Abstract
Microbial metabolites are known to affect immune system, brain, and behavior via activation of pattern recognition receptors such as Toll-like receptor 4 (TLR4). Unlike the effect of the TLR4 agonist lipopolysaccharide (LPS), the role of other TLR agonists in immune-brain communication is insufficiently understood. We therefore hypothesized that the TLR2 agonist lipoteichoic acid (LTA) causes immune activation in the periphery and brain, stimulates the hypothalamic-pituitary-adrenal (HPA) axis and has an adverse effect on blood-brain barrier (BBB) and emotional behavior. Since LTA preparations may be contaminated by LPS, an extract of LTA (LTAextract), purified LTA (LTApure), and pure LPS (LPSultrapure) were compared with each other in their effects on molecular and behavioral parameters 3 h after intraperitoneal (i.p.) injection to male C57BL/6N mice.
The LTAextract (20 mg/kg) induced anxiety-related behavior in the open field test, enhanced the circulating levels of particular cytokines and the cerebral expression of cytokine mRNA, and blunted the cerebral expression of tight junction protein mRNA. A dose of LPSultrapure matching the amount of endotoxin/LPS contaminating the LTAextract reproduced several of the molecular and behavioral effects of LTAextract. LTApure (20 mg/kg) increased plasma levels of tumor necrosis factor-α (TNF-α), interleukin-6 and interferon-γ, and enhanced the transcription of TNF-α, interleukin-1β and other cytokines in the amygdala and prefrontal cortex. These neuroinflammatory effects of LTApure were associated with transcriptional down-regulation of tight junction-associated proteins (claudin 5, occludin) in the brain. LTApure also enhanced circulating corticosterone, but failed to alter locomotor and anxiety-related behavior in the open field test.
These data disclose that TLR2 agonism by LTA causes peripheral immune activation and initiates neuroinflammatory processes in the brain that are associated with down-regulation of BBB components and activation of the HPA axis, although emotional behavior (anxiety) is not affected. The results obtained with an LTA preparation contaminated with LPS hint at a facilitatory interaction between TLR2 and TLR4, the adverse impact of which on long-term neuroinflammation, disruption of the BBB and mental health warrants further analysis.
Keywords: Anxiety, Brain, Corticosterone, Cytokines, Lipopolysaccharide, Lipoteichoic acid, Neuroinflammation, Tight junction-associated proteins, Toll-like receptors
1. Introduction
There is abundant evidence that bacterial infection of peripheral tissues causes innate immune cells to produce pro-inflammatory cytokines which act on the brain to cause sickness as well as molecular and behavioral perturbations (Dantzer et al., 2008). The immune system senses bacterial intrusion via pattern recognition receptors (PRRs) which recognize evolutionarily highly conserved structures on pathogens, so-called pathogen associated molecular patterns (PAMPs). Toll-like receptors (TLRs) represent the best characterized family of PRRs which are expressed on the cell surface and thus can initiate a first-line immune response against invading pathogens (Medzhitov et al., 1997). Toll-like receptor 4 (TLR4), for instance, is responsible for the recognition of lipopolysaccharide (LPS) on the cell wall of gram-negative bacteria (Poltorak et al., 1998). Upon binding of LPS to TLR4, which requires the presence of myeloid differentiation 2, the signaling cascade targets myeloid differentiation primary response protein 88 and results in the release of pro-inflammatory cytokines (Kawai et al., 1999). If LPS is endocytosed, the TRIF-related adaptor molecule/TIR-domain-containing adapter-inducing interferon-β pathway is activated and causes release of type 1 interferons (Kawai and Akira, 2010). Through these immune mediators, LPS is known to cause sickness and evoke signs of anxiety- and depression-like behavior in rodents (Bluthé et al., 1994; O’Connor et al., 2009; Painsipp et al., 2010, 2008; Sulakhiya et al., 2016). In humans, symptoms of depression and anxiety are correlated with LPS exposure and subsequent cytokine release (Vogelzangs et al., 2016), and increased IL-6 and INF-α levels correlate with severity of depression and anxiety (Capuron et al., 2009; Raison et al., 2006).
The family of TLRs comprises 12 members (10 in humans) (Pandey et al., 2015) which are targeted by different PAMPs (Kawai and Akira, 2010). Under conditions of bacterial invasion it is likely that different PRRs are activated in parallel and that the ensuing immune and brain responses are the result of the positive and/or negative interactions between the PRR-mediated reactions. For instance, the sickness response to LPS is enhanced by synergism between TLR4 and the nuclear-binding domain (NOD)-like receptors NOD1 and NOD2, which recognize peptidoglycan elements (Farzi et al., 2015b). In contrast, lipoteichoic acid (LTA) is a major cell wall component of gram-positive bacteria and a PAMP that is primarily recognized by Toll-like receptor 2 (TLR2) (Hermann et al., 2002). LTA is a surface-associated adhesion amphiphile composed of a soluble polymer, consisting of polyhydroxy alkane units, such as ribitol and glycerol, attached to the cell membrane with a diacylglycerol. The sequence of glycerol and ribitol repeat units varies between species (Schneewind and Missiakas, 2014). Bacteriolysis leads to the release of LTA into the bloodstream, which occurs in response to β-lactam antibiotic treatment (Van Langevelde et al., 1998). LTA induces the secretion of cytokines such as IL-1β and TNF-α, which can contribute to the disruption of the blood-brain barrier (BBB) (Boveri et al., 2006). In addition, LTA is required for anchoring microorganisms to brain microvascular endothelial cells that disrupt the BBB (Sheen et al., 2010).
Despite the deleterious impact mediated by LTA on BBB function, the effects of this PAMP on molecular changes in the immune-brain axis and on behavior have not yet been explored. As concerns regarding the contamination of commercial LTA preparations by LPS have been raised (Gao et al., 2001; Morath et al., 2001), the present study was conducted with an extract (LTAextract) and a purified preparation of LTA (LTApure). The effects of these LTA preparations on the immune-brain axis were compared with the effects of ultrapure LPS (LPSultrapure). The first specific aim was to examine whether behavior in the open field, indicative of sickness and/or anxiety, is affected by intraperitoneally (i.p.) injected LTA from Bacillus subtilis. The second aim was to examine the effect on immune activation in the periphery and brain as reflected by the expression of cytokines in the plasma, amygdala and prefrontal cortex. Given that the HPA axis is activated by extrinsic and intrinsic stressors (Borrow et al., 2016) including immune activation (Farzi et al., 2015b; Lehmann et al., 2013), the third aim was to assess the effect of LTA on circulating corticosterone. The fourth aim was to evaluate the potentially deleterious effect of LTA on BBB composition by studying the transcriptional regulation of tight junction-associated proteins in the amygdala and prefrontal cortex.
2. Methods and materials
2.1. Experimental animals
The experiments were performed with 10-week-old male C57BL/6N mice (n=188; 22-27 g body weight) obtained from Charles River (Sulzfeld, Germany). The animals were housed in pairs in a vivarium under controlled conditions: temperature set point at 22 °C, air humidity set point at 50 % and a 12 h light/dark cycle. Tap water and standard laboratory chow were provided ad libitum throughout the experiment.
2.2. Ethics statement
The experimental procedure and number of animals used were approved by the ethical committee at the Federal Ministry of Science, Research, and Economy of the Republic of Austria (BMWF-66.010/0026-WF/II/3b/2014) and conducted according to the Directive of the European Parliament and of the Council of September 22, 2010 (2010/63/EU). The experiments were designed in such a way that both the number of animals used and their suffering was minimized.
2.3. Reagents
LTA from Bacillus subtilis was obtained from two different vendors (Sigma-Aldrich, Vienna, Austria, catalog number L3265, from here on referred to as LTAextract, and Invivogen, Toulouse, France, catalog number tlrl-lta, from here on referred to as LTApure). LPS from Escherichia coli O111:B4 extracted by successive enzymatic hydrolysis steps and purified by the phenol-TEA-DOC extraction protocol (LPSultrapure) was obtained from Invivogen (catalog number tlrl-3pelps). For TLR4 antagonism experiments, the TLR4 antagonist TAK-242 was used (Calbiochem/Merck Millipore, Darmstadt, Germany; catalog number US1614316, resatorvid, ethyl (6R)-6-[N-(2-chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate) (Ii et al., 2006; Kawamoto et al., 2008).
2.4. Activation of TLR2 and TLR4 in the HEK-Blue® reporter cell assay
HEK-Blue® (Invivogen, Toulouse, France) hTLR2 and hTLR4 cells were grown in Dulbecco’s Modified Eagle Medium (Gibco, ThemoFisher, Waltham, MA, USA) containing 4.5 g/l glucose, 2 nM L-glutamine, 10% fetal bovine serum, 1% penicillin-streptomycin and 1 x HEK-Blue® Selection at 37 °C and 5% CO2. After confluency was reached, cells were seeded into 24-well plates, 2.5 x 105 cells/well. Cells were then treated with 102 pg/ml, 104 pg/ml, or 106 pg/ml of LTApure, LTAextract, or LPSultrapure and incubated for 24 h. Sterile, distilled H2O was used as control.
To assess the TLR4 specificity of the agonists, cells were incubated overnight (12 h) with 3 µM TAK-242 dissolved in DMSO. DMSO was used as control at a concentration that did not exceed 0.2%. Following the overnight treatment with TAK-242 or DMSO, LTAextract (106 pg/ml) or LPSultrapure (104 pg/ml, or 106 pg/ml) was added, after which the cells were incubated for 24 h.
