Abstract
Many glucocorticoid (Gc) actions are of rapid onset and therefore require acute regulation of intracellular signaling cascades. Integration of diverse extracellular signals requires cross-talk between intracellular pathways, suggesting the existence of nodes for signal interaction, such as the specialized membrane microdomains caveolae. We have identified rapid Gc-dependent phosphorylation of caveolin, and protein kinase B (PKB)/Akt, in the lung epithelial cell line A549 and found this was dependent on src kinases. There was also activation of PKB downstream molecules glycogen synthase kinase-3β, and mammalian target of rapamycin. Subcellular fractionation colocalized glucocorticoid receptor (GR) and c-src to caveolin-containing membrane fractions. Coimmunoprecipitation studies also identified interactions between GR and caveolin and suggested that the activation function 1 domain within the GR may serve to support an interaction between GR and caveolin. Disruption of lipid raft formation, impairment of caveolin function using dominant-negative caveolin, down-regulation of caveolin-1 using short hairpin RNA or complete ablation of caveolin-1 prevented Gc-induced activation of PKB. Loss of caveolin-1 also prevents Gc activation of glycogen synthase kinase-3β and mammalian target of rapamycin. In contrast, caveolin interference/down-regulation had no effect on Gc transactivation. Functional analysis of caveolin-1 knockdown and knockout cells identified profound loss of Gc-mediated growth inhibition compared with controls, with a requirement for caveolin in order for Gc to regulate cell cycle progression. Therefore, disruption of caveolae leads to dissociation of Gc action, with impaired induction of PKB activation, and cell growth inhibition, but with negligible effects on Gc transactivation. These observations have implications for understanding the diverse physiological actions of Gc.
GLUCOCORTICOIDS (Gc) are steroid hormones that affect virtually all organ systems to influence many aspects of mammalian physiology, including metabolism, growth, and development. In addition, Gc are the most potent antiinflammatory agents known and are widely used in clinical medicine.
Gc act through the ubiquitously expressed intracellular glucocorticoid receptor (GR). The GR resides in the cytoplasm in an inactive state as part of a multiprotein complex, which includes chaperone proteins and immunophilins among others (1, 2, 3, 4).
The ligand-activated GR translocates to the cell nucleus where it dimerizes and binds palindromic glucocorticoid-response elements. The complex then recruits either coactivator or corepressor molecules that can modify chromatin and facilitate or inhibit transcription initiation. Within the nucleus the GR can also interact with other transcription factors, e.g. nuclear factor-κB (5, 6, 7).
In addition, Gc can have effects that occur within minutes and are insensitive to inhibitors of transcription, making a primary transcriptional mechanism unlikely (2, 8, 9). These so-called nongenomic effects of Gc may be mediated, at least in part, by a membrane-associated GR (10, 11, 12). Reported nongenomic effects include activation of phosphatidylinositol 3-kinase (PI3K) (13), MAPKs (14), and c-src (8, 15, 16).
Over the last decade, highly ordered plasma membrane microdomains with particular lipid and protein composition have been defined. There is evidence that these domains, which due to their rigid structure are broadly termed “lipid rafts,” orchestrate some control over intracellular signaling pathways and mediate cross talk between membrane-associated receptors (17, 18, 19).
Current evidence suggests that the estrogen receptor is associated with a particular subset of lipid rafts termed “caveolae” (20, 21). Caveolin-1, the major protein component of caveolae, has been implicated as a structural scaffold for the oligomerization and organization of cytoplasmic signal complexes (22) Interaction with, and modulation by, caveolin-1 has been shown in many signal transduction pathways, including those regulated by receptor and soluble tyrosine kinases (23, 24, 25, 26). Indeed, nongenomic estradiol-dependent stimulation of nitric oxide release is reported to be mediated by estrogen receptors localized within caveolae (27). Additional evidence exists to suggest that androgen receptor signaling is potentiated by overexpression of caveolin-1 (28). This raises the question as to whether a similar system may be in place for regulating nongenomic pathways initiated by Gc.
In this study we examine rapid Gc-dependent phosphorylation events in A549 cells. We colocalize GR with c-src in caveolae and show rapid Gc activation of the PI3K/protein kinase B (PKB) pathway. We further show that caveolin mediates the rapid effects of Gc on PKB, and longer term effects on cell proliferation, but has no role in mediating Gc transactivation.
RESULTS
Treatment with Dexamethasone Promotes the Transient Phosphorylation of PKB
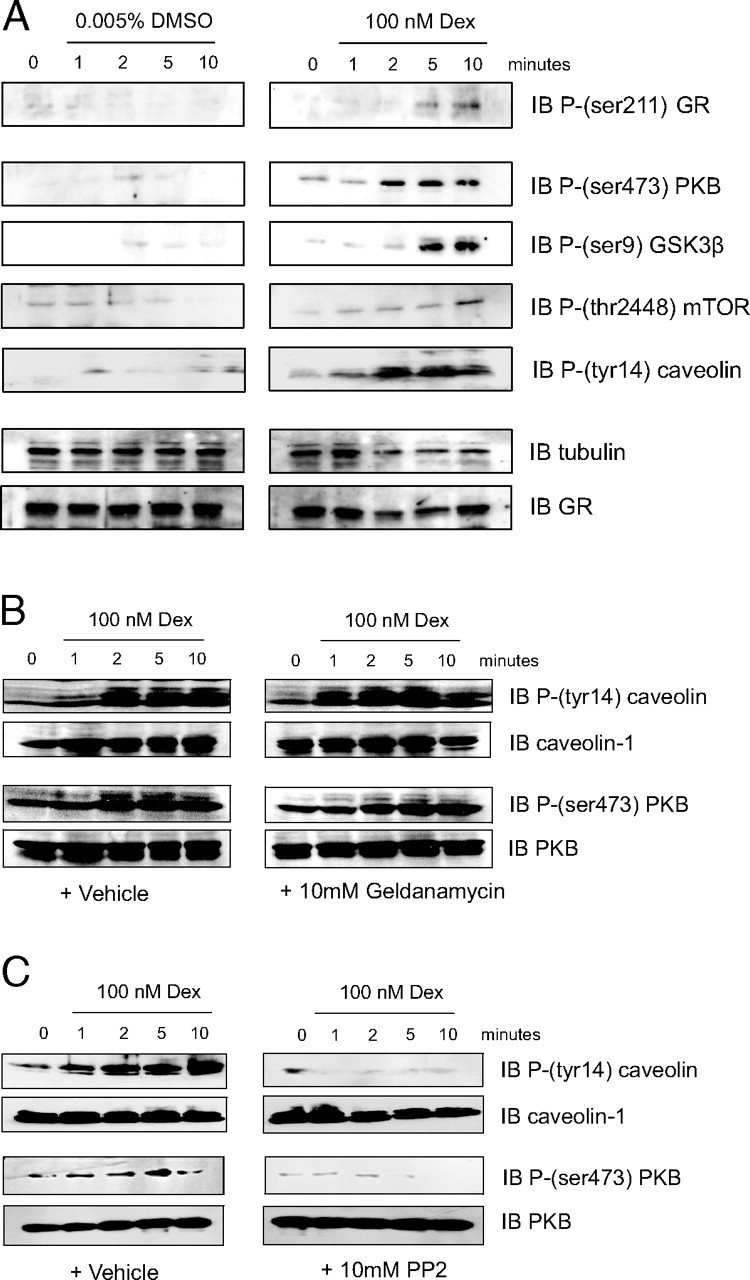
Dexamethasone induced rapid phosphorylation of cytosolic kinase PKB and the integral membrane protein caveolin (Fig. 1A). Induction of PKB phosphorylation was blocked by preincubation with either RU486 or LY294002 (data not shown), suggesting that activation of PKB is both PI3K and Gc specific. Dexamethasone also promoted the rapid phosphorylation of glycogen synthase kinase (GSK)3β and mammalian target of rapamycin (mTOR), two downstream target kinases of PKB. There was no detectable activation of MAPK p38, p42/44, or c-Jun N-terminal kinase in these cells (data not shown). Vehicle treatment alone had no effect on protein phosphorylation (Fig. 1A).
