Abstract
Obestatin was identified as a brain/gut peptide hormone encoded by the ghrelin gene and found to interact with the G protein-coupled receptor, GPR39. We investigated target cells for obestatin based on induction of an early-response gene c-fos in different tissues. After ip injection of obestatin, c-fos staining was found in the nuclei of gastric mucosa, intestinal villi, white adipose tissues, hepatic cords, and kidney tubules. Immunohistochemical analyses using GPR39 antibodies further revealed cytoplasmic staining in these tissues. In cultured 3T3-L1 cells, treatment with obestatin, but not motilin, induced c-fos expression. In these preadipocytes, treatment with obestatin also stimulated ERK1/2 phosphorylation. Because phenotypes of GPR39 null mice are partially consistent with a role of GPR39 in mediating obestatin actions, we hypothesized that inconsistencies on the binding of iodinated obestatin to GPR39 are due to variations in the bioactivity of iodinated obestatin. We obtained monoiodoobestatin after HPLC purification and demonstrated its binding to jejunum, stomach, ileum, pituitary, and white adipose tissue. Furthermore, human embryonic kidney 293T cells transfected with plasmids encoding human or mouse GPR39 or a human GPR39 isoform, but not the ghrelin receptor, exhibited high-affinity binding to monoiodoobestatin. Binding studies using jejunum homogenates and recombinant GPR39 revealed obestatin-specific displacement curves. Furthermore, treatment with obestatin induced c-fos expression in gastric mucosa of wild-type, but not GPR39 null, mice, underscoring a mediating role of this receptor in obestatin actions. The present findings indicate that obestatin is a metabolic hormone capable of binding to GPR39 to regulate the functions of diverse gastrointestinal and adipose tissues.
GHRELIN IS A GH-releasing acylated peptide synthesized in the stomach and found to enhance food intake (1). Based on bioinformatic prediction of a novel peptide encoded by the ghrelin gene and endogenous peptide purification, we identified an amidated peptide of 23 residues from the rat stomach and demonstrated its ability to suppress food intake (2). We also found that obestatin interacts with the orphan receptor GPR39 (2) known to exhibit sequence homology to the ghrelin receptor (3). As recently reviewed (4, 5), questions have been raised regarding the role of obestatin as a hormone mainly due to inconsistencies in its ability to regulate food intake. To provide functional evidence in support of the role of obestatin as a peptide hormone, we investigated the ability of obestatin to induce the expression of an early-response gene c-fos in gastrointestinal and other tissues. Based on c-fos induction in the white adipose tissue after obestatin injection in animals, we further investigated the ability of obestatin to induce c-fos expression and to stimulate ERK1/2 phosphorylation in 3T3-L1 cells, a mouse fibroblastic cell line known for its ability to differentiate into adipocytes (6).
In response to technical comments (7), we indicated that our original peptide preparation was contaminated, and in vitro findings of obestatin binding to G protein-coupled receptor (GPR39)-expressing cells and its stimulation of cAMP and serum response element-mediated responses could not be reproduced using purified obestatin. Despite controversies regarding the role of obestatin as a ligand for GPR39 (7, 8, 9), studies using GPR39 null mice showed altered gastrointestinal and metabolic functions as well as increases in mature body weight and body fat composition (10), suggesting that obestatin could interact with GPR39. Because up to four iodine molecules are incorporated into obestatin during iodination (11, 12), we hypothesized that inconsistent binding of iodinated obestatin to GPR39 (2, 8) is due to variable loss of obestatin bioactivity after iodination. Using HPLC to purify monoiodoobestatin, we now report that labeled obestatin is capable of binding to human embryonic kidney (HEK)293T cells transiently transfected with plasmids encoding human or mouse GPR39.
RESULTS
Obestatin Induction of an Early-Response Gene c-fos in Gastrointestinal and Metabolic Tissues
To investigate target cells for obestatin, adult male mice were given an ip injection of human obestatin. Tissues were collected 3 h later for immunostaining of the early-response gene c-fos, which is known to be induced after different hormonal stimuli (13, 14, 15). As shown in Fig. 1A (a, b, and e), nuclear c-fos staining was found in intestinal villi but not in the crypts of obestatin-treated animals, whereas no specific signal was found in saline-treated animals. Nonspecific staining to secretory substances was found in intestinal lumen of both groups. In addition, immunohistochemical analyses using an antibody against the third extracellular loop of GPR39 showed cytoplasmic staining in intestinal villi (Fig. 1A, panel c). Nonspecific signals were found in the base of crypts in sections incubated with the GPR39 antibody or nonimmune IgG (Fig. 1A, panel d). In the stomach (Fig. 1B, a and b), c-fos staining was found in the nuclei of gastric mucosal cells after obestatin, but not saline, treatment. Again, GPR39 staining was found in the cytoplasm of mucosal cells (Fig. 1B, panel c), but no signal was found after staining with nonimmune IgG (Fig. 1B, panel d). In white adipose tissues, c-fos staining was present in the nuclei of some adipocytes after treatment with obestatin (Fig. 1C, panel a), but not saline (Fig. 1C, panel b). In addition, positive signals were found in the cytoplasm of some adipocytes in sections stained with the GPR39 antibody (Fig. 1C, panel c) but not nonimmune IgG (Fig. 1C, panel d). In the liver, hepatocytes in cords of lobules showed c-fos staining after obestatin, but not saline, treatment (Fig. 1D, a and b). Also, positive signals were found in the cytoplasm of these cells stained with the GPR39 antibodies but not nonimmune IgG (Fig. 1D, c and d). In the kidney, c-fos expression was found in the nuclei of cells in distal and proximal tubules as well as in the glomeruli after obestatin, but not saline, treatment (Fig. 1E, a and b). Also, GPR39 signals were found in the cytoplasm of these cells (Fig. 1E, panel c).
Fig. 1.
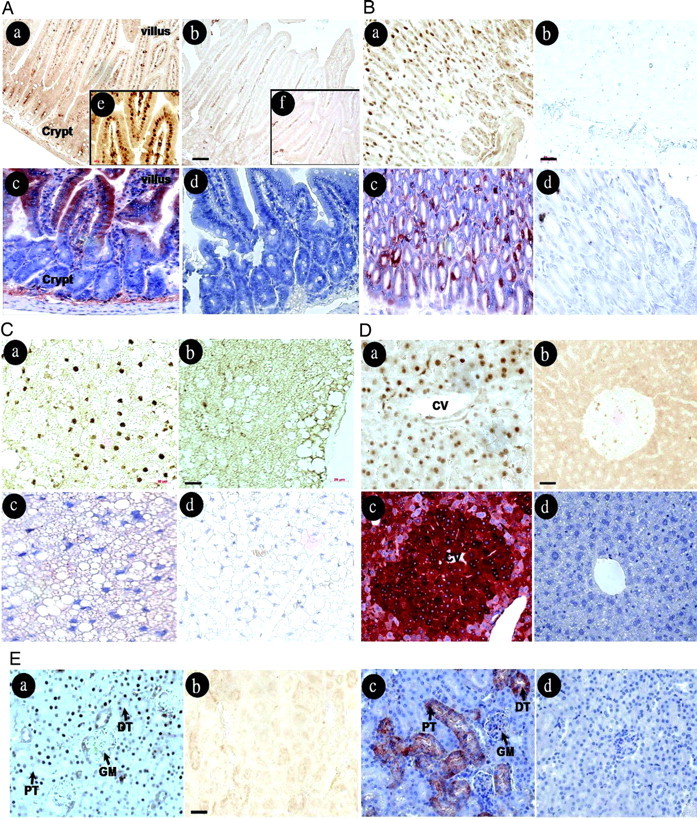
Treatment with Obestatin Induced c-fos Expression in Gastrointestinal and Metabolic Tissues
Adult male mice were treated ip with obestatin (500 nmol/kg body weight in 100 μl saline) or saline for 3 h before immunostaining using c-fos antibodies or GPR39 antibodies. A, Duodenum; B, stomach; C, white adipose tissue; D, liver; E, kidney. For all tissues: a, obestatin-treated group; b, saline-treated group; c, staining with GPR39 antibodies; d, staining with nonimmune IgG. Sections shown in c and d are counterstained using hematoxylin. For duodenum samples, e and f represent areas under a higher magnification. CV, Central vein; PT, proximal tubule; DT: distal tubule; GM: glomeruli. Bar, 50 μm.