For quantitation of TLR2 and TLR4 activation, 180 µl of HEK-Blue® Detection medium was added to a 96-well plate, and 20 µl of supernatant from the treated cells was added. Alkaline phosphatase activity was subsequently measured with a Victor plate reader (PerkinElmer, Rodgau, Germany) at 655 nm.
2.5. Quantitation of endotoxin in LTAextract with the EndoLISA® endotoxin detection assay
To determine the amount of endotoxin present in LTAextract, the EndoLISA® (Hyglos, Bernried am Starnberger See, Germany) endotoxin detection assay with a measurement range of 0.05 - 500 EU/ml was used. The assay was performed according to the manufacturer’s instructions.
2.6. Experimental groups and timelines of the in vivo experiments
The animals were given two weeks to get accustomed to the vivarium at the institute before any experiments were performed. To reduce extrinsic stressors affecting the experiments, they were transferred to the behavioral test room at least 16 h (overnight) before behavioral testing or euthanization.
Preliminary experiments involving 7 groups (n=6-8 per group) of mice were conducted to study the effect of LTAextract in the open field test with regard to dosage and timeline. Initially LTAextract (0.15 mg and 0.5 mg per mouse) or its vehicle (pyrogen-free sterile saline, 8 ml/kg) was administered i.p. 3 h before the open field test was performed. Next, 0.5 mg LTAextract (corresponding to 20 mg/kg in a 25 g mouse) or its vehicle was administered i.p. to test for any behavioral effect in the open-field test 6 h and 27 h post-injection. All further experiments were conducted with the 20 mg/kg LTA dose injected i.p. 3 h prior to behavioral testing or organ collection.
To compare the effects of LTApure, LTAextract, and LPSultrapure, relative to their vehicle, on behavior in the open field, six groups (n=6-8 per group) of mice were employed. For the collection of blood and brains, six additional groups (n=5-9 per group) treated with LTApure, LTAextract, LPSultrapure or their vehicle were used to exclude any potential confounding effects of behavioral tests on molecular marker expression. All three TLR agonists were dissolved in pyrogen-free sterile saline right before administration and injected i.p.. LTApure and LTAextract were administered at a dose of 20 mg/kg and a volume of 5 ml/kg, while LPSultrapure was given at a dose of 1938 EU/kg and a volume of 7.75 ml/kg, corresponding to the amount of 97 EU/mg endotoxin detected in the 20 mg/kg LTAextract dose by means of the EndoLISA® endotoxin quantification assay. The vendor of LPSultrapure does not provide any information on the EU/weight ratio of this preparation. According to a formula given by the vendor of the EndoLISA® assay (1 EU ≈ 100 pg E. coli LPS; www.hyglos.de) the dose of 1938 EU/kg LPSultrapure would roughly correspond to 0.19 mg/kg. This quantity appears plausible, as a dose of 0.1 mg/kg LPS has previously been found to affect behavioral parameters in the LabMaster system (Farzi et al., 2015). As control treatment, pyrogen-free sterile saline was used at a volume of 5 ml/kg. Due to the experimental schedule a maximum of 16 animals could be used per day. Therefore the experiments were performed in a consecutive manner, each treatment group (LTApure, LTAextract, LPSultrapure) being compared with a separate vehicle group, which is also reflected in the statistical analysis (independent samples t test). Injections were made between 9:00 am and 12:00 pm, and the open field test and the plasma/organ collections were performed 3 h after the injection of the respective TLR agonist or its vehicle.
For the in vivo assessment of the effects of TAK-242 on corticosterone levels, six additional groups (n=7-8 per group) of animals were used. The animals received 4 mg/kg of TAK-242 dissolved in 11% DMSO i.p. at a volume of 10 ml/kg, and 30 min later the animals received either 20 mg/kg LTApure or LTAextract as well via i.p. injection. As control treatments, either 11% DMSO (vehicle for TAK-242) or pyrogen-free sterile saline (vehicle for LTApure or LTAextract) were used, respectively (Farzi et al., 2015a). Blood was collected 3 h after the injection of LTApure or LTAextract.
2.7. Open field test
The open field consisted of an opaque grey box (50 x 50 x 30 cm, B x W x H), illuminated by 35 lx at floor level. The central area (CA) was defined as 36 x 36 cm square in the middle, leaving a 7 cm boarder zone on each side. The test was performed individually on each mouse. The animals were placed in the center of the box, and their behavior (time in CA, CA visits, and total traveling distance) was tracked for 5 min by a video camera mounted above the open field and recorded with the VideoMot2 (TSE Systems, Bad Homburg, Germany) software.
2.8. Blood sampling and collection of brains
Animals were deeply anesthetized with pentobarbital (150 mg/kg i.p.), and blood was collected via cardiac puncture. One hundred µl of 3.8% sodium citrate was used as anticoagulant in each single-use syringe. The samples were centrifuged at 7000 rpm and 4 °C for 15 min, then plasma was collected and stored at -70 °C until further processing. After blood collection, brains were collected and immediately frozen for 5 s in 2-methylbutane on dry ice. Brains were wrapped in aluminum foil and stored at -70 °C.
2.9. Circulating corticosterone
Corticosterone levels in plasma were determined via an enzyme-linked immunosorbent assay (Assay Designs, Ann Arbor, Michigan, USA). The manufacturer’s specifications state a sensitivity of 27.0 pg/ml and intra- and inter-assay coefficients of variation of 7.7% and 9.7%, respectively. The assay was performed according to the manufacturer’s instructions.
2.10. Circulating cytokines in plasma
To determine levels of IL-1β (catalog number EPX01A-26002), IL-6 (EPX01A-20603), IL-10 (EPX01A-20614), INF-γ (EPX01A-20606), and TNF-α (EPX01A-20607), the magnetic bead-based ProcartaPlex™ immunoassay (catalog number EPX010-20440-901, eBioscience, San Diego, CA, USA) was used. The fluorescent signal was quantified with the Bio-Plex 200 multiplex suspension array system equipped with Luminex® xMAP® technology and the Bio-Plex 5.0 software (BioRad, Hercules, CA, USA). The assay was performed according to the manufacturer’s instructions.
2.11. Microdissection of amygdala and prefrontal cortex
The brains were microdissected by a trained researcher on a cold plate (Weinkauf Medizintechnik, Forchheim, Germany) at -20 °C (Brunner et al., 2014). The instruments were cleaned with RNase AWAY (Carl Roth, Karlsruhe, Germany) before and in between uses. The prefrontal cortex (Bregma +3.20 to -0.22) and amygdala (Bregma -0.58 to -2.54) were microdissected under a stereomicroscope. The dissected brain samples were transferred to micro packaging tubes with Precellys beads (Peqlab, Erlangen, Germany) and stored at -70 °C until further processing.
2.12. Reverse transcriptase polymerase chain reaction (RT-PCR) and quantitative real-time PCR (qPCR) of amygdala and prefrontal cortex
Amygdala and prefrontal cortex sections were homogenized with the Precellys 24 homogenizer (Peqlab, Erlangen, Germany). Subsequently, RNA was extracted according to the manufacturer’s instructions using the RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany). The RNA concentration in each sample was determined via NanoDrop (Thermo Scientific, DE, USA). Afterwards, 2 µg of RNA were reverse-transcribed with the high capacity cDNA reverse transcription kit (Fisher Scientific, Vienna, Austria) according to the manufacturer’s instructions, using the Mastercycler Gradient (Eppendorf, Hamburg, Germany). Relative quantitation of mRNA levels was performed via qPCR using a LightCycler 480® system with TaqMan gene expression assays for CLDN5 (catalog number Mm00727012_s1), OCLN (Mm00500912_m1), TJP1 (Mm00493699_m1), CCL2 (Mm00441242_m1), IL-1β (Mm00434228_m1), IL-6 (Mm00446190_m1), IL-10 (Mm01288386_m1), INF-γ (Mm01168134_m1), and TNF-α (Mm00443258_m1), and a master mix (catalog number 4369510, Fisher Scientific, Vienna, Austria). Controls without reverse transcriptase were included for each brain area and treatment group. ACTB (Mm00607939_s1), GAPDH (Mm99999915_g1), and PPIL3 (Mm00510343_m1) were used as endogenous reference genes. The 2−ΔΔCt method was used to quantitate target gene levels relative to controls. Differences in treatment groups were expressed as fold changes.
2.13. Statistics
Results were statistically analyzed using GraphPad® Prism5 (GraphPad Software Inc., La Jolla, CA, USA) or SPSS 22 (SPSS Inc., Chicago, IL, USA). Homogeneity of variances was assessed with the Kolmogorov-Smirnov test. Differences between groups were analyzed with the unpaired samples t test. In case a non-parametric test was required, the Mann–Whitney U test or Kruskal Wallis test was used. Due to the consecutive setup of the experiments, ANOVA was not permitted. Probability values of p ⩽ 0.05 were regarded as statistically significant.
3. Results
3.1. LTApure activates TLR2 but not TLR4 in the HEK-Blue® reporter cell assay, while LTAextract activates TLR4, but fails to activate TLR2
Given the concerns regarding the contamination of commercial LTA by LPS (Gao et al., 2001; Morath et al., 2001), the HEK-Blue® reporter cell assay was used to investigate the TLR2/TLR4 specificity of the three TLR agonists under study, LTAextract, LTApure, and LPSultrapure.