Fig. 1.
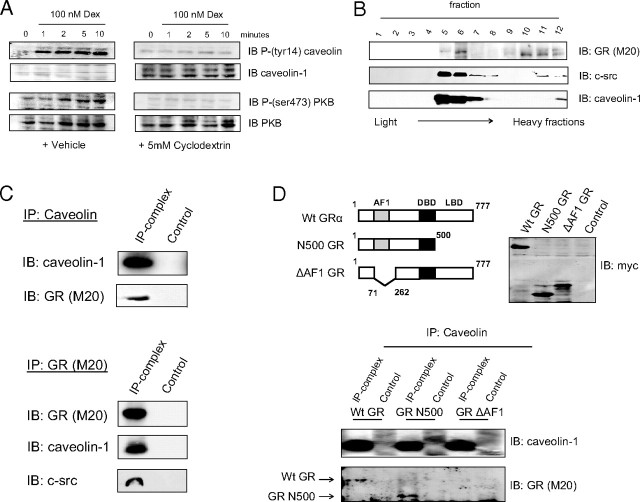
Gc Induce Rapid Phosphorylation of Caveolin and PKB
A549 cells were serum starved for 24 h before treatment with vehicle or 100 nm dexamethasone for the times stated (A), or after pretreatment with the HSP90 antagonist geldanamycin (B) or the c-src inhibitor PP2 (C). Protein (50 μg) was electrophoresed and immunoblotted for phospho-GR, phospho-PKB, phospho-caveolin, phospho-GSK3β, phospho-mTOR (A) or phospho-PKB and phospho-caveolin (B and C). GR and tubulin (A) or PKB and caveolin (B and C) are shown to demonstrate equal loading. Dex, Dexamethasone; DMSO, dimethylsulfoxide; IB, immunoblot.
Phosphorylation of PKB and Caveolin Is Independent of Heat Shock Protein (HSP)90 Association
Geldanamycin, an HSP90 inhibitor, did not prevent dexamethasone induction of PKB phosphorylation (Fig. 1B). To preserve ligand binding affinity, cells were incubated with Gc on ice, before the addition of geldanamycin. Interestingly, the time course of caveolin phosphorylation, but not that of PKB, was marginally left-shifted in the presence of geldanamycin (Fig. 1B), with a more rapid onset, and termination of phosphorylation.
Phosphorylation of PKB and Caveolin Is c-src Dependent
The src inhibitor PP2 (10 μm) abolished Gc-induced phosphorylation of PKB (Fig. 1C). Pretreatment with PP2 also abolished the Gc-induced phosphorylation of caveolin-1 (Fig. 1C); this also suggests a role for c-src in coupling GR/caveolin/ PKB signals.
Gc-Dependent Phosphorylation of PKB and Caveolin Is Lipid Raft Dependent
Treatment with the cholesterol-binding drug methylcyclodextrin disrupts lipid rafts, including caveolae. Pretreatment with methylcyclodextrin clearly abolished the rapid Gc activation of caveolin (Fig. 2A). Because the Gc-induced activation of PKB was also abolished, this suggests not only that membrane domains are important in facilitating rapid signaling in response to dexamethasone, but also that the phosphorylation of caveolin may be required for the Gc-dependent activation of PKB.
Fig. 2.
GR Localizes to Caveolar Fractions through Binding to Caveolin
Serum-starved A549 cells were pretreated with methylcyclodextrin or vehicle and then 100 nm dexamethasone for the times stated. Protein (50 μg) was immunoblotted for phospho-PKB and phospho-caveolin and then PKB and caveolin to show equal loading (A). A549 cells were subjected to subcellular fractionation, and the resulting 12 fractions were electrophoresed and immunoblotted for caveolin, GR, and c-src where higher number fractions contain progressively lower buoyant density homogenate (B). Whole-cell extracts of A549 cells were immunoprecipitated with antibodies against the GR or caveolin before resolution in SDS-PAGE and immunoblotting for GR, caveolin, and c-src. Samples immunoprecipitated with protein A-coated sepharose beads alone are included as controls (C). U20S cells lacking endogenous GR were transfected with full-length GRα, or mutants lacking the ligand-binding or AF1 domains (schematic shown inset) and cell lysates were prepared. These were immunoprecipitated with antibody specific to caveolin-1. Cell extracts and immunoprecipitates were electrophoresed and immunoblotted for myc or GR, respectively (D). Images are representative of three independent experiments. DBD, DNA-binding domain; Dex, dexamethasone; IB, immunoblot; IP, immunoprecipitation; LBD, ligand-binding domain; Wt, wild type.
GR Localizes to Caveolin-Containing Subcellular Fractions through Binding to Caveolin
Subcellular fractionation studies were used to identify GR association with the caveolar membrane marker caveolin-1, and also c-src. Immunoblotting of the 12 fractions obtained from density gradient centrifugation demonstrated that the GR and c-src were present in the same fractions as caveolin in A549 cells (Fig. 2B).
Coimmunoprecipitation studies were then used to examine whether the subcellular distribution of GR and c-src could be mediated through interaction with caveolin. Immunoprecipitation of whole-cell extracts with antibodies against either the GR or caveolin resulted in the specific cross-precipitation of the other molecule (Fig. 2C). However, although GR immunoprecipitated c-src, caveolin did not. The reverse immunoprecipitation, with c-src antibody, did not bring down the GR (Fig. 2C).
U20S cells, which lack endogenous GR, were transfected with wild-type GRα or GR mutants that lack either the ligand-binding domain (N500GR) or activation function (AF)1 domain (ΔAF1GR). Lysate from these cells was subjected to immunoprecipitation with a caveolin-1 antibody, and the precipitates were immunoblotted for GR. Figure 2D shows that caveolin precipitates wild-type GR and N500GR, but not ΔAF1GR, which suggests that the AF1 domain is important for interaction with caveolin.
Phosphorylation of PKB Requires Plasma Membrane-Associated GR
To investigate the contribution of caveolin function to the rapid phosphorylation events detailed above, a dominant-negative caveolin (DN-caveolin) molecule was generated. This comprised the isolated scaffolding domain of caveolin-1, which is reported to mediate the interaction of caveolin with other molecules and so competitively opposes endogenous caveolin action.
Phosphoimmunoblot analysis demonstrates reduced activation of caveolin (P-tyr14), in cells expressing DN-caveolin compared with control-transfected cells (Fig. 3A). In addition, transfection of the DN-caveolin molecule into A549 cells prevented Gc-induced phosphorylation of PKB. Because TNFα signaling requires caveolin, the efficacy of the DN-caveolin molecule was studied in cotransfection studies. These showed that the DN-caveolin blocked TNFα action on an IL 6-luciferase reporter gene (Fig. 3C). Interestingly, the DN-caveolin molecule did not affect the ability of GR to transactivate a simple GR reporter gene (Fig. 3B).
Fig. 3.
