Discosoma sp. red fluorescent protein; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; PHA I, pseudohypoaldosteronism type I; PRA, plasma renin activity; RNAi, RNA interference; shRNA, short hairpin RNA; siMR, short interfering MR; siRNA, short interfering RNA.
References
- 1.Thomas KR, Capecchi MR 1987. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 51:503–512 [DOI] [PubMed] [Google Scholar]
- 2.Iannaccone PM, Taborn GU, Garton RL, Caplice MD, Brenin DR 1994. Pluripotent embryonic stem cells from the rat are capable of producing chimeras. Dev Biol 163:288–292 [DOI] [PubMed] [Google Scholar]
- 3.Zhou Q, Renard JP, Le Friec G, Brochard V, Beaujean N, Cherifi Y, Fraichard A, Cozzi J 2003. Generation of fertile cloned rats by regulating oocyte activation. Science 302:1179. [DOI] [PubMed] [Google Scholar]
- 4.Kissler S, Stern P, Takahashi K, Hunter K, Peterson LB, Wicker LS 2006. In vivo RNA interference demonstrates a role for Nramp1 in modifying susceptibility to type 1 diabetes. Nat Genet 38:479–483 [DOI] [PubMed] [Google Scholar]
- 5.Lu W, Yamamoto V, Ortega B, Baltimore D 2004. Mammalian Ryk is a Wnt coreceptor required for stimulation of neurite outgrowth. Cell 119:97–108 [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Stockton J, Mathis D, Benoist C 2006. Modeling CTLA4-linked autoimmunity with RNA interference in mice. Proc Natl Acad Sci USA 103:16400–16405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dann CT, Alvarado AL, Hammer RE, Garbers DL 2006. Heritable and stable gene knockdown in rats. Proc Natl Acad Sci USA 103:11246–11251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connell JM, Davies E 2005. The new biology of aldosterone. J Endocrinol 186:1–20 [DOI] [PubMed] [Google Scholar]
- 9.Berger S, Bleich M, Schmid W, Cole TJ, Peters J, Watanabe H, Kriz W, Warth R, Greger R, Schütz G 1998. Mineralocorticoid receptor knock-out mice: pathophysiology of Na+ metabolism. Proc Natl Acad Sci USA 95:9424–9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, Chepkova AN, Welzl H, Haas HL, Lipp HP, Schütz G 2006. Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci USA 103:195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronzaud C, Loffing J, Bleich M, Gretz N, Grone HJ, Schütz G, Berger S 2007. Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18:1679–1687 [DOI] [PubMed] [Google Scholar]
- 12.Rubinson DA, Dillon CP, Kwiatkowski AV, Sievers C, Yang L, Kopinja J, Rooney DL, Ihrig MM, McManus MT, Gertler FB, Scott ML, Van Parijs L 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet 33:401–406 [DOI] [PubMed] [Google Scholar]
- 13.van den Brandt J, Wang D, Kwon SH, Heinkelein M, Reichardt HM 2004. Lentivirally generated eGFP-transgenic rats allow efficient cell tracking in vivo. Genesis 39:94–99 [DOI] [PubMed] [Google Scholar]
- 14.Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868–872 [DOI] [PubMed] [Google Scholar]
- 15.Dickins RA, McJunkin K, Hernando E, Premsrirut PK, Krizhanovsky V, Burgess DJ, Kim SY, Cordon-Cardo C, Zender L, Hannon GJ, Lowe SW 2007. Tissue-specific and reversible RNA interference in transgenic mice. Nat Genet 39:914–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowley KA, Morton MJ, Hunter M, Sandle GI 2003. Non-genomic regulation of intermediate conductance potassium channels by aldosterone in human colonic crypt cells. Gut 52:854–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schinke M, Baltatu O, Bohm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M 1999. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci USA 96:3975–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tischner D, Weishaupt A, van den Brandt J, Müller N, Beyersdorf N, Ip CW, Toyka KV, Hünig T, Gold R, Kerkau T, Reichardt HM 2006. Polyclonal expansion of regulatory T cells interferes with effector cell migration in a model of multiple sclerosis. Brain 129:2635–2647 [DOI] [PubMed] [Google Scholar]
- 19.Sato Y, Endo H, Ajiki T, Hakamata Y, Okada T, Murakami T, Kobayashi E 2004. Establishment of Cre/LoxP recombination system in transgenic rats. Biochem Biophys Res Commun 319:1197–1202 [DOI] [PubMed] [Google Scholar]
- 20.van den Brandt J, Kwon SH, Hünig T, McPherson KG, Reichardt HM 2005. Sustained pre-TCR expression in Notch1IC-transgenic rats impairs T cell maturation and selection. J Immunol 174:7845–7852 [DOI] [PubMed] [Google Scholar]