Obestatin Stimulation of c-fos Expression and ERK1/2 Phosphorylation in Cultured Preadipocytes
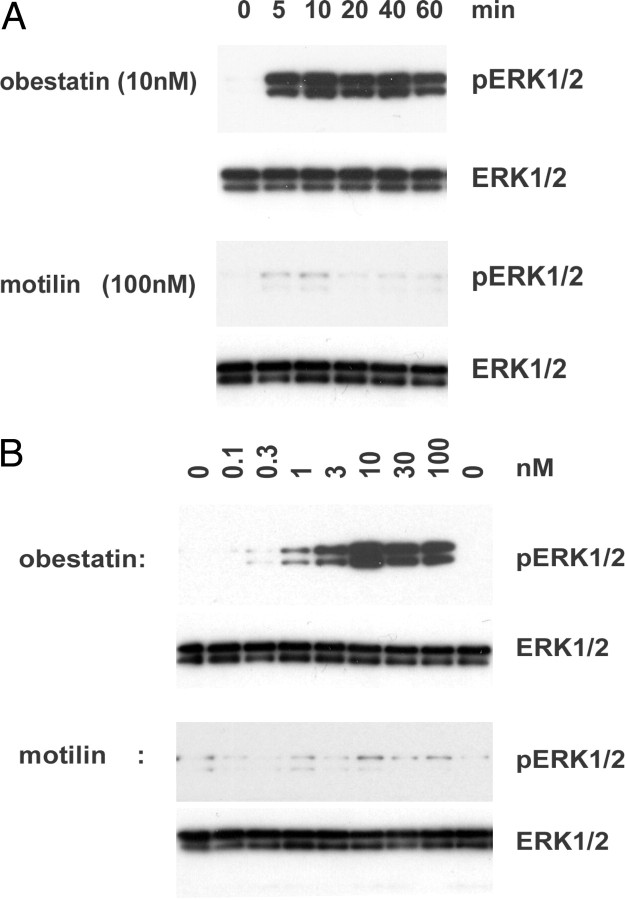
We tested direct actions of obestatin in cultured preadipocytes. As shown in Fig. 2A, treatment of mouse 3T3-L1 cells with human obestatin, but not motilin, induced c-fos protein expression. Analyses using real-time RT-PCR further indicated a time-dependent stimulation of c-fos transcript levels after treatment of these cells with obestatin (Fig. 2B). We further tested the ability of obestatin to stimulate the activation of the ERK pathway. As shown in Fig. 3A, treatment with 10 nm obestatin, but not motilin, led to time-dependent stimulation of ERK1/2 phosphorylation (pERK1/2) showing increases at 5 min after exposure. In contrast, treatment with motilin was ineffective. For cells incubated for 15 min, treatment with increasing amounts of obestatin, but not motilin, led to dose-dependent increases in ERK1/2 phosphorylation with 10 nm obestatin showing maximal stimulation (Fig. 3B). For all tests, total ERK1/2 levels did not change.
Fig. 2.
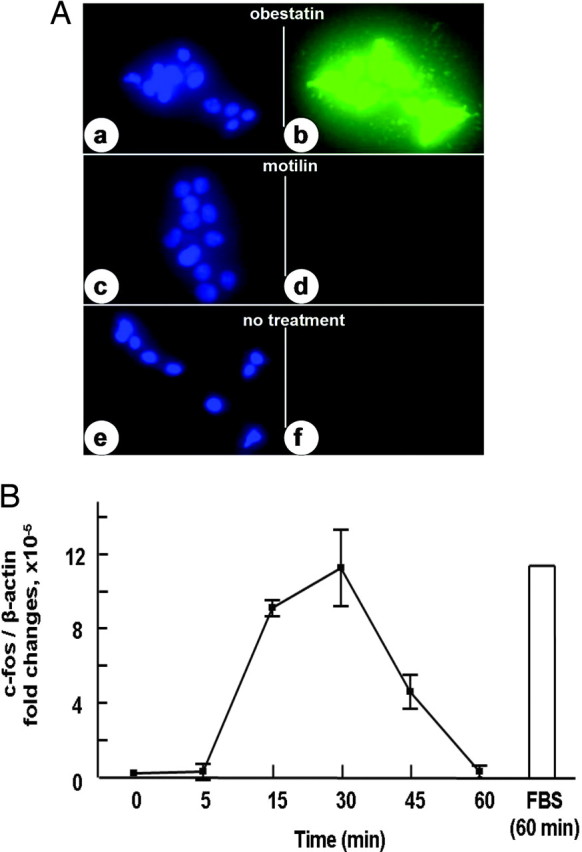
Treatment with Obestatin Induced the Expression of c-fos Protein and Transcripts in Cultured 3T3-L1 Cells
A, c-fos antigen staining. 3T3-L1 cells were treated with 100 nm obestatin (b) or motilin (d) for 1 h before staining using c-fos antibodies for observation under a confocal microscope. Panels a, c, and e represent the staining of cell nuclei using the Hoechst 33342 dye. B, Transcript levels for c-fos. 3T3-L1 cells were incubated in serum-free media overnight before treatment with 30 nm obestatin for different intervals. Transcript levels for c-fos were determined using real-time RT-PCR and normalized based on β-actin levels. FBS, Fetal bovine serum.
Fig. 3.
Treatment with Obestatin Stimulated the Phosphorylation of ERK1/2
A, Time course studies. B, Dose-response analyses. 3T3-1 cells were incubated in serum-free media for 5 h before hormonal treatment and estimation of ERK1/2 phosphorylation using immunoblotting. For time course studies, 10 nm obestatin and 100 nm motilin were used whereas dose-response studies involved hormonal treatment for 15 min. Immunoblots for both phospho-ERK1/2 (pERK1/2) and total ERK1/2 are shown.