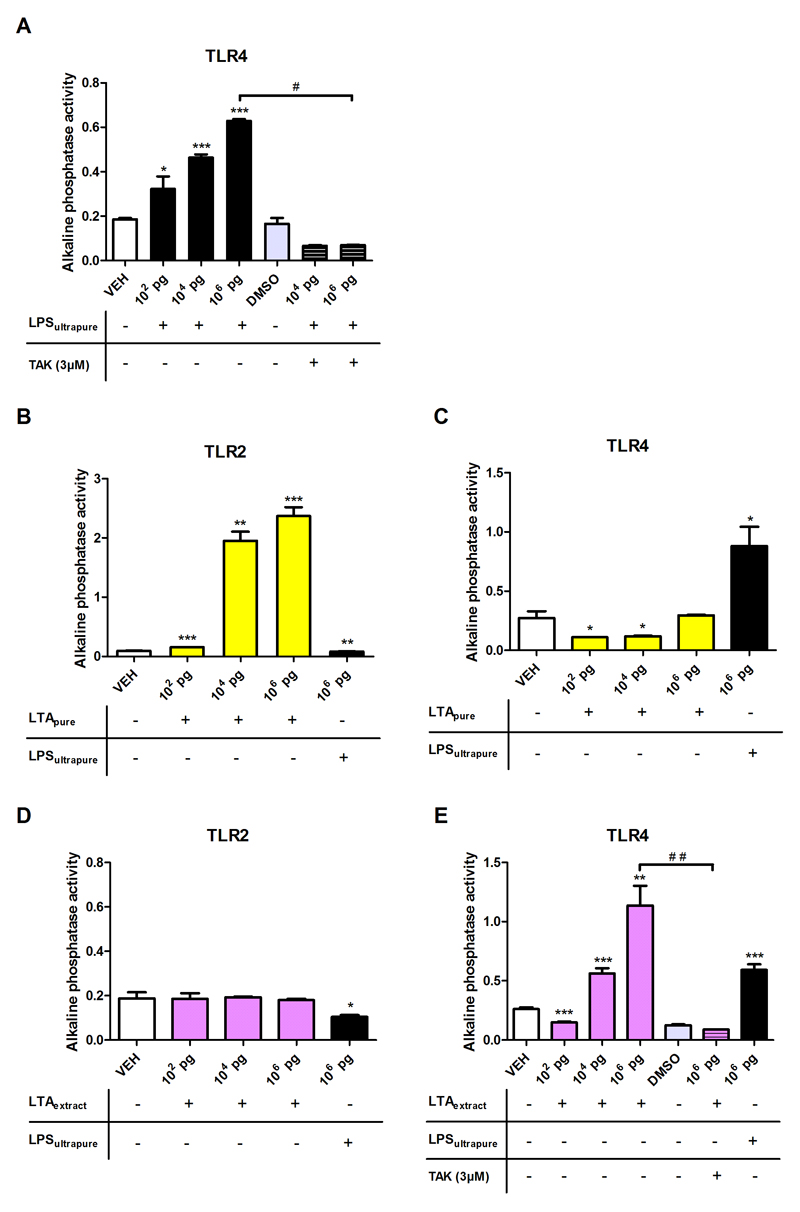
Initial validation of the TLR4 system was performed with LPSultrapure. As expected, LPSultrapure dose-dependently increased secretion of alkaline phosphatase, and this effect was inhibited in the presence of the TLR4 inhibitor TAK-242 (Figure 1A). LTApure dose-dependently activated TLR2-dependent secretion of alkaline phosphatase, whereas LPSultrapure had no effect (Figure 1B). In the TLR4 reporter HEK-Blue® cell line, LTApure was without effect (Figure 1C). LTAextract did not elicit TLR2 signaling (Figure 1D), but activated TLR4 signaling in a concentration-dependent manner, this effect being reduced to baseline in the presence of TAK-242 (Figure 1E).
Figure 1. Effect of LTApure, LTAextract, and LPSultrapure on alkaline phosphatase release in HEK-Blue® TLR2 and TLR4 reporter cells.
HEK-Blue® TLR4 (A,C,E) and TLR2 (B,D) cells were incubated with LPSultrapure (102 pg/ml, 104 pg/ml, 106 pg/ml; A), LTApure (102 pg/ml, 104 pg/ml, 106 pg/ml; B,C), or LTAextract (102 pg/ml, 104 pg/ml, 106 pg/ml; D,E). After a 24 h incubation, alkaline phosphatase activity was determined at 655 nm on a plate reader. TAK-242 (TAK; 3 µM) was added to the cell suspensions 12 h prior incubation with LTAextract or LPSultrapure. The bars represent means + SD from one representative experiment performed in triplicates. *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to vehicle (VEH)-treated cells; #p ≤ 0.05 compared to 106 pg/ml LPSultrapure; ##p ≤ 0.01 compared to 106 pg/ml LTAextract. The data in panels A,B,E were analyzed with the independent samples t test, those in panels C,D with the non-parametric Kruskal-Wallis test.
3.2. Lipopolysaccharide, but not pure lipoteichoic acid, induces anxiety-like behavior in the open field test
The open field test was used to assess anxiety-like behavior in response to systemic application of the TLR agonists under study. The time spent in the CA, and the CA visits were used as a measure of anxiety. More time spent in and more visits to the CA indicated less anxiety. The locomotor activity deduced from the total traveling distance was used as a measure of sickness. Preliminary experiments (Table 1) showed that LTAextract at a dose of 0.5 mg/mouse, but not 0.15 mg/mouse, enhanced anxiety-like behavior as reflected by a significant diminution of the time spent in the CA and the number of CA visits. This effect of LTAextract at 0.5 mg per mouse (corresponding to 20 mg/kg in a 25 g mouse) was observed 3 h, but not 6 h or 27 h, post-administration (Table 1).
Table 1. Dose- and time-dependent effect of LTAextract on anxiety-like behavior in the open field test.
The open field test was performed 3, 6 or 27 h after i.p. treatment of mice with LTAextract at the dose/mouse indicated or its vehicle (VEH). The figures represent means + SEM, n = 6-8; *p ≤ 0.05 compared to VEH-treated mice, independent samples t test.
| Treatment | VEH | LTAextract | LTAextract | VEH | LTAextract | VEH | LTAextract |
|---|---|---|---|---|---|---|---|
| Dose injected [mg] | 0.15 | 0.5 | 0.5 | 0.5 | |||
| Time of open field test post injection [h] | 3 | 6 | 27 | ||||
| Time in central area [min] | 1.32 | 0.88 | 0.63* | 0.98 | 1.08 | 0.85 | 1.06 |
| Central area visits | 28.83 | 24.05 | 16.88* | 17.57 | 20.71 | 20.17 | 22.43 |
| Total traveling distance [m] | 2.25 | 2.05 | 1.92 | 2.08 | 2.03 | 2.29 | 2.04 |
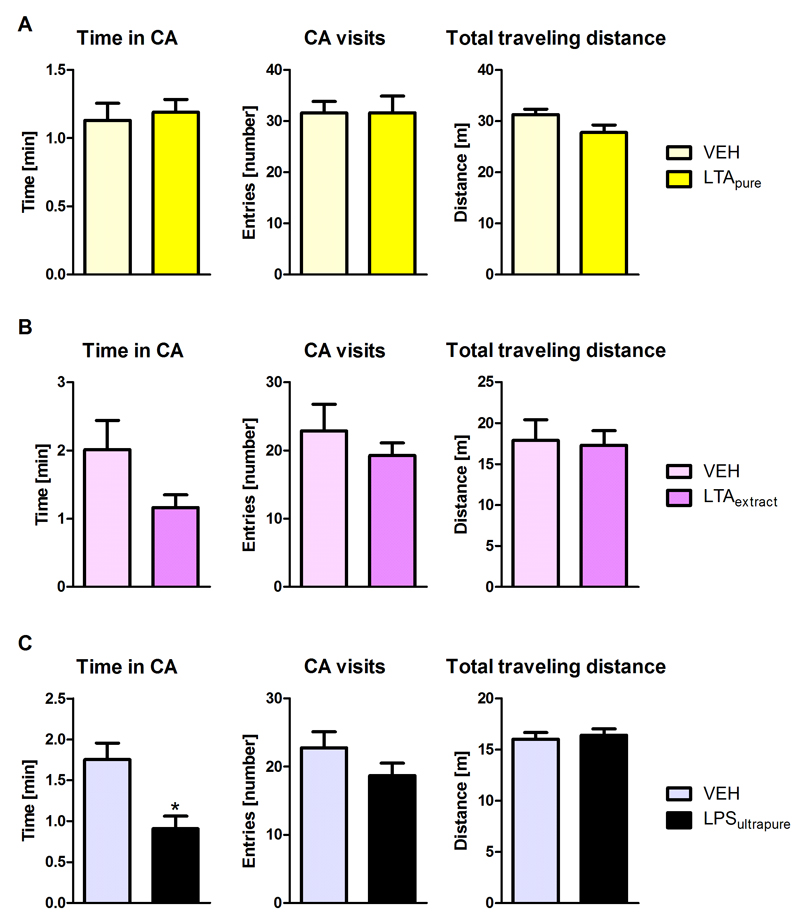
Next we compared the effects of LTAextract, LTApure, and LPSultrapure on the behavior in the open field test. LTApure had no significant effect on any of the three parameters measured (Figure 2A). In line with the data shown in Table 1, LTAextract clearly tended to reduce the time in the CA although the effect did not reach statistical significance (p=0.0941) in this set of experiments. CA visits and total traveling distance remained largely unaffected by LTAextract (Figure 2B). Systemic administration of LPSultrapure significantly reduced the time in the CA (p=0.0106, Figure 2C), but had no significant effect on CA visits or traveling distance. These results indicate that LPSultrapure is anxiogenic, while LTApure has no effect on anxiety-like behavior. LTAextract induced a behavioral pattern more similar to LPSultrapure than LTApure.