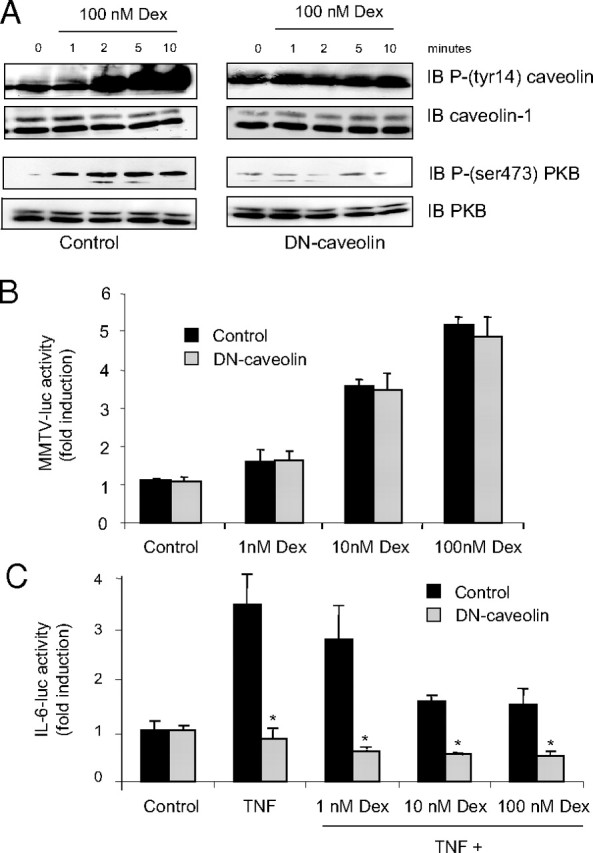
Membrane-Associated Caveolin-1 Is Required for Gc-Induced Protein Phosphorylation But Not Gene Activation in A549 Cells
A549 cells were transiently transfected with dominant negative Caveolin. Cells were treated 24 h post transfection with 100 nm dexamethasone or vehicle for the times indicated. Cells were lysed and 50 μg of protein was electrophoresed and immunoblotted for phospho-PKB and phosphocaveolin, after which immunoblots were stripped and reprobed with antibodies against PKB and caveolin (A). Immunoblots are representative of three independent experiments. Cells were transfected with dominant negative caveolin, or a control vector together with MMTV-luc (B) or a TNFα-responsive IL-6-luc reporter (C). Cells were divided and treated with 10 ng/ml TNFα, dexamethasone, or vehicle as indicated for 16 h and then harvested and assayed for luciferase activity. Graphs depict mean ± sem of three replicates. Experiments were performed at least twice. *, P < 0.05 compared with control transfection. Dex, Dexamethasone; IB, immunoblot; MMTV, mouse mammary tumor virus.
Caveolin-1 Is Not Required for GR Transactivation
To further explore the role of caveolin-1 in mediating Gc action, caveolin-1 knockdown cells were generated. Cells were cotransfected with short hairpin RNA (shRNA)-caveolin-1 and pcDNA3, single clone cell lines selected with neomycin, expanded, and screened for caveolin expression by immunoblot. Figure 4A shows immunoblots of the three clones with variant caveolin expression selected for subsequent study. The first clone expresses equivalent caveolin to wild-type A549 cells (100 ± 10%), and the other two clones have significantly reduced caveolin expression (44 ± 5%; 18 ± 4% wild-type caveolin expression); however, GR expression is not significantly altered in any of the three clones.
Fig. 4.
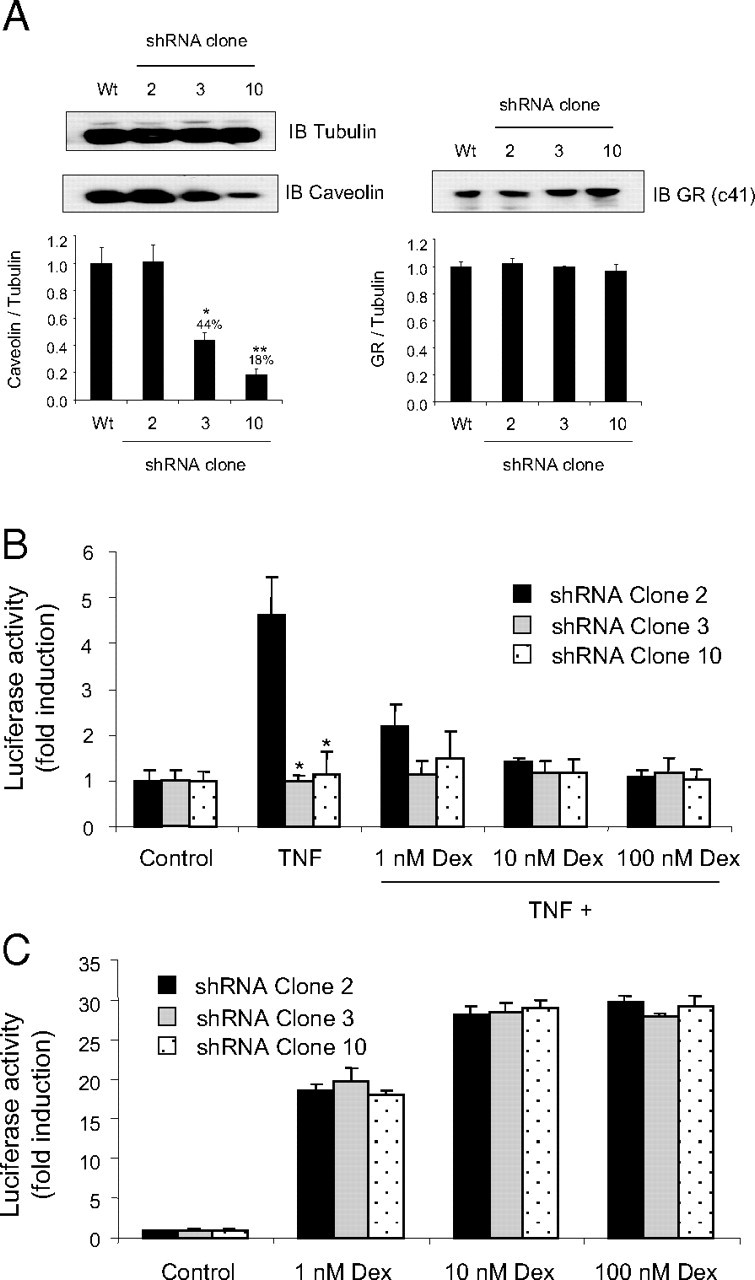
Cells with Variant Caveolin Expression Have Similar Gc-Induced Gene Activation
A549 cells were stably transfected with shRNA targeting caveolin-1. Single-clone cell lines were isolated, lysed, and screened for caveolin expression by immunoblot. Immunoblot images were analyzed by densitometry, and three clones with variant caveolin expression were selected for subsequent study (A). shRNA-A549 cells were transfected with either IL-6-luc (B) or TAT3-luc (C) together with CMV Renilla reporter gene construct (to control for transfection efficiency) (5 54 55 ). Cells were divided and treated with 10 ng/ml TNFα (B only), dexamethasone (B and C), or vehicle as indicated for 16 h and then harvested and assayed for luciferase activity. Graphs depict mean ± sem of four independent experiments performed in triplicate. *, P < 0.05 compared with control cells (shRNA-A549 clone 2). IB, Immunoblot; Wt, wild type.
Because the DN-caveolin molecule abolished TNFα signaling, shRNA-A549 cells were first analyzed for Gc-induced reporter gene activity on an IL-6-luc reporter construct as a functional validation of the caveolin knockdown cell model. Caveolin-1 knockdown blocked TNFα induction of the IL-6-Luc reporter (Fig. 4B). In contrast, a simple, positive Gc reporter gene was unaffected by altered caveolin expression (Fig. 4C).
Caveolin-1 Couples Gc Signals to the PI3K/PKB Pathway
To explore the functional role of caveolin in Gc action, caveolin knockdown cells were analyzed by immunoblot (Fig. 5A). In these clones there was a dose-dependent effect of caveolin on the phosphorylation of both GR(Ser211) and PKB, with induction of basal phosphorylation of both. As a consequence, the relative induction after treatment with ligand is significantly reduced when compared with the corresponding serum-starved controls. To further validate these results, primary, thymic fibroblasts were obtained from wild-type and caveolin-1 knockout mice. These cells were treated with dexamethasone and analyzed by immunoblot. Although there was induction of PKB and GR phosphorylation in the caveolin(+/+) cells in response to dexamethasone treatment, the caveolin(−/−) cells appeared largely unresponsive (Fig. 5B). There was comparable induction of basal phosphorylation of both GR and PKB similar to that seen in the knockdown cells. Interestingly, although the downstream PKB targets, GSK3β and mTOR, were phosphorylated in the caveolin(+/+) cells after treatment with dexamethasone, these proteins were not detectably phosphorylated in the caveolin(−/−) cells under the same experimental conditions (Fig. 5C). In keeping with the altered baseline activity of PKB in the caveolin(−/−) cells, both GSK3β and mTOR displayed increased basal phosphorylation, suggesting downstream consequences of the altered PKB activity.