Binding of Monoiodoobestatin to Diverse Tissues and GPR39-Expressing Cells
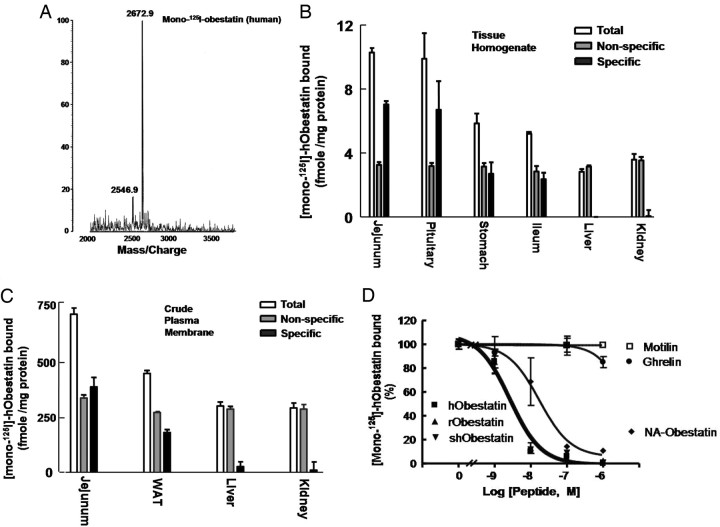
Synthetic human amidated obestatin was iodinated and purified in a C18 analytical column using HPLC by Phoenix Pharmaceuticals, Inc. After separation, the peak fraction containing monoiodoobestatin was confirmed by matrix-assisted laser desorption/ionization, time-of-flight mass spectrometry, showing a molecular mass of 2672.9 (Fig. 4A). Under the present iodination condition, Tyr16 in obestatin is the preferred residue to be modified. In addition, a minor peak corresponding to noniodinated obestatin (molecular mass 2546.9) was also detected. Using this tracer, obestatin binding to tissue homogenates was tested. Samples were incubated with 12 nm monoiodoobestatin without (total binding) or with (nonspecific binding) a 200-fold excess of unlabeled obestatin. As shown in Fig. 4B, specific binding was found in jejunum, pituitary, stomach, and ileum with minimal binding in the liver and kidney. To avoid lipid interference of the binding assay, we prepared crude plasma membrane fractions of white adipose tissues. As shown in Fig. 4C, specific obestatin binding was found in the membrane fractions of jejunum and white adipose tissue with minimal binding in liver and kidney. Furthermore, monoiodoobestatin binding to jejunum homogenates was found to be hormone specific with increasing amounts of obestatin from human, rodent, and sheep origins showing similar displacement curves and nonamidated-human obestatin being less effective (Fig. 4D). In addition, inclusion of ghrelin and motilin led to minimal competition.
Fig. 4.
Binding of Monoiodoobestatin to Diverse Tissues
A, Characterization of monoiodoobestatin using mass spectrometry. Human obestatin was iodinated using the Iodogen method and fractionated using HPLC by Phoenix Pharmaceutical. After fractionation, the peak fraction containing monoiodoobestatin was confirmed by mass spectrometry (molecular mass of 2672.9). B, Binding of monoiodoobestatin to tissue homogenates. Jejunum and other tissues were obtained from adult male rats and homogenized. Tissue extracts were incubated with 12 nm monoiodoobestatin with or without 200-fold excess of unlabeled obestatin for 16 h at 23 C. After incubation, samples were centrifuged for 20 min at 1000 × g, and pellets were washed twice in ice-cold PBS before quantitation of radioactivity using a γ-spectrophotometer. Specific binding was calculated by subtracting nonspecific binding from total binding. C, Binding of monoiodoobestatin to crude plasma membrane factions. For plasma membrane binding, tissue homogenates were centrifuged to collect crude plasma membrane fractions. After resuspension, samples were incubated for 16 h at 23 C before filtration and counting. WAT, White adipose tissue. D, Competition tests. Jejunal homogenates were incubated with 10 nm monoiodoobestatin with or without increasing doses of different peptides at 23 C for 16 h before washing twice and counting. NA, Nonamidated; h, human; r, rat; sh, sheep.
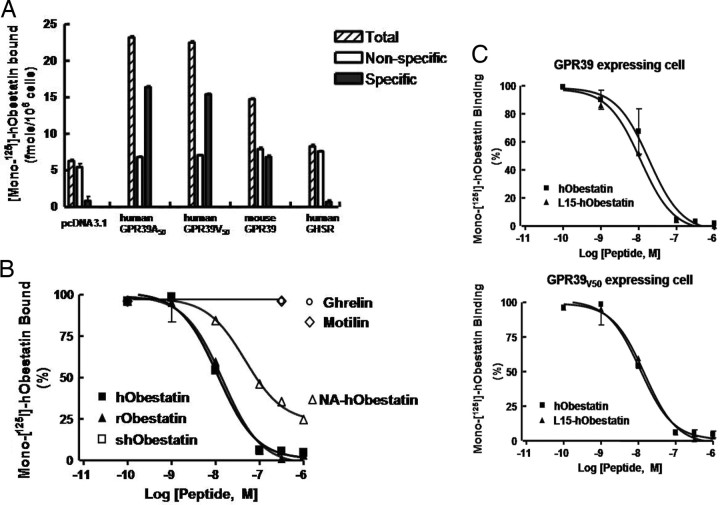
The ability of monoiodoobestatin to bind recombinant GPR39 was also tested. As shown in Fig. 5A, HEK293T cells transiently transfected with plasmids encoding wild-type human GPR39(A50) and mouse GPR39 exhibited specific obestatin binding. Based on searches in the National Center for Biotechnology Information SNP (single nucleotide polymorphism) database (http://www.ncbi.nlm.nih.gov/projects/SNP/), we found an SNP in human GPR39 (SNP ID: rs2241764; average heterozygosity, 0.471) wherein a polymorphism in nucleotide 149 (C to T) changes alanine (A50) to valine (V50) located in the first transmembrane region of human GPR39. We employed site-directed mutagenesis to generate the human GPR39(V50) isoform and demonstrated its specific binding to obestatin (Fig. 5A). Furthermore, obestatin binding to cells expressing human wild-type GPR39(A50) was displaced by obestatin peptides from human, rodent, and sheep origins (Fig. 5B). In contrast, nonamidated human obestatin was less effective whereas ghrelin and motilin were ineffective.
Fig. 5.
Binding of Monoiodoobestatin to Recombinant GPR39 Receptors
A, Obestatin binding to human and mouse GPR39. HEK293T cells were transiently transfected with expression vectors encoding human GPR39 (wild type, A50), an isoform of human GPR39 (V50), mouse GPR39, human GH secretagogue receptor, or the empty pcDNA3 vector. Cells were incubated with 12 nm monoiodoobestatin with or without 200-fold excess of nonlabeled human obestatin at 23 C for 16 h. After incubation, cells were centrifuged at 1000 × g for 10 min and washed with 2 ml of buffer followed by three washes with 1 ml buffer before counting. B, Hormonal specificity of obestatin binding to human GPR39. HEK293T cells expressing wild-type human GPR39 were incubated with 10 nm monoiodoobestatin with or without increasing doses of different peptides at 23 C for 16 h before washing and counting. C, Comparison of the binding of two obestatin isoforms to two isoforms of human GPR39. HEK293T cells were transiently transfected with expression vectors encoding wild-type human GPR39 or human GPR39V50. Cells were incubated with 6 nm monoiodoobestatin with different doses of unlabeled human obestatin or human L15-obestatin at 23 C for 16 h. After incubation, cells were washed as described above. NA, Nonamidated; h, human; r, rat, sh, sheep.