Figure 2. Effect of LTApure, LTAextract, and LPSultrapure on anxiety-like behavior in the open field test.
The graphs depict the time spent in the central area (CA), the number of CA visits, and the total traveling distance during a 5 min test period. The open field test was performed 3 h after i.p. treatment with LTApure (20 mg/kg; A), LTAextract (20 mg/kg; B), LPSultrapure (1938 EU/kg ≈ 0.19 mg/kg; C) or the respective vehicle (VEH). The bars represent means + SEM, n = 8-10; *p ≤ 0.05 compared to VEH-treated mice, independent samples t test.
3.3. LTApure, LTAextract, and LPSultrapure increase circulating corticosterone
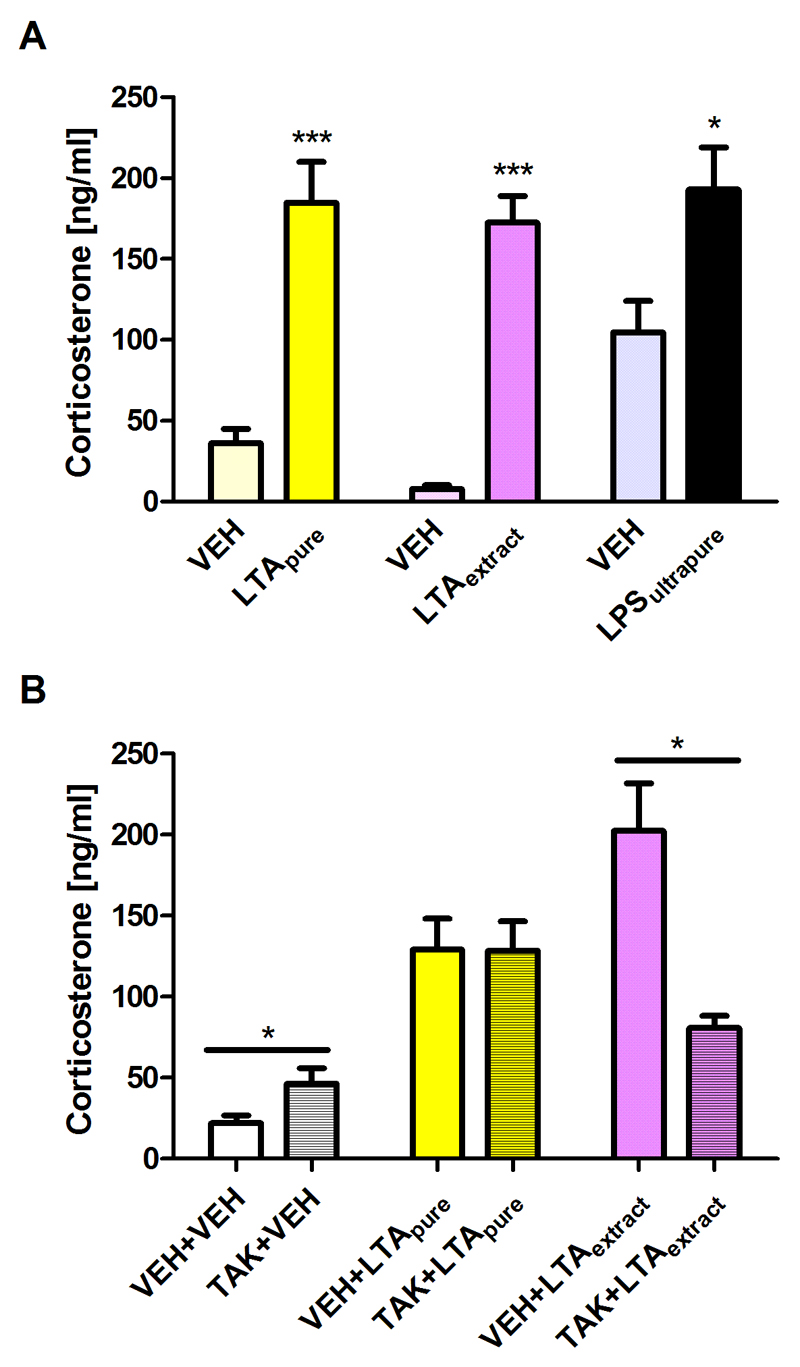
Given that LPS increases circulating corticosterone (Farzi et al., 2015b), the plasma concentration of this glucocorticoid was measured to quantify the effects of LTApure and LTAextract, relative to LPSultrapure, on HPA axis activity. As is shown in Figure 3A, LTApure, LTAextract, and LPSultrapure all significantly increased plasma corticosterone compared to the respective vehicle.
Figure 3. Effect of LTApure, LTAextract, and LPSultrapure on plasma corticosterone levels.
Panel A depicts circulating corticosterone levels measured 3 h after i.p. treatment with LTApure (20 mg/kg), LTAextract (20 mg/kg), LPSultrapure (1938 EU/kg ≈ 0.19 mg/kg) or the respective vehicle (VEH). Panel B depicts circulating corticosterone levels measured 3 h after i.p. treatment with LTApure (20 mg/kg) or LTAextract (20 mg/kg), TAK-242 (TAK; 4 mg/kg) or its VEH being administered 30 min before LTApure or LTAextract was given. The bars represent means + SEM, n = 6-8; *p ≤ 0.05, ***p ≤ 0.001 compared to VEH-treated mice, independent samples t test.
3.4. The TLR4 antagonist TAK-242 attenuates the increase in plasma corticosterone levels induced by LTAextract but not LTApure
To investigate the effects of LTApure and LTAextract on TLR4 signaling in vivo, TAK-242 was administered i.p. 30 min before administering LTApure or LTAextract. The basal levels of circulating corticosterone were slightly, but significantly, elevated by TAK-242 in comparison to the vehicle control (Figure 3B). The effect of LTApure to increase plasma corticosterone remained unaffected by TAK-242 (Figure 3B). In contrast, the effect of LTAextract to raise plasma corticosterone was significantly blunted by TAK-242 (Figure 3B).
3.5. LTApure, LTAextract, and LPSultrapure increase circulating cytokines
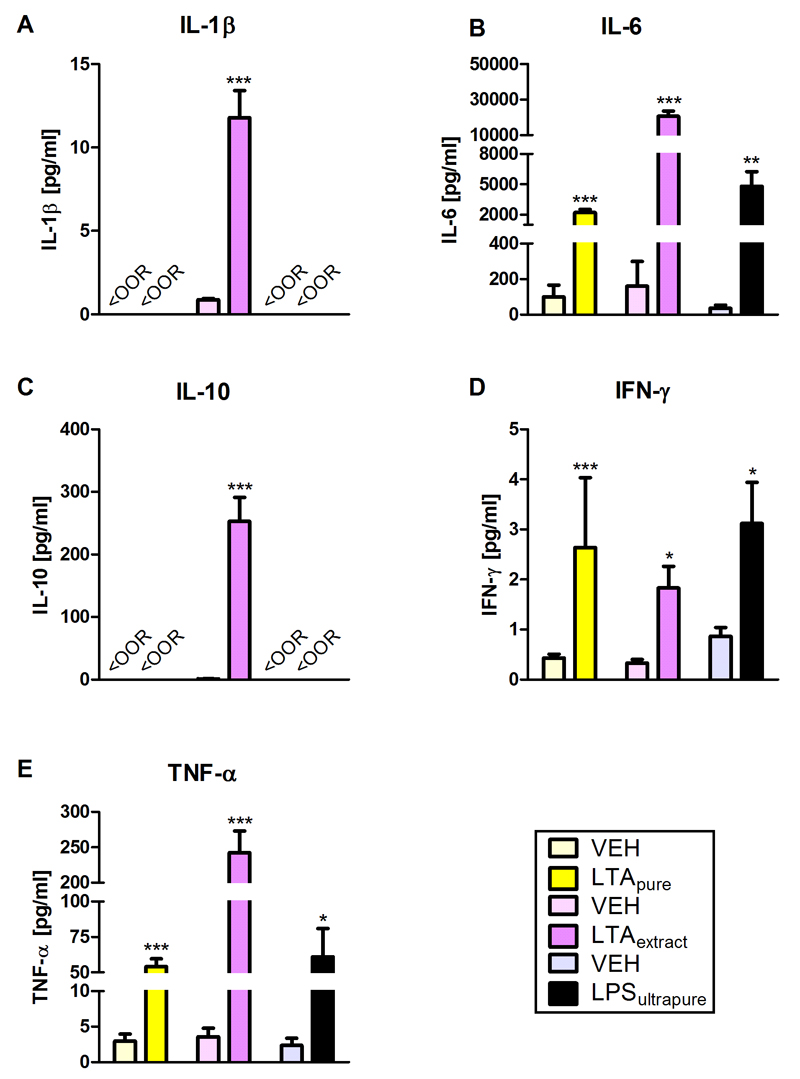
Circulating cytokines were determined to gauge the effect of LTApure, LTAextract, and LPSultrapure on peripheral immune activation. IL-6, INF-γ, and TNF-α were significantly increased 3 h after treatment with the TLR agonists compared to the respective vehicles (Figure 4B,D,E). The plasma concentrations of IL-1β and IL-10 in mice treated with LTApure and LPSultrapure or the respective controls were below the detection limit (Figure 4A,C). In contrast, LTAextract significantly increased IL-1β and IL-10 concentrations, relative to vehicle (Figure 4A,C).