Fig. 5.
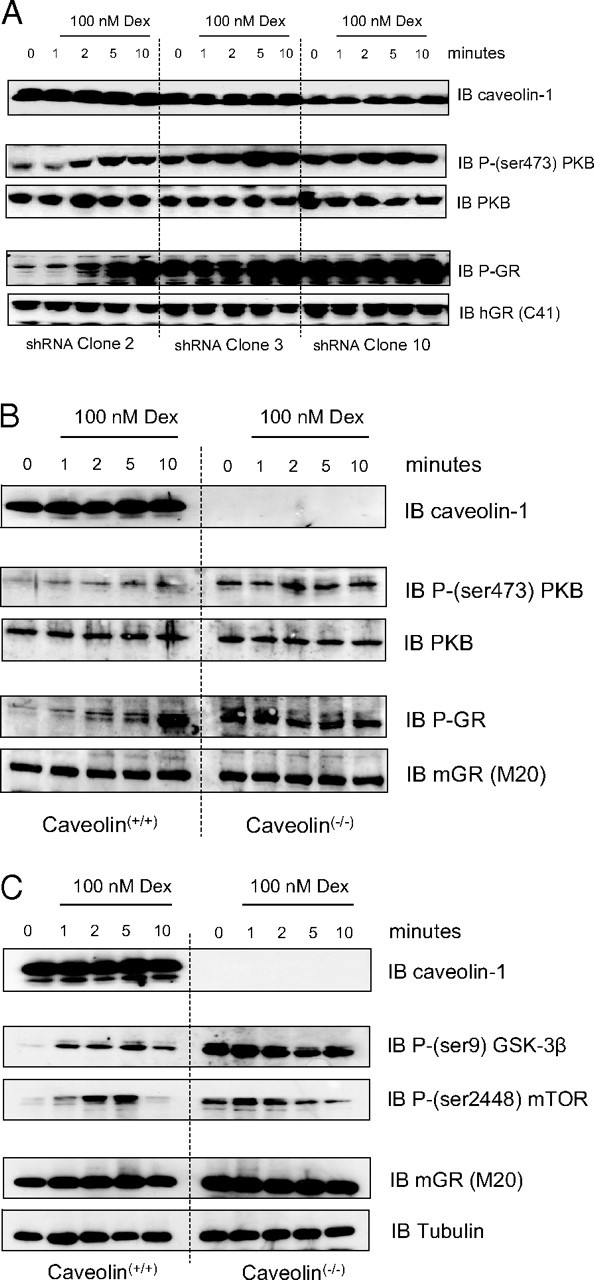
Expression of Caveolin-1 Is Required for Gc Signaling via the PI3K/PKB Pathway
shRNA-A549 (A) and caveolin-1 knockout (B) cells were treated with either 100 nm dexamethasone or vehicle. Protein (50 μg) was immunoblotted for phospho-GR, and phospho-PKB, after which immunoblots were stripped and reprobed with antibodies against GR, PKB, and caveolin. Images are representative of three independent experiments. Protein (50 μg) from dexamethasone-treated wild-type and caveolin-1 knockout cells was also immunoblotted for phospho-GSK3β and phospho-mTOR (C) where GR and tubulin expression is shown to indicate equal protein loading. Dex, Dexamethasone; IB, immunoblot.
Gc-Mediated Growth Arrest Requires the Expression of Caveolin-1
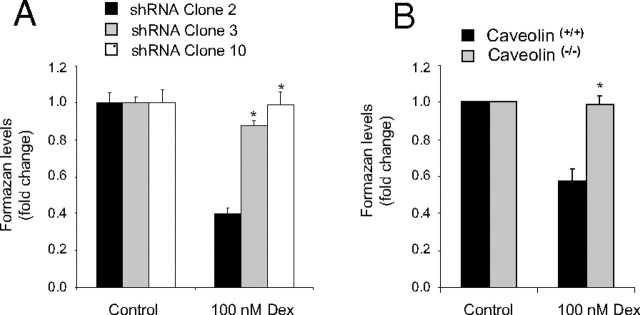
Caveolin knockdown (Fig. 6A) and caveolin knockout (Fig. 6B) cells were cultured in media containing either 100 nm dexamethasone or vehicle for 5 d and then subjected to 3(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay to measure cellular proliferation. Although treatment with dexamethasone significantly inhibited cellular proliferation in control cells (shRNA clone 2 and caveolin(+/+)), the antiproliferative actions of dexamethasone were reduced or completely absent in the caveolin knockdown or knockout clones, respectively.
Fig. 6.
Gc-Mediated Growth Arrest Requires the Expression of Caveolin-1
shRNA-A549 (A) and caveolin-1 knockout (B) cells were cultured in media containing either 100 nm dexamethasone or vehicle for 5 d and then subjected to MTS assay as a measure of Gc-regulated cellular proliferation. In each case graphs depict mean ± sem of four independent experiments completed in triplicate. *, P < 0.05 compared with control cells (shRNA-A549 clone 2 or caveolin(+/+)). Dex, Dexamethasone.
Gc-Mediated Cell Cycle Arrest Requires the Expression of Caveolin-1
Caveolin knockdown cells were cultured in media containing either 100 nm dexamethasone or vehicle for 5 d and then subjected to cell cycle analysis by fluorescence-activated cell sorting (FACS) (Fig. 7). Whereas treatment with dexamethasone induced growth arrest and accumulation in G1/S in control cells (shRNA clone 2), the antiproliferative actions of dexamethasone were reduced or completely absent in the caveolin knockdown clones (clones 3 and 10). A similar lack of dexamethasone effect was observed in the caveolin knockout cells compared with wild-type controls (data not shown).
Fig. 7.
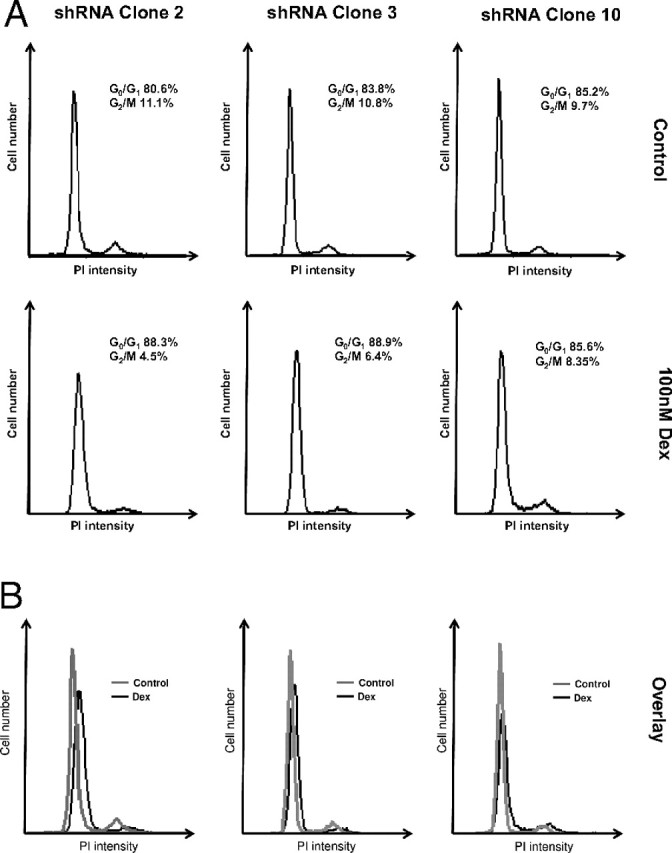
Gc-Mediated Cell Cycle Arrest Requires the Expression of Caveolin-1
shRNA-A549 cells were cultured in media containing either 100 nm dexamethasone or vehicle for 5 d and then subjected to cell cycle analysis by FACS. Images are representative of duplicates from three independent experiments. Individual traces (A) and overlays (B) are shown. Dex, Dexamethasone; PI, propidium iodide.