Based on the National Center for Biotechnology Information SNP database searches of the human ghrelin gene, we also found a 308A to T polymorphism (SNP ID: rs4684677; average heterozygosity, 0.070) that changes glutamine (Q15) to leucine (L15) in the mature peptide of obestatin. Analyses of displacement curves using both isoforms of GPR39 and two human obestatin peptides indicated similar binding kinetics with estimated dissociation constant (Kd) values of approximately 1 nm (Fig. 5C).
Obestatin Induction of c-fos Expression in Wild-Type, But Not GPR39 Null, Mice
Because the phenotypes of GPR39 null mice are partially consistent with the role of GPR39 as a receptor for obestatin (10), we tested the ability of obestatin to stimulate c-fos expression in GPR39 null animals. As shown in Fig. 6, treatment of wild-type mice with obestatin increased nuclear staining of c-fos in the mucosal layer of the stomach as compared with saline-treated controls. In contrast, treatment with obestatin failed to induce c-fos staining in GPR39 null mice. These findings were confirmed in five sets of animals and are consistent with a role of GPR39 in mediating obestatin actions in vivo. Immunostaining using GPR39 antibodies further demonstrated the expression of GPR39 proteins in the cytoplasm of gastric cells in wild-type, but not mutant, mice, and the specificity of the present GPR39 antibodies (Fig. 6, lower panels).
Fig. 6.
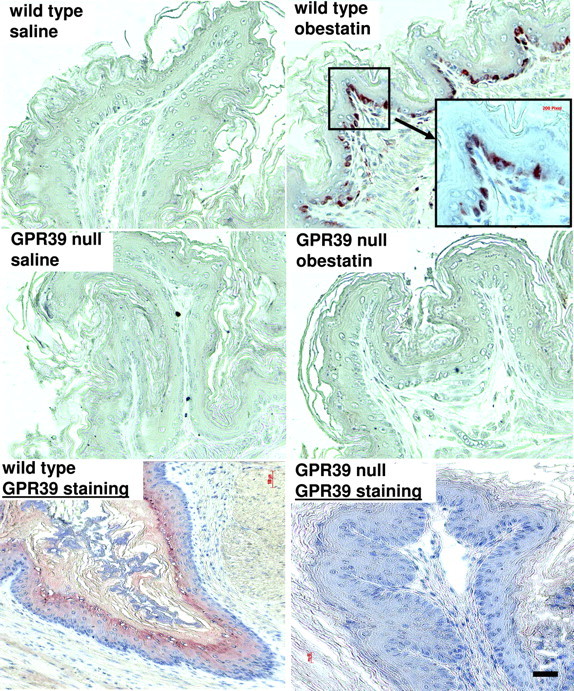
Treatment with Obestatin Induced c-fos Expression in Wild-Type, But Not GPR39 Null, Mice
Adult female mice (4–5 months of age) were genotyped after PCR of genomic DNA. Animals were injected ip with obestatin (500 nmol/kg body weight in 100 μl saline). Stomach tissues were dissected from these mice 1 h after injection for c-fos staining. Tissue sections were treated with 0.01 m sodium citrate at 95 C for 15 min followed by 0.1% trypsin at 37 C for 15 min before immunostaining using affinity-purified, rabbit polyclonal c-fos antibodies (1:300 dilution; Santa Cruz Biotechnology). Signals were detected using the Histostain-SP Kit. Similar results were obtained in five sets of animals. Lower panels, To verify the expression of GPR39 proteins, immunohistochemical staining was performed using gastric sections from wild-type and GPR39 null mice. Bar, 100 μm.
DISCUSSION
The present findings demonstrate the ability of obestatin to induce early-response gene expression in stomach, intestine, white adipose tissue, liver, and kidney, supporting its role as a gastrointestinal and metabolic hormone. Observed binding of monoiodoobestatin to GPR39-expressing cells and the inability of obestatin to induce c-fos expression in the stomach of GPR39 null mice suggest that GPR39 is at least one of the receptors for obestatin. In cultured 3T3-L1 cells, treatment with obestatin induces c-fos expression and activates the MAPK pathway, providing a cell model by which to study obestatin-signaling mechanisms.
Obestatin as a Hormone
Treatment with obestatin induced the expression of c-fos in the nuclei of cells in intestinal villi, gastric mucosa, white adipose tissue, liver, and kidney. Although one cannot rule out the possibility that the observed induction of c-fos is due to secondary stimulation after the initial actions of obestatin in a different cell type, the present findings are consistent with the role of obestatin as a hormone. Despite reports showing minimal effects of obestatin in the modulation of gastrointestinal motility in rats (16, 17, 18), obestatin treatment induced c-fos expression in gastrointestinal tissues (Fig. 1), and treatment of a human gastric tumor cell line with obestatin stimulated ERK phosphorylation and cell proliferation (47). These data provide the basis for further studies on the roles of obestatin in gastrointestinal physiology. Due to the analyses of only one of many early-response genes, the present survey of obestatin target tissues is incomplete. Although we did not find an induction of c-fos or c-jun in the pancreas (data not shown), GPR39 transcripts are expressed in pancreas (10, 19). In addition, obestatin staining was found in pancreatic islets (20), and treatment with obestatin stimulated the secretion of glucagon and inhibited the secretion of pancreatic peptide, somatostatin, and insulin from isolated pancreatic islets in mice and rats (21). In contrast to the inhibitory effects of ghrelin, treatment with obestatin also stimulated the secretion of pancreatic juice enzymes through the vagal pathway (22). Furthermore, iodinated obestatin was found to bind to pancreatic β-cell lines and human islets, to promote the survival of these cells, as well as to stimulate downstream responses including the phosphorylation of ERK1/2 and Akt (23). These data suggest that obestatin is likely a paracrine hormone in the pancreas.
Although several laboratories could not demonstrate obestatin suppression of food intake (8, 24, 25, 26, 27), other studies demonstrated that ip injection of obestatin suppressed basal or ghrelin-stimulated food intake in mice (28, 29) and rats (30). After intracerebroventricular injection, obestatin also inhibited food intake in rats (31). Because a recent study (32) demonstrated the importance of handling, acclimatization, and habituation of rodents during the investigation of the anorexic effects of gastrointestinal hormones, it is likely that discrepant findings on the obestatin regulation of food intake are due to variations in experimental procedures similar to earlier reports on the anorexic role of peptide YY (3–36) (15, 33). With the present demonstration of gastrointestinal tissues as targets for obestatin, additional studies using refined experimental protocols could clarify the role of obestatin on food intake.
In addition to its actions in peripheral tissues, obestatin could also activate neurons in different brain regions. Intracerebroventricular injections of obestatin inhibited thirst (34) and vasopressin secretion (35), suppressed food intake (31), regulated sleep (36), decreased anxiety, and improved memory (31). Coupled with the ability of obestatin to activate cortical neurons (37), and to stimulate the proliferation and downstream signaling of human retinal pigment epithelial cells (38), these findings underscore the diverse functions of obestatin as a neuropeptide.
We previously concentrated immunoreactive obestatin from the stomach of 30 rats and verified its unique sequence according to Edman sequencing (2). In the first 20 amino acids sequenced, two residues are distinct from those of human obestatin and matched with the rat sequence. At the time, no rodent obestatin was synthesized. Although a recent report could not detect endogenous immunoreactive obestatin in rat stomach extracts (39), another study demonstrated the existence of alternatively spliced ghrelin transcripts in the human stomach, encoding exclusively obestatin together with a signal peptide for secretion (40). Future studies are needed to reveal the regulation of obestatin biosynthesis, processing, and secretion.