Figure 4. Effect of LTApure, LTAextract, and LPSultrapure on plasma cytokine levels.
The graphs depict circulating levels of interleukin-1β (IL-1β; A), interleukin-6 (IL-6; B), interleukin-10 (IL-10; C), interferon-γ (INF-γ; D), and tumor necrosis factor-α (TNF-α; E). LTApure (20 mg/kg), LTAextract (20 mg/kg), LPSultrapure (1938 EU/kg ≈ 0.19 mg/kg) or the respective vehicle (VEH) was administered 3 h before the cytokines were assayed. The symbol <OOR refers to values below the detection limit. The bars represent means + SEM, n = 6-9; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to VEH-treated mice, independent samples t test.
3.6. LTApure, LTAextract, and LPSultrapure differentially affect transcription of cytokines and tight junction-associated proteins in the amygdala and prefrontal cortex
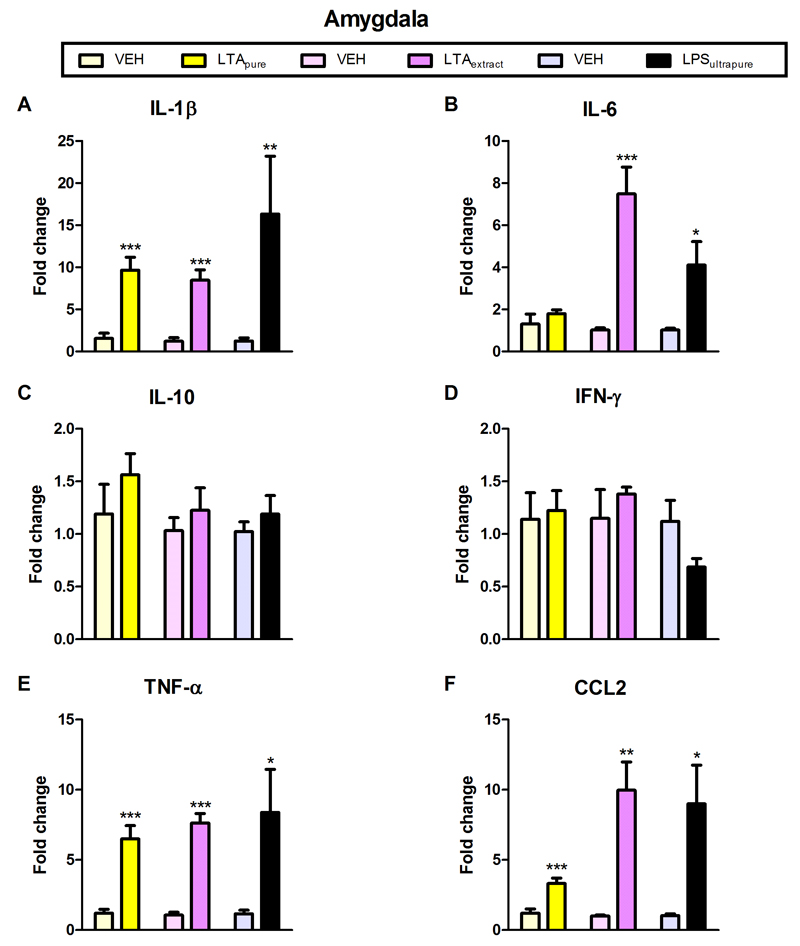
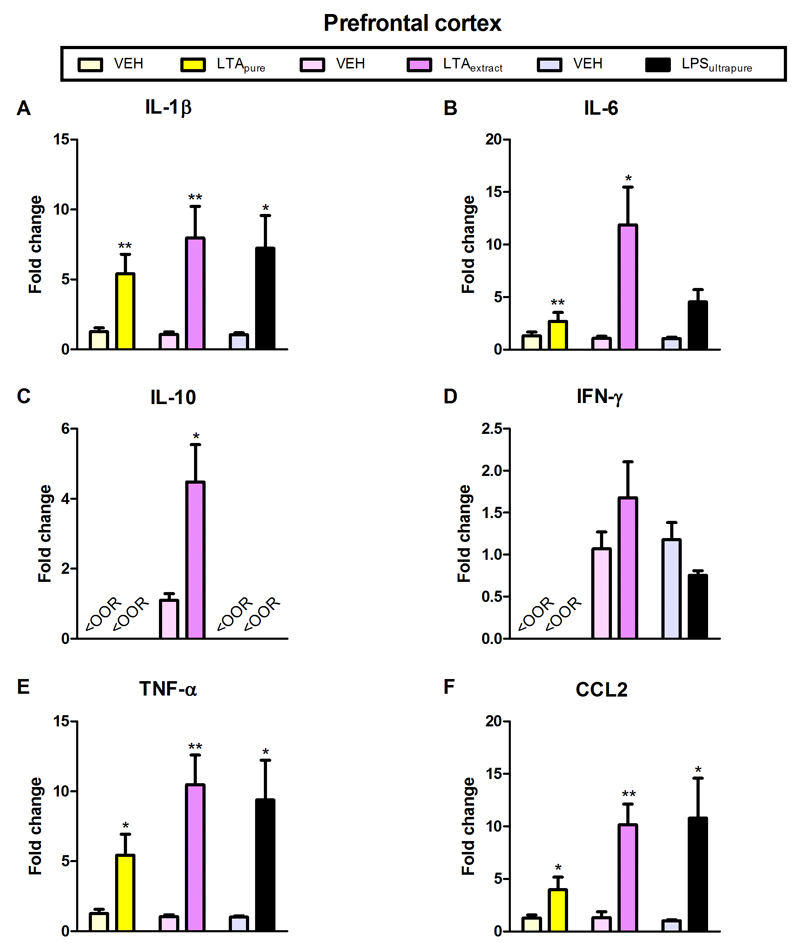
Amygdala and prefrontal cortex were chosen as test brain regions based on their important role in anxiety (Maroun, 2013; Robinson et al., 2016). Furthermore, anxiety is known to be influenced by cytokines (Vogelzangs et al., 2016). LTApure induced a significant increase in the expression of IL-1β, TNFα, and CCL2 mRNA in the amygdala and prefrontal cortex, as well as of IL-6 mRNA in the prefrontal cortex (Figures 5A,E,F and 5A,B,E,F). In contrast to LTApure, LTAextract stimulated cytokine mRNA expression in additional brain regions and/or to a larger extent (Figures 5 and 6). LTAextract significantly enhanced the expression of IL-1β, IL-6, TNFα, and CCL2 mRNA in the amygdala and prefrontal cortex, and of IL-10 in the prefrontal cortex (Figures 5A,B,E,F and 6A,B,C,E,F). The effect of LTAextract to raise IL-6 and CCL2 mRNA expression in the amygdala (Figure 5B,F) and IL-6, IL-10, TNFα, and CCL2 mRNA expression in the prefrontal cortex (Figure 6B,C,E,F) was more pronounced than that of LTApure. LPSultrapure significantly increased IL-1β, TNFα, and CCL2 mRNA expression in the amygdala and prefrontal cortex, as well as of IL-6 mRNA in the amygdala (Figures 5A,B,E,F and 6A,E,F).
Figure 5. Effect of LTApure, LTAextract, and LPSultrapure on cytokine mRNA expression in the amygdala.
The graphs depict the expression of interleukin-1β (IL-1β; A), interleukin-6 (IL-6; B), interleukin-10 (IL-10; C), interferon-γ (INF-γ; D), tumor necrosis factor-α (TNF-α; E), and chemokine (C-C motif) ligand 2 (CCL2; F) mRNA. LTApure (20 mg/kg), LTAextract (20 mg/kg), LPSultrapure (1938 EU/kg ≈ 0.19 mg/kg) or the respective vehicle (VEH) was administered 3 h before the cytokines were assayed. mRNA transcription is expressed as fold change relative to VEH-treated mice. The bars represent means + SEM, n = 5-7; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to VEH-treated mice, independent samples t test.
Figure 6. Effect of LTApure, LTAextract, and LPSultrapure on cytokine mRNA expression in the prefrontal cortex.
The graphs depict the expression of interleukin-1β (IL-1β; A), interleukin-6 (IL-6; B), interleukin-10 (IL-10; C), interferon-γ (INF-γ; D), tumor necrosis factor-α (TNF-α; E), and chemokine (C-C motif) ligand 2 (CCL2; F) mRNA. LTApure (20 mg/kg), LTAextract (20 mg/kg), LPSultrapure (1938 EU/kg ≈ 0.19 mg/kg) or the respective vehicle (VEH) was administered 3 h before the cytokines were assayed. The symbol <OOR refers to values below the detection limit. mRNA transcription is expressed as fold change relative to VEH-treated mice. The bars represent means + SEM, n = 5-7; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to VEH-treated mice, independent samples t test.