DISCUSSION
Studies presented here support the existence of acute, non-transcription-dependent Gc effects on key target cells. In particular we show involvement of c-src, and the PI3K pathway to PKB, an important regulator of cell cycle progression and survival (13, 30, 31, 32). Furthermore, we show Gc activation of the PKB downstream molecules GSK3β, and mTOR, supporting the existence of a coherent intracellular signaling cascade initiated by Gc.
In A549 cells Gc induces tyrosine 14 phosphorylation of caveolin, a recognition site for src kinase. There was also serine phosphorylation of PKB, indicative of PI3K activation. Indeed, use of PI3K inhibitors prevents this up-regulation of PKB phosphorylation, as did src inhibition, which also abolished tyrosine 14 phosphorylation of caveolin.
Heat shock protein complexes in the cytoplasm are important both for the maturation of c-src before its membrane integration and also for retention of high-affinity ligand binding activity of the GR (33). For this reason we explored HSP90 function by using the pharmacological inhibitor, geldanamycin (34). Geldanamycin did not inhibit phosphorylation of PKB or phosphorylation of caveolin. However, we consistently noted that geldanamycin reduced the latent period between ligand binding and induction of phosphorylation of caveolin, but not PKB. This implies that disruption of heat shock protein function dissociates Gc activity, resulting in preferential engagement of certain cell-signaling pathways.
Because inhibition of caveolin function disrupted engagement of cell signaling pathways by Gc, we also sought to disrupt lipid raft formation by using methylcyclodextrin. This compound selectively depletes lipid rafts, including caveolae, of their cholesterol content and resulted in complete loss of both phosphorylation of caveolin and PKB in response to Gc.
Because src family kinases have previously been implicated in Gc action in A549 cells (8) and are a potential mediating step between GR activation and both PI3K function and caveolin phosphorylation, we fractionated cell homogenates to look for segregation of GR with plasma membrane fractions. There are clearly two peaks of GR sedimentation, one which cosediments with caveolin and src and the other with heavier, cytosolic and nuclear fractions. This does not necessarily suggest that the GR is physically embedded within the membrane but rather it may indicate association with the inner leaflet of the plasma membrane, perhaps by protein-protein interaction. Indeed the estrogen receptor has been found to interact with caveolin-1 (21, 35), and an earlier report suggested association of the GR with membrane lipid rafts (36).
To further explore these molecular interactions, immunoprecipitation studies were undertaken in whole-cell extracts. These showed coprecipitation of GR and caveolin. It was also possible to demonstrate the presence of c-src within the GR immunoprecipitate. Interestingly, we were not able to immunoprecipitate src using the caveolin antibody, but it is already established that coupling between src and caveolin is dependent on lipid interaction with an intact plasma membrane (37). Therefore, it is possible that the detergent preparation used to generate cell extracts irreversibly disrupts this molecular association, or that the molecular interaction obscures the immunological epitope. Expression of GR deletants showed that the GR AF-1 domain within the N terminus [amino acids (aa) 71–262] was required for caveolin interaction, confirming the specificity of this interaction and also defining the GR motif. This domain of the GR has, to date, eluded crystallographic structural definition but harbors protein interaction motifs for transcriptional comodulator proteins. There is evidence for physical interaction between progesterone receptor B isoform, mediated by its N-terminal SH3 domain, and also of ER with c-src (38, 39, 40, 41). Indeed, scaffolding proteins that enhance or stabilize such interactions have been described: p130Cas, and MNAR (16, 40, 42, 43).
To explore the role of caveolin in mediating the signaling effects of Gc we overexpressed a caveolin fragment comprising the isolated scaffolding domain. This has previously been reported to function as a dominant-negative molecule opposing caveolin function (44). Because TNF signaling has been shown to be partly mediated via caveolin, we used TNF regulation of a simple reporter gene to demonstrate dominant-negative activity (45). These studies clearly showed a marked inhibition of TNF induction of the IL-6 reporter gene when the dominant-negative molecule was expressed. Similarly, the dominant negative caveolin efficiently opposed the induction of tyrosine phosphorylation of caveolin and the serine phosphorylation of PKB. There was no effect on transactivation of a simple reporter gene.
Knockdown of caveolin-1 expression by shRNA was predicted to yield similar results to those seen using the dominant-negative construct. Clones with defined caveolin knockdown were shown to have a similar loss of TNFα signaling to an IL-6 reporter gene as cells expressing the caveolin dominant negative and also to have no effect on transactivation of simple GR reporter genes, indicating pathway specificity for the caveolin-mediated mechanism. The most striking change was the loss of Gc induction of phosphor-Ser211GR, and of phosphor-PKB. The phosphor-Ser211 is ligand dependent and probably mediated by cyclin-dependent kinases (46, 47). The loss of induction was clearly due to increased basal levels of phosphorylation of both the proteins, which suggests that a function for caveolin in regulating basal activity of the pathway, possibly by regulating protein phosphatase activity (48, 49). The altered activity of PKB clearly translated further downstream because we also show similarly altered phosphorylation profiles for the PKB target proteins, GSK3β and mTOR. Importantly, there were differences seen between the effects of acute reduction in caveolin function, by transient expression of DN-caveolin, and chronic loss of caveolin-1 expression.
The human lung cell line A549 responds well to inflammatory signals, including TNFα, and also is growth inhibited by Gc. Loss of caveolin expression prevented Gc inhibition of proliferation, an effect mediated by a block in G1/S transition, over a 5-d incubation. Therefore, there is a major role for caveolin and caveolin-dependent signaling cascades in mediating this Gc effect. Recent data support integration between signaling cascades to determine integrated cell responses (50). Our data now show that divergent, membrane-proximal signals initiated by Gc are required to mediate effects previously ascribed to the nuclear actions of GR alone.
There is much current interest in understanding how Gc ligands exert their diverse effects, with novel ligand structures selectively engaging particular patterns of gene and cell function regulation (51). We now present data to support a role for caveolin in modulating Gc action. This may be by harnessing intracellular signaling cascades to modulate the interaction surfaces between the GR and many comodulator proteins, e.g. by phosphorylation (52). Understanding how activation of intracellular signaling kinases by extracellular factors [such as TNFα activation of p38kinase (53)] modulates GR function and how engagement of signaling kinases directly by the GR, promoted by caveolin, affects Gc action is important for both physiology and the development of new therapeutics.
MATERIALS AND METHODS
Unless otherwise stated all chemical reagents were purchased from Sigma (Dorset, UK).
Antibodies
Anti-tubulin (1:5000) was from Sigma; anti-mGR (M20, 1:1000), anticaveolin-1 (1:2000), and anti-c-src (1:1000) were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); anti-PKB (1:1000), anti-Phospho-(ser473)-PKB (1:1000), anti-Phospho-(ser211)-GR (1:1000), anti-Phospho-(ser9)-GSK3β (1:2000), and anti-Phospho-(ser2448)-mTOR (1:1000) were purchased from Cell Signaling Technology (Beverly, MA); anti-Phospho-(tyr14)-caveolin (1:1000) and anti-hGR (clone 41, 1:2000) came from BD Transduction Laboratories (Oxfordshire, UK); horseradish peroxidase-conjugated antimouse (1:5000) and antirabbit (1:2000–1:5000) were purchased from GE Healthcare (Buckinghamshire, UK).