Obestatin Interaction with GPR39
GPR39 was discovered as an orphan receptor with sequence homology to GH secretagogue receptor, the receptor for ghrelin (3). GPR39 is expressed in gastrointestinal tissues, and GPR39 null mice showed metabolic abnormalities (10). Using monoiodoobestatin, we demonstrated the ability of obestatin to interact with recombinant GPR39 and its competition by obestatin peptides of human, sheep, and rodent origins whereas non-amidated human obestatin was less effective. Because sheep and rodent obestatin differ from their human counterpart by 7 and 3 (of the 23) residues, respectively, these data demonstrated that a core of obestatin sequence is important for receptor interactions. Observed weaker affinity of nonamidated obestatin to GPR39 is consistent with structural analyses of the obestatin peptide, suggesting its amidated C terminus is important for conformational stability (41).
Moechars et al. (10) found body weight increases and altered gastrointestinal phenotypes in GPR39 null mice and concluded that the findings are partially consistent with the role of GPR39 as the obestatin receptor. In contrast, another report found normal food intake and a lack of short-term body weight changes in GPR39 null mice (27). Here, we showed that treatment with obestatin induced c-fos expression in wild-type, but not GPR39 null, mice, thus providing further support of the ligand-receptor relationship between obestatin and GPR39. In addition, immunohistochemical analyses using an antibody against the third extracellular loop of GPR39 indicated the colocalization of GPR39 with different obestatin target cells expressing c-fos. Of interest, the same cells in the stomach and small intestine were also found to exhibit LacZ staining in GPR39 mutant mice in which the receptor-coding sequence was replaced by the LacZ reporter (10). Also, GPR39 transcripts are expressed in stomach and small intestine (19). These data are consistent with the observed binding of monoiodoobestatin to stomach, jejunum, and ileum. Although LacZ expression in white adipose tissue of transgenic mice was not analyzed, the observed obestatin binding to white adipose tissue and obestatin induction of c-fos correspond to the expression of GPR39 mRNA (19) and protein in adipose tissues. For the anterior pituitary, we did not observe c-fos induction but found GPR39 staining in adult male mice (data not shown). Although we also observed monoiodoobestatin binding to anterior pituitary of adult male rats, GPR39 transcripts were found to be low (8, 19). This discrepancy could be related to receptor protein stability and requires further analyses. Consistent with earlier studies on the expression of GPR39 transcripts in liver and kidney (10, 19), we observed GPR39 staining and obestatin induction of c-fos in these tissues. However, we detected minimal binding of monoiodoobestatin to these tissues. It is likely that the present binding conditions (23 C, 16 h) are not optimal for receptor stability in these tissues. A role of endogenous obestatin also cannot be ruled out. Future studies using autoradiographic analyses are useful.
GPR39 was found to be constitutively active and activated by zinc ions (8). Because HEK293T cells expressing GPR39 are capable of binding obestatin but no clear downstream signaling was detected, one cannot rule out the possibility that a coreceptor or other components are needed for signal transduction by obestatin. For other G protein-coupled receptors, interacting partners for the ACTH receptor (42) and for receptors of the adrenomedullin subfamily (43) have been reported.
We tested the ability of two human variants of GPR39 to interact with two variants of human obestatin. The frequency of the L15 obestatin variant allele (Gln90 of preproghrelin) was found to be comparable among individuals with different body weights (44). Although the present binding studies did not reveal changes in the binding affinity of L15-obestatin to the two isoforms of GPR39, future studies on the signaling potency of the L15 variant are of interest.
Obestatin Actions in Adipocytes
Treatment with obestatin induced c-fos expression in the white adipose tissue and cultured preadipocytes. Similar to studies using human retinal pigment epithelial cells (38), obestatin treatment also stimulated ERK1/2 phosphorylation in 3T3-L1 cells. This preadipocyte cell line provides a valuable model for analyzing ligand signaling by obestatin and for testing different obestatin agonists and antagonists. Of interest, GPR39 expression in white adipose tissues of rats was up-regulated during fasting whereas GPR39 levels were decreased in cultured mouse embryonic fibroblast cell lines (related to 3T3-L1) during adipogenesis (19). In human adipose tissues, decreased GPR39 expression was found in patients with obesity-associated type 2 diabetes mellitus (45). These findings suggest a possible role of GPR39 in adipogenesis.
The present findings of the obestatin induction of c-fos expression in gastrointestinal tissues and its ability to regulate downstream responses in preadipocytes, coupled with the binding of obestatin to recombinant GPR39 and the phenotypes of GPR39 null mice, underscore the roles of the obestatin-GPR39 ligand-signaling system in the regulation of metabolic functions. Future investigation on the physiological roles of this brain/gut hormone and its relationship with ghrelin are of interest.
MATERIALS AND METHODS
Materials
Obestatin peptides from different species and other peptides were purchased from Phoenix Pharmaceuticals, Inc. (Belmont, CA) or synthesized at the Protein and Nucleic Acid facility at Stanford University. Each peptide was purified by reversed-phase HPLC, and the peptide sequence was verified by amino acid analysis and mass spectrometry.
Animals
C57BL6 male mice from Stanford animal facility (8 wk old) were used for c-fos induction tests. Adult male Sprague Dawley rats (Charles River Laboratories, Inc., Wilmington, MA) were used for ligand receptor assays. GPR39 null mice and their wild-type siblings were developed in collaboration with Lexicon Genetics, Inc. (Woodlands, TX) as described previously (10). All animals were housed in an environment with controlled light, humidity, and temperature in accordance with institutional animal care guidelines.
Immunohistochemical Staining for c-fos and GPR39 Antigens
Male mice were injected ip with obestatin (500 nmol/kg body weight in 100 μl saline) or saline. At 3 h after injection, mice were anesthetized with isoflurane and perifused through the tail vein with 0.9% NaCl containing 2% sodium nitrite, followed by 4% paraformaldehyde-Kreb’s PBS. Tissues were removed and then immersed in 25% sucrose solution in distilled water at 4 C for 4–6 d. Fresh sucrose solution was replaced daily. Tissues were embedded with paraffin and cut into 25-mm-thick sections. After soaking in xylene and series of ethanol, tissue sections were treated with 0.01 m sodium citrate at 95 C for 15 min followed by 0.1% trypsin at 37 C for 15 min before immunostaining using affinity-purified, rabbit polyclonal antibodies against synthetic peptides corresponding to the N terminus of human c-fos (1:100 dilution, Abcam, Cambridge, MA; or 1:300 dilution, Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Signals were detected using the Histostain-SP Kit (Zymed Laboratories, Inc., South San Francisco, CA). For immunohistochemical analyses of GPR39, GPR39 antibodies (1:100 dilution, Abcam) against the third extracellular loop of GPR39 were used.
To investigate c-fos induction in GPR39 null mice, adult female wild-type and mutant mice (4–5 months of age) were genotyped after PCR of genomic DNA (10). Animals were injected ip with 100 μl saline or obestatin (500 nmol/kg body weight). Stomach was obtained at 1 h after injection and fixed using Bouin’s solution before embedding in paraffin for making slides. After shipping to Stanford, immunostaining was performed as described earlier.