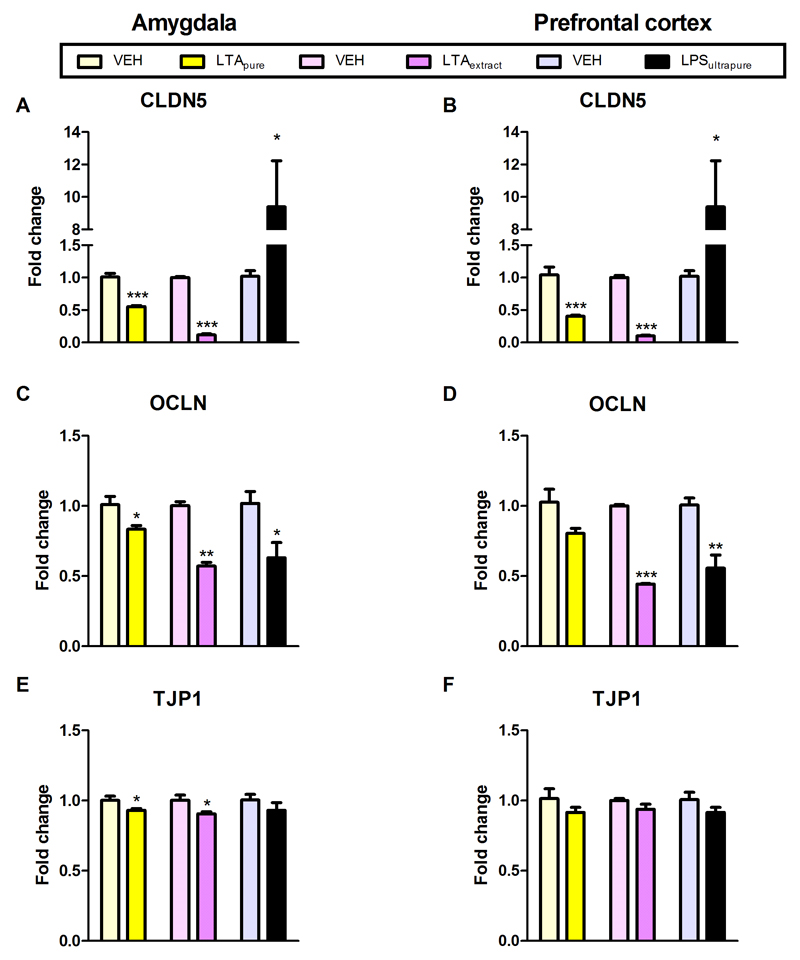
To evaluate the impact of the TLR agonists on gene products governing blood-brain-barrier composition, the mRNA expression of tight junction-associated CLDN5, OCLN, and TJP1 was quantitated by qPCR. CLDN5 mRNA expression in the amygdala and prefrontal cortex was significantly decreased by LTApure and LTAextract, while LPSultrapure significantly increased CLDN5 mRNA in both amygdala and prefrontal cortex (Figure 7A,B).OCLN mRNA expression in the amygdala was decreased by all three TLR agonists under study (Figure 7C), OCLN mRNA expression in the prefrontal cortex was decreased by LTAextract and LPSultrapure, but not by LTApure (Figure 7D). TJP1 mRNA expression in the amygdala was slightly decreased by LTApure and LTAextract, whereas TJP1 mRNA in the prefrontal cortex remained unaffected Figure 7E,F).
Figure 7. Effect of LTApure, LTAextract, and LPSultrapure on tight junction-associated protein mRNA expression in the amygdala (A,C,E) and prefrontal cortex (B,D,F).
The graphs depict the expression of claudin 5 (CLDN5; A,B), occludin (OCLN; C,D), and tight junction protein 1 (TJP1; E,F) mRNA. LTApure (20 mg/kg), LTAextract (20 mg/kg), LPSultrapure (1938 EU/kg ≈ 0.19 mg/kg) or the respective vehicle (VEH) was administered 3 h before the tight junction-associated proteins were assayed. mRNA transcription is expressed as fold change relative to VEH-treated mice. The bars represent means + SEM, n = 5-7; *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 compared to VEH-treated mice, independent samples t test.
4. Discussion
The current data reveal several important advances relating to the communication between the peripheral immune system and brain: (1) TLR2 agonism by purified LTA (LTApure) from Bacillus subtilis causes immune activation in the periphery and brain as reflected by a rise of distinct cytokines in plasma and increased cytokine expression in the amygdala and prefrontal cortex. (2) These immunologic changes are accompanied by a potentially deleterious effect of LTA on BBB composition as mirrored by a decrease in the expression of tight junction-associated proteins in the brain. (3) Another effect of LTA-induced TLR2 stimulation manifests itself in a rise of circulating corticosterone, indicative of activation of the HPA axis. (4) These molecular responses to TLR2 agonism do not extend to behavioral alterations related to sickness and anxiety, as has been reported in response to stimulation of other PRRs such as TLR4, at least not at the LTA doses studied here. (5) The analysis of the biologic effects of LTA preparations with submaximal purity can substantially be confounded by contaminations with endotoxin/LPS. (6) A comparison of the immunologic and cerebral effects of an LTA extract with those of purified LTA and LPS hints at a facilitatory interaction of the TLR2 and TLR4 agonists present in the LTAextract, which may be of translational relevance to the consequences of bacterial translocation and infection.
4.1. Dosage and purity of LTA
Previous work has shown that the TLR2 agonist LTA (purity not specified) causes activation of peripheral as well as cerebral microglial immune cells (Huang et al., 2013; Lim et al., 2013; Neher and Brown, 2007; Oberg et al., 2011) and triggers the release of pro-inflammatory cytokines (Medzhitov and Janeway, 1998). These effects were confirmed by the present in vivo study in which the plasma concentrations of IL-6, INF-γ, and TNF-α were significantly enhanced 3 h after i.p. administration of LTApure and LTAextract. The dose of 20 mg/kg LTA and the measurement time points used here were chosen in view of the preliminary results summarized in Table 1. In this setting, LTAextract shortened the time spent in the CA of the open field 3 h, but not 6 and 27 h post-treatment. In addition, analysis of the effects of the TLR4 agonist LPS has shown, that cytokine production and sickness behavior are maximal between 1 - 6 h post-injection (Dantzer et al., 2008; Layé et al., 1994). A minimum of 3 h between treatment administration and analysis was deemed necessary to exclude confounding effects of the stress of handling and injection.
Stimulation of murine PRRs such as TLR4 by LPS has previously been found to activate the HPA axis and cause the release of corticosterone (Farzi et al., 2015b; Lehmann et al., 2013). The present study showed that LTA likewise enhances the plasma concentrations of corticosterone, an effect that is triggered both by LTAextract and LTApure. In view of the concerns regarding the contamination of commercial LTA preparations by LPS (Gao et al., 2001; Morath et al., 2001) the involvement of TLR4 in the effect of LTA on HPA axis activity was probed with the TLR4 antagonist TAK-242 (Ii et al., 2006; Kawamoto et al., 2008). At the dose of 4 mg/kg (Farzi et al., 2015a; Wang et al., 2013) TAK-242 blunted the rise of plasma corticosterone induced by LTAextract, but not that by LTApure, which indicates that the effect of LTAextract is largely due to contamination by a TLR4 agonist, most likely LPS. This lack of specificity of LTAextract for TLR2 was confirmed in TLR2/TLR4 HEK-Blue® reporter assays in which LTAextract, like LPSultrapure, stimulated TLR4, whereas LTApure activated TLR2, but not TLR4. Corroborating these pharmacologic properties, TAK-242 at the concentration of 3 µM (concentration indicated in product leaflet by Invivogen) prevented stimulation of TLR4 by LTAextract and LPSultrapure. In view of the observations that, unlike LTApure, LTAextract displayed affinity for TLR4, the immunologic, cerebral and behavioral effects of these two LTA preparations were strictly analyzed in parallel with those of LPSultrapure at a dose matching the amount of contaminating LPS present in LTAextract.
Currently we have no conclusive explanation why LTAextract, unlike LTApure, failed to stimulate TLR2 in the HEK-Blue® reporter assay (Figure 1A) although at the same time it was able to stimulate TLR4 (Figure 1B). We speculate that the interaction of LTA with TLR2 in HEK-Blue® cells is negatively regulated by unknown components of the LTAextract and/or peculiarities in cellular transduction mechanisms, given that LTA signaling is determined by many factors other than TLR2. Thus, the TLR2-mediated activation of immune cells by LTA is enhanced by lipopolysaccharide-binding protein and CD-14 but is independent of TLR4 and the co-receptor MD-2 (Schröder et al., 2003). In order to activate the TLR2 signaling cascade, LTA must be present in an active form (Lebeer et al., 2010), although it has been argued that TLR2 is not necessary for LTA-induced secretion of IL-8 after TLR2 is blocked with a TLR2 antibody (Hattar et al., 2006). In view of these circumstances, the failure of LTAextract to stimulate TLR2 in the reporter assay may be due to (1) an inappropriate conformation of LTA to activate TLR2 and its signaling cascade, (2) the presence of unknown components in the LTAextract that interfere with TLR2 activation and transduction, and/or (3) a negative regulation of TLR2 signaling by excess LPS in the in vitro system. The presence of appreciable amounts of endotoxin/LPS in the LTAextract, as shown by the HEK-Blue® reporter cell assay, is affirmed by the ability of the selective TLR4 inhibitor TAK-242 (Ii et al., 2006; Kawamoto et al., 2008) to suppress the activity of the LTAextract (Figure 1E).
4.2. Effect of LTA on circulating and cerebral cytokines as well as BBB proteins
Purified and undamaged LTA from S. aureus has been reported to stimulate the release of pro-inflammatory cytokines such as TNF-α, INF-γ, CCL2 (Kang et al., 2012), and interleukins 1, 5, 6, and 8 from immune cells (Ginsburg, 2002). This was corroborated in the current study where plasma IL-6, INF-γ, and TNF-α were significantly enhanced by LTApure, LTAextract, and LPSultrapure. The effect of LPS on circulating cytokine levels is in line with another report in which low doses of LPS (0.1 mg/kg) have been found to elevate plasma levels of IL-1β and IL-6 (Farzi et al., 2015b). In the current study it is particularly worth noting that the production of IL-1β and anti-inflammatory IL-10 was stimulated by LTAextract only and that the rise of plasma IL-6 and TNF-α was multiple times higher after treatment with LTAextract than after treatment with either LTApure or LPSultrapure. These observations are suggestive of a facilitatory interaction between LTA, LPS and possibly other PAMPs present in LTAextract. Synergies between activation of TLR4 by LPS and stimulation of TLR2 by diverse agonists have previously been noted in various cellular systems (Beutler et al., 2001; Xu et al., 2007).