DN-Caveolin
A dominant negative caveolin molecule was generated by PCR amplification of the scaffolding domain of human caveolin-1 (forward primer, 5′-gaa ttc gac ggc att tgg aag gcc agc-3′; reverse primer, 5′-ctc gag gcg gta aaa cca gta ttt cgt c-3′). The resulting PCR fragment was then cloned into pCRII-TOPO vector following the manufacturers recommendations (Invitrogen, Carlsbad, CA), and then subcloned into cytomegalovirus (CMV)-tag1 c-myc tagged vector (Stratagene, La Jolla, CA). This results in expression of a protein fragment from aa 82–102, which binds caveolin partner proteins but cannot associate with the plasma membrane.
GR Deletants
Full-length hGRα cDNA was digested with BglII to remove the N-terminal transactivation domain aa 71–263 (ΔAF1), or with EcoRI to remove the majority of the ligand-binding domain aa 500–777 (N500) before cloning into a myc-tagged vector (pCMVTAG3B, Stratagene).
Cell Culture and Maintenance
A549 human lung epithelial cells, U20S human osteosarcoma cells (European Collection of Cell Cultures, Wiltshire, UK) and immortalized primary fibroblasts from caveolin-1 knockout mice (a generous gift of Professor Richard Anderson, University of Texas Southwestern Medical Center, Dallas, TX) were cultured in DMEM supplemented with 10% charcoal dextran-stripped fetal calf serum (Invitrogen, Glasgow, UK) and 0.04 mm alanyl-glutamine in a humidified atmosphere of 5% carbon dioxide at 37 C.
Stable shRNA Caveolin Transfection
A plasmid containing an shRNA sequence to target caveolin-1 (a generous gift of Dr. Melissa Westwood, University of Manchester, Manchester, UK) was used to generate stable caveolin-1 knockdown A549 cells. A549 cells were cotransfected with 10 μg shRNA plasmid and a plasmid conferring mammalian antibiotic resistance (pcDNA3, Invitrogen) at a ratio of 10:1 using Fugene 6 (Roche Life Sciences, Basel, Switzerland). Cells were transferred to media containing neomycin 24 h post transfection, and cell clones expressing pcDNA3 were isolated and expanded. pcDNA3-positive clones were then screened for caveolin expression by immunoblot.
Transient Transfection
For phosphoimmunoblot analysis, cells were transfected with 3 μg dominant negative Caveolin plasmid using Fugene 6. After transfection cells were serum starved (24 h), and treated with dexamethasone, as indicated, before processing for phosphoimmunoblot analysis as described below.
For immunoprecipitation studies, U20S cells were transfected with 3 μg wild-type or mutant GR plasmid using Fugene 6. Twenty-four hours after transfection, cell lysates were processed for immunoprecipitation/ immunoblot analysis as described below.
For reporter gene assays, cells were cotransfected with 3 μg DN-caveolin plasmid and either 2 μg TAT3-luciferase, or 2 μg IL-6-luciferase reporter gene constructs together with 0.5 μg CMV-Renilla luciferase (to correct for transfection efficiency) using Fugene 6 (Roche). After 24-h serum withdrawal, cells were treated with 100 nm dexamethasone and/or 10 ng/ml TNFα (Sigma) as indicated before assay for luciferase activity (Promega Corp., Madison, WI).
Treatments
Cells were serum starved for 24 h, pretreated with 10 μm PP2 (Calbiochem, La Jolla, CA) for 30 min, or 5 mm methyl-cyclodextrin (Sigma) for 1 h. Cells were incubated with vehicle or 100 nm dexamethasone (Sigma) for 1–10 min (as indicated in Results) at 37 C, 5% CO2 before lysis. For experiments using the HSP90 inhibitor geldanamycin (Invitrogen), A549 cells were precooled on ice for 10 min and incubated with vehicle or 100 nm dexamethasone on ice for 30 min. After inclusion of 10 μm geldanamycin for a further 30 min (on ice), cells were returned to 37 C for 1–10 min (as indicated in Results) before lysis.
Preparation of Cell Lysates
Cell extracts were prepared by scraping cells washed with PBS (Oxoid, Hampshire, UK) into RIPA lysis buffer (50 mm TrisCl, pH 7.4; 1% NP40; 0.25% Na-deoxycholate; 150 mm NaCl, 1 mm EDTA) and Complete protease inhibitor (Roche), with phosphatase inhibitor cocktail 1 and 2 (Sigma) before centrifugation for 30 min at 10,000 × g and 4 C. The supernatants were harvested, assayed for protein content (Bio-Rad Laboratories, Hercules, CA) and diluted in reducing loading buffer [0.125 m TrisCl (pH 6.8), 0.1% sodium dodecyl sulfate (SDS), 20% glycerol, 0.2% β-mercaptoethanol, 0.001% bromophenol blue] before boiling for 5 min.
Subcellular Fractionation
A549 cells were washed twice in PBS, scraped into ice-cold PBS, and pelleted for 5 min at 1500 × g. After homogenization in 1 ml 500 mm Na2CO3 with 12 strokes of a Dounce homogenizer, the solution was centrifuged at 1000 × g for 10 min, the postnuclear supernatant collected and assayed for protein content (Bradford reagent, Bio-Rad). Total protein (1 mg) was adjusted to 2 ml volume, combined with equal volumes of 80% sucrose in MBS (25 mm Mes, pH 6.5; 0.15 m NaCl) and laid in the bottom of a Beckman ultracentrifuge tube (331374). The homogenate was then overlaid with 4 ml 35% sucrose, and 4 ml 5% sucrose (both in MBS containing 0.25 m Na2CO3), and the samples were centrifuged at 84,000 × g in a Beckman SW40Ti swinging bucket rotor for 18 h at 4 C.
Twelve 1-ml fractions were collected, the protein precipitated with 10% trichloroacetic acid and boiled for 5 min in reducing loading buffer (0.125 m Tris, pH 6.8; 0.1% SDS; 20% glycerol; 0.2% β-mercaptoethanol; 0.001% bromophenol blue) before immunoblotting.
Immunoprecipitation
Cell extracts (500 μg protein) were precleared with 50 μl protein A-coated sepharose beads (Santa Cruz Biotechnology) for 30 min at room temperature. These beads were pelleted by centrifugation at 5000 × g for 20 sec and discarded. In the test samples, supernatant was incubated with 2 μg primary antibody and 50 μl protein A-coated sepharose beads overnight at 4 C. The control sample supernatants were incubated with 50 μl protein A-coated sepharose beads alone. After this incubation, both sets of samples were centrifuged (5000 × g for 20 sec) to pellet the protein A-coated sepharose beads. The supernatants were collected, precipitated with 10% trichloroacetic acid, and boiled in reducing loading buffer for 5 min. The pellets were washed three times in ice-cold PBS and boiled for 5 min in reducing loading buffer, and the beads were removed before electrophoresis by centrifugation at 5000 × g for 20 sec.
Immunoblot Analysis
Cell extracts (50 μg protein) or immunoprecipitates were electrophoresed on SDS/acrylamide gels and transferred to 0.2-μm nitrocellulose membranes overnight at 4 C. Membranes were blocked for 6 h (0.15 m NaCl, 1% dried milk, 0.1% Tween 20) and incubated with primary antibodies (diluted in blocking buffer) overnight at 4 C. After three 10-min washes (88 mm Tris, pH 7.8; 0.25% dried milk; 0.1% Tween 20), membranes were incubated with a species-specific horseradish peroxidase-conjugated secondary antibody (diluted in wash buffer) for 1 h at room temperature and washed a further three times, each for 10 min. Immunoreactive proteins were visualized on Kodak Biomax film (Eastman Kodak, Rochester, NY) using enhanced chemiluminescence (ECL Advance, GE Healthcare), and the protein molecular masses were established using calibrated full range (10–250 kDa) Precision Plus standards (Bio-Rad).