Immunofluorescence Staining of c-fos in 3T3-L1 Cells
3T3-L1 cells were cultured on a coverslip in six-well plates until 50–70% confluent. Cells were treated with 100 nm obestatin for 1 h and rinsed twice in PBS before fixing in 4% paraformaldehyde in PBS for 20 min at 23 C. After three washes with PBS, cells were incubated with prewarmed antigen retrieval buffer (100 mm sodium citrate, pH 6.0) at 95 C for 20 min. After further rising three times in PBS and incubation in 0.1% Triton X-100 in PBS for 15 min at 23 C, cells were treated with 10% goat serum for 1 h at 23 C. This was followed by overnight incubation of cells with the c-fos antibodies (1:300; Abcam) at 4C. After reaction, cells were rinsed in 1% goat serum in PBS three times before treatment with fluorophore-conjugated secondary antibodies for 2 h at 23 C under darkness. Cells were then washed three times in 1% goat serum for 10 min before staining of cell nuclei using the Hoechst 33342 dye (Invitrogen, San Diego, CA) for visualization under a fluorescence microscope.
Culture of 3T3-L1 Cells and Quantitative Real-Time RT-PCR
3T3-L1 cells were obtained from American Type Culture Collection (catalog no. CL-173; Manassas, VA) and maintained in DMEM containing 10% calf serum, 100 U/ml penicillin, and 100 U/ml streptomycin. To determine c-fos transcript levels in 3T3-L1 cells, cells were preincubated under serum-free conditions for 16 h before hormonal treatment. Total RNA was extracted from cells using the RNeasy kit (QIAGEN Science, Valencia, CA), and genomic DNA was eliminated using deoxyribonuclease digestion before reverse transcription using a Sensiscript RT kit (QIAGEN Science). Primers were designed using Primer Express 2.0 software (Applied Biosystems, Foster City, CA). Standard curves for c-fos and β-actin transcripts were generated by serial dilutions of individual cDNAs. The primer pairs used were: c-fos forward, 5′-TGCGCAAAAGTCCTGTGTGT-3′; c-fos reverse, 5′-GGGACAGCCTTTCCTACTACCAT-3′; β-actin forward, 5′-CATTGCTGACAGGATGCAGAAG-3′; β-actin reverse, 5′-CTCAGGAGG-AGCAATGATCTTGA-3′.
Real-time PCR was performed using iQ SYBR Green Supermix (Bio-Rad Laboratories, Inc., Hercules, CA), and β-actin levels were used for copy number normalization. The assays were performed on a Smart Cycler TD System (Cepheid, Sunnyvale, CA) with an initial enzyme activation step of 15 min at 95 C, followed by 45 cycles of two-step PCR (94 C, 15 sec; 60 C, 60 sec). PCR products were checked for specificity by melting curve analyses (60–95 C in 0.2 C increments) and subsequently using 1.5% agarose gel electrophoresis to verify the absence of primer dimers. Data are presented as relative expression, normalized to β-actin (46). Results represent mean ± se of fold changes of normalized expression.
Immunoblotting Analysis of ERK1/2
3T3-L1 cells were preincubated in serum-free conditions for 5 h before hormonal treatment. After treatment, cells were lysed in a mammalian protein extraction buffer (Pierce Chemical Co., Rockford, IL), containing Halt Protease Inhibitors, PhosSTOP (Roche, Indianapolis, IN) and 5 mm EDTA. After 10 min agitation at 23 C, samples were centrifuged at 11,000 × g for 10 min. Supernatants were collected and protein concentrations were determined by the Bradford assay (Bio-Rad Laboratories) using BSA as the standard. Samples were then stored at −20 C until further use. Cell lysates (7.5 μg protein/well) were separated by SDS-PAGE and electrotransferred to Immobilon-P membranes (Millipore Corp., Bedford, MA). After blocking for 1 h in 5% (wt/vol) nonfat dry milk in Tris-buffered saline containing 0.05% (vol/vol) Tween 20, membranes were incubated overnight in a buffer containing 3% BSA and monoclonal phospho-specific anti-ERK1/2 antibodies (Santa Cruz Biotechnology). After the membranes were washed, signals were detected after 2 h incubation with a horseradish peroxidase-conjugated secondary antibody (sc-2005; Santa Cruz Biotechnology) for visualization using Immobilon Chemiluminescent Horseradish Peroxidase Substrate (Millipore Corp.). To estimate total ERK content, blots were stripped in Restore PLUS Western Blot Stripping Buffer (Thermo Scientific, Rockford, IL), blocked, and incubated with polyclonal antibodies against ERK2 (sc-154; Santa Cruz Biotechnology) before signal development. Figures shown are representative of three independent experiments.
Radioligand Binding Assays
Human obestatin was iodinated using the Iodogen tube method and purified using HPLC by Phoenix Pharmaceutical, Inc. (Belmont, CA). Briefly, 20 μg of obestatin were dissolved in the phosphate buffer (pH 7.4) to reach a final concentration of 0.2 nm. Peptide solution (10 μl) was reacted with 7 μl (10 μCi) of [125I] Na in the Iodogen tube at room temperature for 5 min before termination of the reaction by adding 50 μl of 10% KI and 250 μl of 50% acetic acid. Iodinated peptides were injected into a C-18 analytical column and purified using a linear gradient of 0–60% CH3CN in 1% trifluoroacetic acid after HPLC fractionation (total run time of 40 min with a flow rate of l ml/min). Collected fractions (0.5 ml/30 sec/tube) were monitored by a γ counter, and the peak fractions containing monoiodoobestatin were confirmed by matrix-assisted laser desorption/ionization, time-of-flight mass spectrometry and showed a specific activity of 1187 Ci/mmol.
For radioligand binding assays, rat jejunum or other tissues were washed with buffer A (20 mm HEPES; 5 mm EDTA; 1 mm dithiothreitol; 10 μm amidinophenyl-methanesulfonyl fluoride; 5 mg/liter leupeptin; 100 mm KCl, pH 7.5), cut into small pieces, and homogenized using a motorized homogenizer. After protein content had been estimated, 1 mg protein/100 μl was incubated with labeled obestatin with or without 200-fold excess of unlabeled obestatin. The reactants were incubated overnight (16–18 h) at 23 C. After 2 ml chilled PBS-0.1% BSA was added, the homogenates were centrifuged at 1000 × g for 20 min. The supernatant was decanted and tubes kept at 4 C. Tubes were vortexed once before addition of 2 ml of PBS-0.1% BSA, and the procedure was repeated twice before radioactivities in the pellet were estimated. For plasma membrane binding, tissue homogenates were centrifuged at 1000 × g for 5 min, and the supernatant was further centrifuged at 300,000 × g for 1 h at 4 C to collect pellets containing crude plasma membranes. After resuspension using a Dounce homogenizer, samples were incubated for 16 h at 23 C before filtration using a GF/C filter (Whatman, Inc., Florham Park, NJ) for radioactivity counting.