The LTA-evoked peripheral immune activation extends to the central nervous system in which the transcription of both cytokines and tight junction-associated proteins was significantly altered. The molecular analysis focused on mRNA quantitation, because this parameter provides a better reflection of the dynamics of intervention-induced gene expression. LTApure increased the expression of IL-1β, TNFα, and CCL2 mRNA in the amygdala and prefrontal cortex and that of IL-6 mRNA in the prefrontal cortex. These findings indicate that systemic administration of LTA induces neuroinflammation in two brain areas (amygdala and prefrontal cortex) that are relevant to emotional-affective behavior such as anxiety (Maroun, 2013; Robinson et al., 2016). Since LTApure, LTAextract and LPSultrapure all increased peripheral circulating TNF-α levels, it is conceivable that this cytokine is one of the master regulators transferring inflammation from the periphery to the brain. This conclusion is supported by the finding that mice lacking TNF receptors (TNFR1/R2-/-) do not develop neuroinflammation (Qin et al., 2007). Thus, systemic administration of LPS or TNF-α is unable to elevate TNF-α, CCL2, and IL-1β mRNA levels in the brain of TNFR1/2-/- mice (Qin et al., 2007). As LTApure, LTAextract and LPSultrapure all elevated the circulating level of IL-6, and LTAextract and LPSultrapure also enhanced its expression in the amygdala, IL-6 may be another cytokine that significantly contributes to immune signaling between periphery and brain. This contention is in keeping with the emerging evidence that IL-6 plays an important role in neuroinflammation and associated mental disturbances (Burton et al., 2013; Qian et al., 2014; Spooren et al., 2011). The observation that, relative to LTApure or LPSultrapure, LTAextract stimulated cytokine mRNA expression in additional brain regions and/or to a larger extent suggests that neuroinflammatory processes in the brain may be facilitated by concomitant activation of TLR2 and TLR4, although to a lesser extent than in the periphery.
Peripheral as well as cerebral immune activation by both LTAextract and LTApure went in parallel with a decreased transcription of the tight junction-associated proteins CLDN5 and OCLN, while transcription of TJP1 remained almost unaffected. Of note, LTAextract was consistently more efficacious in attenuating the expression of CLDN5 and OCLN than LTApure, which is at variance with the activity of LPSultrapure to elevate the expression of CLDN5 mRNA. Although we do not know whether these changes in mRNA expression translate to changes in actual BBB integrity and function, it is emerging from other studies that the effect of TLR2 and TLR4 agonists on tight junction-associated protein mRNA expression in the brain is related to neuroinflammation and its deleterious impact on the BBB. In vitro studies have revealed that TNF-α, IL-1β, and IL-6 (all being elevated in the present study) increase the permeability of brain endothelial cell monolayers (De Vries et al., 1996). It is obvious from these findings that cytokines can induce aberrant BBB function, an activity that could be exploited to increase delivery of pharmaceuticals to the brain (Wardill et al., 2016). CCL2 (also elevated in the present study), which is a critical mediator of inflammation within and outside the central nervous system (Bennett et al., 2003), can disrupt BBB function via C-C chemokine receptor type 2-mediated signaling pathways that lead to myosin light chain hyperphosphorylation (Yao and Tsirka, 2014).
Further work has shown that highly purified LTA from S. aureus concentration- and time-dependently influences BBB integrity via activation of glial cells in a co-culture model of bovine brain capillary endothelial cells and rat primary glial cells (Boveri et al., 2006). One report holds that nitric oxide, TNF-α, and IL-1β produced by activated glial cells affect the BBB (Boveri et al., 2006), while another report suggests that the main BBB offenders are TNF-α, IL-1β, and IL-6 (De Vries et al., 1996). While Boveri et al. (2006) failed to observe a significant change in CLDN and OCLN expression following treatment with highly purified LTA from S. aureus in an in vitro BBB model, Singh et al. (2007) found a decrease of OCLN mRNA after LTA (purity not specified) treatment of endothelial cells. In spite of these inconsistencies it has been shown that LTA is crucial for S. aureus to adhere to and invade brain endothelial cells via surface anchoring (Sheen et al., 2010). The current finding of a pronounced decrease in CLDN5 and OCLN mRNA expression in the amygdala and prefrontal cortex, along with an increase in pro-inflammatory cytokine expression, supports the contention that TLR2-mediated neuroinflammation is closely related to a disruption of the molecular BBB composition. It should not go unnoticed that the effect of LTA on tight junctions is region-dependent, given that LTA from E. hirae has been shown to ameliorate TLR2-mediated intestinal epithelial tight junction impairment (Miyauchi et al., 2008).
4.3. Effect of LTA on HPA axis activity and anxiety-like behavior
Induction of neuroinflammatory processes in the brain by TLR4 agonists such as LPS is known to induce a syndrome of behavioral changes known as sickness response, followed by an increase in anxiety- and depression-like behavior (Bluthé et al., 1994; Dantzer, 2004). Although TLR2 agonism also causes immune activation in the brain and attenuates the expression of BBB components as shown here, there is scarce information on any behavioral alterations due to LTA-induced TLR2 agonism. TLR2 appears to play a role in the anxiety-like behavior of a murine schizophrenia model, given that anxiety-like behavior in the open field and elevated plus maze test is reduced in TLR2 knockout mice (Park et al., 2015). In addition, cytokines are thought to participate in the etiology of anxiety disorders (Vogelzangs et al., 2016). The present study, however, indicates that the molecular changes induced by LTApure in the brain do not translate to any behavioral changes in the open field test. In contrast, LTAextract was able to enhance anxiety-like behavior, an effect that is likely due to the LPS contamination, as LPSultrapure likewise had an anxiogenic action. Importantly, the doses of LTApure, LTAextract and LPSultrapure examined in this study were too low to induce overt sickness behavior, which would be reflected by a decrease of the traveling distance in the open field (Painsipp et al., 2010).
Activation of the HPA axis by internal and external stressors is known to have an impact on emotional-affective behavior including anxiety (Jacobson, 2014). As shown here, the plasma concentration of corticosterone was enhanced by LTApure, LTAextract and LPSultrapure as measured 3 h post-injection. Stimulation of murine PRRs such as TLR4 by LPS has previously been found to activate the HPA axis and cause the release of corticosterone (Farzi et al., 2015b; Lehmann et al., 2013), and a similar observation has been made with LTA (species and purity not specified) at a dose of 1 mg/kg (Bergt et al., 2013). Our finding indicates that stimulation of the HPA axis by systemic LTA does not result in short-term changes of anxiety-like behavior. It awaits to be investigated, however, whether the profound molecular changes induced by TLR2 agonism in the brain manifest themselves in long-term alterations of brain function and yet-to-be-identified behavioral traits.
5. Conclusions
Our work has shown that the effects of LTA on immune-brain communication depend to a good deal on the source and purity of the LTA preparation under study. This limitation can be overcome by the use of a highly purified LTA preparation, biologic validation of its selectivity as a TLR2 agonist and careful analysis of the effects that potential LPS/endotoxin contaminations may contribute. Through this approach we have been able to disclose that selective stimulation of TLR2 by purified LTA not only causes peripheral immune activation but also initiates neuroinflammatory processes in the brain as mirrored by transcriptional up-regulation of pro-inflammatory cytokines. These neuroinflammatory processes take place in parallel with a transcriptional down-regulation of tight junction-associated proteins, which points to a deleterious effect on the molecular composition of the BBB. In context with other studies we conclude that TNF-α and IL-6 may be a major regulators of the transition from peripheral immune stimulation to neuroinflammation and disruption of the BBB. The molecular changes evoked by TLR2 activation extend to activation of the HPA axis but have little impact on emotional behavior, unless TLR2 is concomitantly activated with TLR4, which appears to boost peripheral and cerebral immune activation and to enhance anxiety. In a translational perspective, the joint effect of different PRR agonists is of pathophysiologic relevance under conditions of bacterial invasion or translocation when different PRRs are activated in parallel. Constituents of the vast intestinal microbiota will also cause immune activation if the intestinal mucosal barrier is disrupted and allows for translocation of microbial constituents (Garrett et al., 2010; Inman et al., 2012; Kelly et al., 2015; Maslanik et al., 2012). There is increasing evidence that a disturbed interaction between the gut microbiota and the intestinal immune system has an impact on mental health (Kelly et al., 2015; Sampson and Mazmanian, 2015).
Acknowledgments
Raphaela Mayerhofer, Esther E. Fröhlich, and Nora Kogelnik received funding from the Austrian Science Fund FWF (W1241) and the Medical University Graz through the PhD Program Molecular Fundamentals of Inflammation (DK-MOLIN). Peter Holzer received funding from the Austrian Science Fund (FWF project P 25912-B23). The results contained in this work form part of the PhD thesis of Raphaela Mayerhofer. The technical assistance of Martina Hatz and Theresa Maierhofer (Center for Medical Research, Medical University of Graz) is greatly appreciated.