MTS Assay
Cells were grown in DMEM supplemented with vehicle or 100 nm dexamethasone. Cells were trypsinized 5 d later, aliquots seeded in 96-well plates, and cells were allowed to adhere overnight. CellTiter 96 AQueous Assay reagent (20 μl) (Promega) was added to each well and cells were incubated for 1 h at 37 C. The reaction was terminated after the addition of 25 μl 10% SDS, and formazan production (which is proportional to cell number) was measured at 490 nm.
FACS Analysis
Cells were grown in DMEM supplemented with vehicle or 100 nm dexamethasone. Cells were trypsinized 5 d later, and the cell suspension was combined with equal volumes of 100% ice-cold ethanol for 1 h at 4 C. Cells were pelleted at 1500 × g for 10 min and resuspended in 200 μl PBS. DNAse-free RNAse A (50 μl, 1 mm) was added to each sample and incubated at room temperature for 30 min. Propidium iodide (50 μl, 1 mm) was added before analysis. In each case, 10,000 cells were counted.
Acknowledgments
We thank Dr. Melissa Westwood for helpful advice, and for the caveolin-1 hairpin plasmid. We also thank Professor Richard Anderson for kind donation of caveolin knockout cells.
NURSA Molecule Pages:
Coregulators: CAV1;
Ligands: Dexamethasone | RU486;
Nuclear Receptors: GR.
Footnotes
This work was supported by the Wellcome Trust. H.G. was supported by a Biotechnology and Biological Sciences Research Council Collaborative Award in Science and Engineering award. D.W.R. was supported by a Glaxo SmithKline fellowship.
Disclosure Statement: The authors have nothing to disclose
First Published Online February 28, 2008
L.M. and A.B. contributed equally to this work and should both be considered first authors.
Abbreviations: aa, Amino acids; AF, activation function; CMV, cytomegalovirus; DN-caveolin, dominant-negative caveolin; FACS, fluorescence-activated cell sorting; Gc, glucocorticoids; GR, glucocorticoid receptor; GSK, glycogen synthase kinase; HSP, heat shock protein; mTOR, mammalian target of rapamycin; MTS, 3(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, PI3K, phosphatidylinositol 3-kinase; PKB, protein kinase B; SDS, sodium dodecyl sulfate; shRNA, short hairpin RNA.
References
- 1.Morishima Y, Murphy PJM, Li DP, Sanchez ER, Pratt WB 2000. Stepwise assembly of a glucocorticoid receptor·hsp90 heterocomplex resolves two sequential ATP-dependent events involving first hsp70 and then hsp90 in opening of the steroid binding pocket. J Biol Chem 275:18054–18060 [DOI] [PubMed] [Google Scholar]
- 2.Croxtall JD, van Hal PT, Choudhury Q, Gilroy DW, Flower RJ 2002. Different glucocorticoids vary in their genomic and non-genomic mechanism of action in A549 cells. Br J Pharmacol 135:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, Ye S, Bartlam M, Shen B, Rao Z 2004. 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Natl Acad Sci USA 101:8348–8353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pratt WB, Toft DO 2003. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med 228:111–133 [DOI] [PubMed] [Google Scholar]
- 5.Garside H, Stevens A, Farrow S, Normand C, Houle B, Berry A, Maschera B, Ray D 2004. Glucocorticoid ligands specify different interactions with NF-κB by allosteric effects on the glucocorticoid receptor DNA binding domain. J Biol Chem 279:50050–50059 [DOI] [PubMed] [Google Scholar]
- 6.Nissen RM, Yamamoto KR 2000. The glucocorticoid receptor inhibits NFκB by interfering with serine-2 phosphorylation of the RNA polymerase II carboxy-terminal domain. Genes Dev 14:2314–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Refojo D, Liberman AC, Holsboer F, Arzt E 2001. Transcription factor-mediated molecular mechanisms involved in the functional cross-talk between cytokines and glucocorticoids. Immunol Cell Biol 79:385–394 [DOI] [PubMed] [Google Scholar]
- 8.Croxtall, JD, Choudhury Q, Flower RJ 2000. Glucocorticoids act within minutes to inhibit recruitment of signalling factors to activated EGF receptors through a receptor-dependent, transcription-independent mechanism. Br J Pharmacol 130:289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Wang YX, Zhou J, Long F, Sun HW, Liu Y, Chen YZ, Jiang CL 2005. Rapid non-genomic inhibitory effects of glucocorticoids on human neutrophil degranulation. Inflamm Res 54:37–41 [DOI] [PubMed] [Google Scholar]
- 10.Buttgereit F, Straub RH, Wehling M, Burmester GR 2004. Glucocorticoids in the treatment of rheumatic diseases: an update on the mechanisms of action. Arthritis Rheum 50:3408–3417 [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Watson CS, Gametchu B 1999. Association of the glucocorticoid receptor alternatively-spliced transcript 1A with the presence of the high molecular weight membrane glucocorticoid receptor in mouse lymphoma cells. J Cell Biochem 74:430–446 [PubMed] [Google Scholar]
- 12.Gametchu B, Watson CS, Wu S 1993. Use of receptor antibodies to demonstrate membrane glucocorticoid receptor in cells from human leukemic patients. FASEB J 7:1283–1292 [DOI] [PubMed] [Google Scholar]
- 13.Hafezi-Moghadam A, Simoncini T, Yang Z, Limbourg FP, Plumier JC, Rebsamen MC, Hsieh CM, Chui DS, Thomas KL, Prorock AJ, Laubach VE, Moskowitz MA, French BA, Ley K, Liao JK 2002. Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat Med 8:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solito E, Mulla A, Morris JF, Christian HC, Flower RJ, Buckingham JC 2003. Dexamethasone induces rapid serine-phosphorylation and membrane translocation of annexin 1 in a human folliculostellate cell line via a novel nongenomic mechanism involving the glucocorticoid receptor, protein kinase c, phosphatidylinositol 3-kinase, and mitogen-activated protein kinase. Endocrinology 144:1164–1174 [DOI] [PubMed] [Google Scholar]
- 15.Barletta F, Wong CW, McNally C, Komm BS, Katzenellenbogen B, Cheskis BJ 2004. Characterization of the interactions of estrogen receptor and MNAR in the activation of cSrc. Mol Endocrinol 18:1096–1108 [DOI] [PubMed] [Google Scholar]
- 16.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ 2002. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA 99:14783–14788 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Abrami L, Fivaz M, Kobayashi T, Kinoshita T, Parton RG, van der Goot FG 2001. Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J Biol Chem 276:30729–30736 [DOI] [PubMed] [Google Scholar]
- 18.D'Alessio A, Al Lamki RS, Bradley JR, Pober JS 2005. Caveolae participate in tumor necrosis factor receptor 1 signaling and internalization in a human endothelial cell line. Am J Pathol 166:1273–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller G, Frick W 1999. Signalling via caveolin: involvement in the cross-talk between phosphoinositolglycans and insulin. Cell Mol Life Sci 56:945–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I 1999. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor α localized in caveolae. Biochem Biophys Res Commun 263:257–262 [DOI] [PubMed] [Google Scholar]
- 21.Razandi M, Oh P, Pedram A, Schnitzer J, Levin ER 2002. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Mol Endocrinol 16:100–115 [DOI] [PubMed] [Google Scholar]
- 22.Okamoto T, Schlegel A, Scherer PE, Lisanti MP 1998. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem 273:5419–5422 [DOI] [PubMed] [Google Scholar]
- 23.Rybin VO, Xu X, Steinberg SF 1999. Activated protein kinase C isoforms target to cardiomyocyte caveolae: stimulation of local protein phosphorylation. Circ Res 84:980–988 [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Butz S, Ying Ys, Anderson RGW 1997. Tyrosine kinase receptors concentrated in caveolae-like domains from neuronal plasma membrane. J Biol Chem 272:3554–3559 [DOI] [PubMed] [Google Scholar]