Site-directed mutagenesis was performed to change Ala50 to Val in GPR39 by using two pairs of overlapping primers coding for the mutated nucleotide to generate variant GPR39 cDNA fragments based on the PCR amplification of the wild-type construct (upstream primer 1, TCAGAATTCATGGCTTCACCCAGCCTCC; downstream primer 1, TGACCCGAATGGTGACGCTGTT; upstream primer 2, AACAGCGTCACCA- TTCGGGTCA; downstream primer 2, TCATCTAGATCAAACTTCATGCTCCTGAA; underlined sequences represent altered nucleotides). After confirmation of intended mutation by sequencing, the fragment was used to replace the wild-type sequences after subcloning with flanking restriction enzyme sites. For cell binding assays, HEK293T cells were transfected with plasmids encoding human or rat GPR39 using lipofectamine 2000. About 1,000,000 cells per tube were used instead of tissue homogenates, and the incubation (23 C, 16 h) and binding procedures were the same as for tissue homogenates.
Footnotes
The laboratory of A.J.W.H. is supported by a grant from Johnson & Johnson Pharmaceutical Research (2005–2008).
Disclosure Statement: A.J.W.H. and J.V.Z. submitted patents on obestatin and GPR39; K.V.K., L.V.D., and D.M. are employed by Johnson & Johnson Pharmaceutical Research and Development; L.V.D. and D.M. are inventors on patents related to GPR39. The remaining authors have nothing to disclose.
First Published Online March 12, 2008
Abbreviations: GPR39, G protein-coupled receptor; HEK, human embryonic kidney; SNP, single nucleotide polymorphism.
References
- 1.Kojima M, Kangawa K 2005. Ghrelin: structure and function. Physiol Rev 85:495–522 [DOI] [PubMed] [Google Scholar]
- 2.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ 2005. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake. Science 310:996–999 [DOI] [PubMed] [Google Scholar]
- 3.McKee KK, Tan CP, Palyha OC, Liu J, Feighner SD, Hreniuk DL, Smith RG, Howard AD, Van der Ploeg LH 1997. Cloning and characterization of two human G protein-coupled receptor genes (GPR38 and GPR39) related to the growth hormone secretagogue and neurotensin receptors. Genomics 46:426–434 [DOI] [PubMed] [Google Scholar]
- 4.Gourcerol G, St-Pierre DH, Tache Y 2007. Lack of obestatin effects on food intake: should obestatin be renamed ghrelin-associated peptide (GAP)? Regul Pept 141:1–7 [DOI] [PubMed] [Google Scholar]
- 5.Garg A 2007. The ongoing saga of obestatin: is it a hormone? J Clin Endocrinol Metab 92:3396–3398 [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Umek RM, McKnight SL 1991. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev 5:1538–1552 [DOI] [PubMed] [Google Scholar]
- 7.Chartrel N, Alvear-Perez R, Leprince J, Iturrioz X, Reaux-Le Goazigo A, Audinot V, Chomarat P, Coge F, Nosjean O, Rodriguez M, Galizzi JP, Boutin JA, Vaudry H, Llorens-Cortes C 2007. Comment on “Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin’s effects on food intake.” Science 315:766; author reply 766 [DOI] [PubMed] [Google Scholar]
- 8.Holst B, Egerod KL, Schild E, Vickers SP, Cheetham S, Gerlach LO, Storjohann L, Stidsen CE, Jones R, Beck-Sickinger AG, Schwartz TW 2007. GPR39 Signaling is stimulated by zinc ions but not by obestatin. Endocrinology 148:13–20 [DOI] [PubMed] [Google Scholar]
- 9.Lauwers E, Landuyt B, Arckens L, Schoofs L, Luyten W 2006. Obestatin does not activate orphan G protein-coupled receptor GPR39. Biochem Biophys Res Commun 351:21–25 [DOI] [PubMed] [Google Scholar]
- 10.Moechars D, Depoortere I, Moreaux B, de Smet B, Goris I, Hoskens L, Daneels G, Kass S, Ver Donck L, Peeters T, Coulie B 2006. Altered gastrointestinal and metabolic function in the GPR39-obestatin receptor-knockout mouse. Gastroenterology 131:1131–1141 [DOI] [PubMed] [Google Scholar]
- 11.Vergote V, Bode S, Peremans K, Vanbree H, Baert B, Slegers G, Burvenich C, De Spiegeleer B 2007. Analysis of iodinated peptides by LC-DAD/ESI ion trap mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 850:213–220 [DOI] [PubMed] [Google Scholar]
- 12.Vergote V, Baert B, Vandermeulen E, Peremans K, van Bree H, Slegers G, Burvenich C, De Spiegeleer B 2008. LC-UV/MS characterization and DOE optimization of the iodinated peptide obestatin. J Pharm Biomed Anal 46:127–136 [DOI] [PubMed] [Google Scholar]
- 13.Hoffman GE, Smith MS, Verbalis JG 1993. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 14:173–213 [DOI] [PubMed] [Google Scholar]
- 14.Halazonetis TD, Georgopoulos K, Greenberg ME, Leder P 1988. c-Jun dimerizes with itself and with c-Fos, forming complexes of different DNA binding affinities. Cell 55:917–924 [DOI] [PubMed] [Google Scholar]
- 15.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR 2002. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature 418:650–654 [DOI] [PubMed] [Google Scholar]
- 16.Bassil AK, Haglund Y, Brown J, Rudholm T, Hellstrom PM, Naslund E, Lee K, Sanger GJ 2007. Little or no ability of obestatin to interact with ghrelin or modify motility in the rat gastrointestinal tract. Br J Pharmacol 150:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Smet B, Thijs T, Peeters TL, Depoortere I 2007. Effect of peripheral obestatin on gastric emptying and intestinal contractility in rodents. Neurogastroenterol Motil 19:211–217 [DOI] [PubMed] [Google Scholar]
- 18.Depoortere I, Thijs T, Moechars D, De Smet B, Ver Donck L, Peeters TL 2008. Effect of peripheral obestatin on food intake and gastric emptying in ghrelin-knockout mice. Br J Pharmacol 153:1550–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egerod KL, Holst B, Petersen PS, Hansen JB, Mulder J, Hokfelt T, Schwartz TW 2007. GPR39 splice variants versus antisense gene LYPD1: expression and regulation in gastrointestinal tract, endocrine pancreas, liver, and white adipose tissue. Mol Endocrinol 21:1685–1698 [DOI] [PubMed] [Google Scholar]
- 20.Zhao CM, Furnes MW, Stenstrom B, Kulseng B, Chen D 2008. Characterization of obestatin- and ghrelin-producing cells in the gastrointestinal tract and pancreas of rats: an immunohistochemical and electron-microscopic study. Cell Tissue Res 331:575–587 [DOI] [PubMed] [Google Scholar]
- 21.Qader SS, Hakanson R, Rehfeld JF, Lundquist I, Salehi A 2008. Proghrelin-derived peptides influence the secretion of insulin, glucagon, pancreatic polypeptide and somatostatin: a study on isolated islets from mouse and rat pancreas. Regul Pept 146:230–237 [DOI] [PubMed] [Google Scholar]