- 25.Podar K, Tai YT, Cole CE, Hideshima T, Sattler M, Hamblin A, Mitsiades N, Schlossman RL, Davies FE, Morgan GJ, Munshi NC, Chauhan D, Anderson KC 2003. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem 278:5794–5801 [DOI] [PubMed] [Google Scholar]
- 26.Couet J, Sargiacomo M, Lisanti MP 1997. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem 272:30429–30438 [DOI] [PubMed] [Google Scholar]
- 27.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, Mendelsohn ME, Anderson RGW, Shaul PW 2000. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res 87:E44–E52 [DOI] [PubMed]
- 28.Lu ML, Schneider MC, Zheng Y, Zhang X, Richie JP 2001. Caveolin-1 interacts with androgen receptor. A positive modulator of androgen receptor mediated transactivation. J Biol Chem 276:13442–13451 [DOI] [PubMed] [Google Scholar]
- 29.Hong S, Huo H, Xu J, Liao K 2004. Insulin-like growth factor-1 receptor signaling in 3T3-L1 adipocyte differentiation requires lipid rafts but not caveolae. Cell Death Differ 11:714–723 [DOI] [PubMed] [Google Scholar]
- 30.Limbourg FP, Liao JK 2003. Nontranscriptional actions of the glucocorticoid receptor. J Mol Med 81:168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sasson R, Shinder V, Dantes A, Land A, Amsterdam A 2003. Activation of multiple signal transduction pathways by glucocorticoids: protection of ovarian follicular cells against apoptosis. Biochem Biophys Res Commun 311:1047–1056 [DOI] [PubMed] [Google Scholar]
- 32.Wang LL, Ou CC, Chan JYH 2005. Receptor-independent activation of GABAergic neurotransmission and receptor-dependent nontranscriptional activation of phosphatidylinositol 3-kinase/protein kinase Akt pathway in short-term cardiovascular actions of dexamethasone at the nucleus tractus solitarii of the rat. Mol Pharmacol 67:489–498 [DOI] [PubMed] [Google Scholar]
- 33.Ziemiecki A, Catelli MG, Joab I, Moncharmont B 1986. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun 138:1298–1307 [DOI] [PubMed] [Google Scholar]
- 34.Galigniana MD, Scruggs JL, Herrington J, Welsh MJ, Carter-Su C, Housley PR, Pratt WB 1998. Heat shock protein 90-dependent (geldanamycin-inhibited) movement of the glucocorticoid receptor through the cytoplasm to the nucleus requires intact cytoskeleton. Mol Endocrinol 12:1903–1913 [DOI] [PubMed] [Google Scholar]
- 35.Schlegel A, Wang C, Katzenellenbogen BS, Pestell RG, Lisanti MP 1999. Caveolin-1 potentiates estrogen receptor α (ERα) signaling. Caveolin-1 drives ligand-independant nuclear translocation and activation of ERα. J Biol Chem 274:33551–33556 [DOI] [PubMed] [Google Scholar]
- 36.Jain S, Li Y, Kumar A, Sehgal PB 2005. Transcriptional signaling from membrane raft-associated glucocorticoid receptor. Biochem Biophys Res Commun 336:3–8 [DOI] [PubMed] [Google Scholar]
- 37.Lee H, Woodman SE, Engelman JA, Volonté D, Galbiati F, Kaufman HL, Lublin DM, Lisanti MP 2001. Palmitoylation of caveolin-1 at a single site (Cys-156) controls its coupling to the c-src tyrosine kinase. Targeting of dually acylated molecules (GPI-linked, transmembrane, or cytoplasmic) to caveolae effectively uncouples c-src and caveolin-1 (TYR-14). J Biol Chem 276:35150–35158 [DOI] [PubMed] [Google Scholar]
- 38.Edwards DP, Boonyaratanakornkit V 2003. Rapid extranuclear signaling by the estrogen receptor (ER): MNAR couples ER and Src to the MAP kinase signaling pathway. Mol Interv 3:12–15 [DOI] [PubMed] [Google Scholar]
- 39.Migliaccio A, Piccolo D, Castoria G, Di Domenico M, Bilancio A, Lombardi M, Gong W, Beato M, Auricchio F 1998. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J 17:2008–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, Barone MV, Ametrano D, Zannini MS, Abbondanza C, Auricchio F 2000. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. EMBO J 19:5406–5417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boonyaratanakornkit V, Scott MP, Ribon V, Sherman L, Anderson SM, Maller JL, Miller WT, Edwards DP 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol Cell 8:269–280 [DOI] [PubMed] [Google Scholar]
- 42.Castoria G, Barone MV, Di Domenico M, Bilancio A, Ametrano D, Migliaccio A, Auricchio F 1999. Non-transcriptional action of oestradiol and progestin triggers DNA synthesis. EMBO J 18:2500–2510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabodi S, Moro L, Baj G, Smeriglio M, Di Stefano P, Gippone S, Surico N, Silengo L, Turco E, Tarone G, Defilippi P 2004. p130Cas interacts with estrogen receptor α and modulates non-genomic estrogen signaling in breast cancer cells. J Cell Sci 117:1603–1611 [DOI] [PubMed] [Google Scholar]
- 44.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC 2000. In vivo delivery of the caveolin-1 scaffolding domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6:1362–1367 [DOI] [PubMed] [Google Scholar]
- 45.Ono K, Iwanaga Y, Hirayama M, Kawamura T, Sowa N, Hasegawa K 2004. Contribution of caveolin-1α and Akt to TNF-α-induced cell death. Am J Physiol Lung Cell Mol Physiol 287:L201–L209 [DOI] [PubMed]
- 46.Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ 1997. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol 17:3947–3954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Z, Frederick J, Garabedian MJ 2002. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J Biol Chem 277:26573–26580 [DOI] [PubMed] [Google Scholar]
- 48.Li L, Ren CH, Tahir SA, Ren C, Thompson TC 2003. Caveolin-1 maintains activated Akt in prostate cancer cells through scaffolding domain binding site interactions with and inhibition of serine/threonine protein phosphatases PP1 and PP2A. Mol Cell Biol 23:9389–9404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caselli A, Mazzinghi B, Camici G, Manao G, Ramponi G 2002. Some protein tyrosine phosphatases target in part to lipid rafts and interact with caveolin-1. Biochem Biophys Res Commun 296:692–697 [DOI] [PubMed] [Google Scholar]
- 50.Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol, PF, Glass CK, Rosenfeld MG, Rose DW 2006. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 124:615–629 [DOI] [PubMed] [Google Scholar]
- 51.Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq C, Yamamoto KR 2004. From the cover: chromatin immunoprecipitation (ChIP) scanning identifies primary glucocorticoid receptor target genes. Proc Natl Acad Sci USA 101:15603–15608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng FF, Wu RC, Smith CL, O'Malley BW 2005. Rapid estrogen-induced phosphorylation of the SRC-3 coactivator occurs in an extranuclear complex containing estrogen receptor. Mol Cell Biol 25:8273–8284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Szatmary Z, Garabedian MJ, Vilcek J 2004. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem 279:43708–43715 [DOI] [PubMed] [Google Scholar]
- 54.Alourfi Z, Donn RP, Stevens A, Berry A, McMaster A, Ray DW 2005. Glucocorticoids suppress macrophage migration inhibitory factor (MIF) expression in a cell-type-specific manner. J Mol Endocrinol 34:583–595 [DOI] [PubMed] [Google Scholar]
- 55.Stevens A, Garside H, Berry A, Waters C, White A, Ray D 2003. Dissociation of steroid receptor coactivator 1 and nuclear receptor corepressor recruitment to the human glucocorticoid receptor by modification of the ligand-receptor interface: the role of tyrosine 735. Mol Endocrinol 17:845–859 [DOI] [PubMed] [Google Scholar]