- 22.Kapica M, Zabielska M, Puzio I, Jankowska A, Kato I, Kuwahara A, Zabielski R 2007. Obestatin stimulates the secretion of pancreatic juice enzymes through a vagal pathway in anaesthetized rats—preliminary results. J Physiol Pharmacol 58(Suppl 3):123–130 [PubMed] [Google Scholar]
- 23.Granata R, Settanni F, Gallo D, Trovato L, Biancone L, Cantaluppi V, Nano R, Annunziata M, Campiglia P, Arnoletti E, Ghe C, Volante M, Papotti M, Muccioli G, Ghigo E 2008. Obestatin promotes survival of pancreatic β-cells and human islets and induces expression of genes involved in the regulation of β-cell mass and function. Diabetes 57:967–979 [DOI] [PubMed] [Google Scholar]
- 24.Nogueiras R, Pfluger P, Tovar S, Arnold M, Mitchell S, Morris A, Perez-Tilve D, Vazquez MJ, Wiedmer P, Castaneda TR, Dimarchi R, Tschop M, Schurmann A, Joost HG, Williams LM, Langhans W, Dieguez C 2007. Effects of obestatin on energy balance and growth hormone secretion in rodents. Endocrinology 148:21–26 [DOI] [PubMed] [Google Scholar]
- 25.Gourcerol G, Million M, Adelson DW, Wang Y, Wang L, Rivier J, St-Pierre DH, Tache Y 2006. Lack of interaction between peripheral injection of CCK and obestatin in the regulation of gastric satiety signaling in rodents. Peptides 27:2811–2819 [DOI] [PubMed] [Google Scholar]
- 26.Seoane LM, Al-Massadi O, Pazos Y, Pagotto U, Casanueva FF 2006. Central obestatin administration does not modify either spontaneous or ghrelin-induced food intake in rats. J Endocrinol Invest 29:RC13–RC15 [DOI] [PubMed]
- 27.Tremblay F, Perreault M, Klaman LD, Tobin JF, Smith E, Gimeno RE 2006. Normal food intake and body weight in mice lacking the G protein-coupled receptor GPR39. Endocrinology 148:501–506 [DOI] [PubMed] [Google Scholar]
- 28.Green BD, Irwin N, Flatt PR 2007. Direct and indirect effects of obestatin peptides on food intake and the regulation of glucose homeostasis and insulin secretion in mice. Peptides 28:981–987 [DOI] [PubMed] [Google Scholar]
- 29.Zizzari P, Longchamps R, Epelbaum J, Bluet-Pajot MT 2007. Obestatin partially affects ghrelin stimulation of food intake and GH secretion in rodents. Endocrinology 148:1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bresciani E, Rapetti D, Dona F, Bulgarelli I, Tamiazzo L, Locatelli V, Torsello A 2006. Obestatin inhibits feeding but does not modulate GH and corticosterone secretion in the rat. J Endocrinol Invest 29:RC16–RC18 [DOI] [PubMed]
- 31.Carlini VP, Schioth HB, Debarioglio SR 2007. Obestatin improves memory performance and causes anxiolytic effects in rats. Biochem Biophys Res Commun 352:907–912 [DOI] [PubMed] [Google Scholar]
- 32.Abbott CR, Small CJ, Sajedi A, Smith KL, Parkinson JR, Broadhead LL, Ghatei MA, Bloom SR 2006. The importance of acclimatisation and habituation to experimental conditions when investigating the anorectic effects of gastrointestinal hormones in the rat. Int J Obes (Lond) 30:288–292 [DOI] [PubMed] [Google Scholar]
- 33.Tschop M, Castaneda TR, Joost HG, Thone-Reineke C, Ortmann S, Klaus S, Hagan MM, Chandler PC, Oswald KD, Benoit SC, Seeley RJ, Kinzig KP, Moran TH, Beck-sickinger AG, Koglin N, Rodgers RJ, Blundell JE, Ishii Y, Beattie AH, Holch P, Allison DB, Raun K, Madsen K, Wulff BS, Stidsen CE, et al 2004. Physiology: does gut hormone PYY3–36 decrease food intake in rodents? Nature [Erratum (2004) 431:1038] 430:1; discussion 2 [DOI] [PubMed] [Google Scholar]
- 34.Samson WK, White MM, Price C, Ferguson AV 2007. Obestatin acts in brain to inhibit thirst. Am J Physiol Regul Integr Comp Physiol 292:R637–R643 [DOI] [PubMed]
- 35.Samson WK, White MM, Price C, Ferguson AV 2008. Obestatin inhibits vasopressin secretion: evidence for a physiological role in the control of fluid homeostasis. J Endocrinol 196:559–564 [DOI] [PubMed] [Google Scholar]
- 36.Szentirmai E, Krueger JM 2006. Obestatin alters sleep in rats. Neurosci Lett 404:222–226 [DOI] [PubMed] [Google Scholar]
- 37.Dun SL, Brailoiu GC, Brailoiu E, Yang J, Chang JK, Dun NJ 2006. Distribution and biological activity of obestatin in the rat. J Endocrinol 191:481–489 [DOI] [PubMed] [Google Scholar]
- 38.Camina JP, Campos JF, Caminos JE, Dieguez C, Casanueva FF 2006. Obestatin-mediated proliferation of human retinal pigment epithelial cells: regulatory mechanisms. J Cell Physiol 211:1–9 [DOI] [PubMed] [Google Scholar]
- 39.Bang AS, Soule SG, Yandle TG, Richards AM, Pemberton CJ 2007. Characterisation of proghrelin peptides in mammalian tissue and plasma. J Endocrinol 192:313–323 [DOI] [PubMed] [Google Scholar]
- 40.Seim I, Collet C, Herington AC, Chopin LK 2007. Revised genomic structure of the human ghrelin gene and identification of novel exons, alternative splice variants and natural antisense transcripts. BMC Genomics 8:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scrima M, Campiglia P, Esposito C, Gomez-Monterrey I, Novellino E, D'Ursi AM 2007. Obestatin conformational features: a strategy to unveil obestatin’s biological role? Biochem Biophys Res Commun 363:500–505 [DOI] [PubMed] [Google Scholar]
- 42.Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, Huebner A, Cheetham ME, Clark AJ 2005. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nat Genet 37:166–170 [DOI] [PubMed] [Google Scholar]
- 43.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM 1998. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393:333–339 [DOI] [PubMed] [Google Scholar]
- 44.Hinney A, Hoch A, Geller F, Schafer H, Siegfried W, Goldschmidt H, Remschmidt H, Hebebrand J 2002. Ghrelin gene: identification of missense variants and a frameshift mutation in extremely obese children and adolescents and healthy normal weight students. J Clin Endocrinol Metab 87:2716. [DOI] [PubMed] [Google Scholar]
- 45.Catalan V, Gomez-Ambrosi J, Rotellar F, Silva C, Gil MJ, Rodriguez A, Cienfuegos JA, Salvador J, Fruhbeck G 2007. The obestatin receptor (GPR39) is expressed in human adipose tissue and is down-regulated in obesity-associated type 2 diabetes mellitus. Clin Endocrinol (Oxf) 66:598–601 [DOI] [PubMed] [Google Scholar]
- 46.Livak KJ, Schmittgen TD 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-ΔΔC(T)) method. Methods 25:402–408 [DOI] [PubMed] [Google Scholar]
- 47.Pazos Y, Alvarez CJ, Camiña JP, Casanueva FF 2007. Stimulation of extracellular signal-related kinases and proliferation in the human gastric cancer cells KATO-III by obestatin. Growth Factors 25:373–381 [DOI] [PubMed] [Google Scholar]