Abbreviations
- ACTB
beta actin
- BBB
blood-brain barrier
- CA
central area
- CCL2
chemokine (C-C motif) ligand 2
- CLDN5
claudin 5
- DMSO
dimethyl sulfoxide
- EU
endotoxin unit
- GAPDH
glycerinaldehyde-3-phosphate-Dehydrogenase
- HEK
human embryonic kidney
- HPA
hypothalamic-pituitary-adrenal
- hTLR
human Toll-like receptor
- IFN
interferon
- IL
interleukin
- i.p.
intraperitoneal
- LPS
lipopolysaccharide
- LTA
lipoteichoic acid
- NOD
nucleotide-binding oligomerization domain
- OCLN
occludin
- PAMP
pathogen associated molecular patterns
- PFCT
prefrontal cortex
- PPIL3
peptidyl-prolyl cis-trans isomerase-like 3
- PRR
pattern recognition receptor
- TJP1
tight junction protein 1
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
References
- Bennett JL, Elhofy A, Canto MCD, Tani M, Ransohoff RM, Karpus WJ. CCL2 transgene expression in the central nervous system directs diffuse infiltration of CD45 high CD11b + monocytes and enhanced Theiler ’ s murine encephalomyelitis virus – induced demyelinating disease. 2003:623–636. doi: 10.1080/13550280390247551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler E, Gelbart T, West C. Synergy between TLR2 and TLR4: a safety mechanism. Blood Cells Mol Dis. 2001;27:728–30. doi: 10.1006/bcmd.2001.0441. [DOI] [PubMed] [Google Scholar]
- Bluthé RM, Walter V, Parnet P, Layé S, Lestage J, Verrier D, Poole S, Stenning BE, Kelley KW, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. doi: 10.1016/0165-5728(94)90309-3. [DOI] [PubMed] [Google Scholar]
- Borrow AP, Stranahan AM, Suchecki D, Yunes R. Neuroendocrine Regulation of Anxiety: Beyond the HPA Axis. J Neuroendocrinol. 2016 doi: 10.1111/jne.12403. [DOI] [PubMed] [Google Scholar]
- Boveri M, Kinsner A, Berezowski V, Lenfant AM, Draing C, Cecchelli R, Dehouck MP, Hartung T, Prieto P, Bal-Price A. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: Role of pro-inflammatory cytokines and nitric oxide. Neuroscience. 2006;137:1193–1209. doi: 10.1016/j.neuroscience.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Brunner SM, Farzi A, Locker F, Holub BS, Drexel M, Reichmann F, Lang AA, Mayr JA, Vilches JJ, Navarro X, Lang R, et al. GAL3 receptor KO mice exhibit an anxiety-like phenotype. Proc Natl Acad Sci U S A. 2014;111:7138–43. doi: 10.1073/pnas.1318066111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MD, Rytych JL, Freund GG, Johnson RW. Central inhibition of interleukin-6 trans-signaling during peripheral infection reduced neuroinflammation and sickness in aged mice. Brain Behav Immun. 2013;30:66–72. doi: 10.1016/j.bbi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behaviour: A neuroimmune response to activation of innate immunity. Eur J Pharmacol. 2004;500:399–411. doi: 10.1016/j.ejphar.2004.07.040. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries HE, Blom-Roosemalen MCM, Van Oosten M, De Boer AG, Van Berkel TJC, Breimer DD, Kuiper J. The influence of cytokines on the integrity of the blood-brain barrier in vitro. J Neuroimmunol. 1996;64:37–43. doi: 10.1016/0165-5728(95)00148-4. [DOI] [PubMed] [Google Scholar]
- Farzi A, Halicka J, Mayerhofer R, Fröhlich EE, Tatzl E, Holzer P. Toll-like receptor 4 contributes to the inhibitory effect of morphine on colonic motility in vitro and in vivo. Sci Rep. 2015a;5:9499. doi: 10.1038/srep09499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzi A, Reichmann F, Meinitzer A, Mayerhofer R, Jain P, Hassan AM, Fröhlich EE, Wagner K, Painsipp E, Rinner B, Holzer P. Synergistic effects of NOD1 or NOD2 and TLR4 activation on mouse sickness behavior in relation to immune and brain activity markers. Brain Behav Immun. 2015b;44:106–120. doi: 10.1016/j.bbi.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Gordon JI, Glimcher LH. Homeostasis and Inflammation in the Intestine. Cell. 2010 doi: 10.1016/j.cell.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2:171–179. doi: 10.1016/S1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- Hattar K, Grandel U, Moeller A, Fink L, Iglhaut J, Hartung T, Morath S, Seeger W, Grimminger F, Sibelius U. Lipoteichoic acid (LTA) from Staphylococcus aureus stimulates human neutrophil cytokine release by a CD14-dependent, Toll-like-receptor-independent mechanism: Autocrine role of tumor necrosis factor-[alpha] in mediating LTA-induced interleukin-8 generatio. Crit Care Med. 2006;34:835–841. doi: 10.1097/01.CCM.0000202204.01230.44. [DOI] [PubMed] [Google Scholar]
- Hermann C, Spreitzer I, Schröder NWJ, Morath S, Lehner MD, Fischer W, Schütt C, Schumann RR, Hartung T. Cytokine induction by purified lipoteichoic acids from various bacterial species - Role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-γ release. Eur J Immunol. 2002;32:541–551. doi: 10.1002/1521-4141(200202)32:2<541::AID-IMMU541>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Huang BR, Tsai CF, Lin HY, Tseng WP, Huang SS, Wu CR, Lin C, Yeh WL, Lu DY. Interaction of inflammatory and anti-inflammatory responses in microglia by Staphylococcus aureus-derived lipoteichoic acid. Toxicol. Appl Pharmacol. 2013;269:43–50. doi: 10.1016/j.taap.2013.03.004. [DOI] [PubMed] [Google Scholar]
- Ii M, Matsunaga N, Hazeki K, Nakamura K, Takashima K, Seya T, Hazeki O, Kitazaki T, Iizawa Y. A novel cyclohexene derivative, ethyl (6R)-6-[N-(2-Chloro-4-fluorophenyl)sulfamoyl]cyclohex-1-ene-1-carboxylate (TAK-242), selectively inhibits toll-like receptor 4-mediated cytokine production through suppression of intracellular signaling. Mol Pharmacol. 2006;69:1288–1295. doi: 10.1124/mol.105.019695. [DOI] [PubMed] [Google Scholar]
- Inman CF, Laycock GM, Mitchard L, Harley R, Warwick J, Burt R, van Diemen PM, Stevens M, Bailey M. Neonatal Colonisation Expands a Specific Intestinal Antigen-Presenting Cell Subset Prior to CD4 T-Cell Expansion, without Altering T-Cell Repertoire. PLoS ONE. 2012;7(3):e33707. doi: 10.1371/journal.pone.0033707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L. Hypothalamic-pituitary-adrenocortical axis: Neuropsychiatric aspects. Compr Physiol. 2014;4:715–738. doi: 10.1002/cphy.c130036. [DOI] [PubMed] [Google Scholar]
- Gao Jian Jun, Xue Q, Zuvanich EG, Haghi KR, Morrison DC. Commercial preparations of lipoteichoic acid contain endotoxin that contributes to activation of mouse macrophages in vitro. Infect Immun. 2001;69:751–757. doi: 10.1128/IAI.69.2.751-757.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S-S, Kim HJ, Jang MS, Moon S, In Lee S, Jeon JH, Baik JE, Park O-J, Son YM, Kim GR, Joo D, et al. Gene expression profile of human peripheral blood mononuclear cells induced by Staphylococcus aureus lipoteichoic acid. Int Immunopharmacol. 2012;13:454–460. doi: 10.1016/j.intimp.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity. 1999;11:115–122. doi: 10.1016/S1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–84. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Ii M, Kitazaki T, Iizawa Y, Kimura H. TAK-242 selectively suppresses Toll-like receptor 4-signaling mediated by the intracellular domain. Eur J Pharmacol. 2008;584:40–48. doi: 10.1016/j.ejphar.2008.01.026. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, Hyland NP. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front Cell Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layé S, Parnet P, Goujon E, Dantzer R. Peripheral administration of lipopolysaccharide induces the expression of cytokine transcripts in the brain and pituitary of mice. Mol Brain Res. 1994;27:157–162. doi: 10.1016/0169-328X(94)90197-X. [DOI] [PubMed] [Google Scholar]
- Lebeer S, Vanderleyden J, De Keersmaecker SCJ. Host interactions of probiotic bacterial surface molecules: comparison with commensals and pathogens. Nat Rev Microbiol. 2010;8:171–184. doi: 10.1038/nrmicro2297. [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Mustafa T, Eiden AM, Herkenham M, Eiden LE. PACAP-deficient mice show attenuated corticosterone secretion and fail to develop depressive behavior during chronic social defeat stress. Psychoneuroendocrinology. 2013;38:702–715. doi: 10.1016/j.psyneuen.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim H, Kim D, Lee SJ. Toll-like receptor 2 mediates peripheral nerve injury-induced NADPH oxidase 2 expression in spinal cord microglia. J Biol Chem. 2013;288:7572–7579. doi: 10.1074/jbc.M112.414904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun M. Medial prefrontal cortex: multiple roles in fear and extinction. Neuroscientist. 2013;19:370–383. doi: 10.1177/1073858412464527. [DOI] [PubMed] [Google Scholar]
- Maslanik T, Tannura K, Mahaffey L, Loughridge AB, Benninson L, Ursell L, Greenwood BN, Knight R, Fleshner M. Commensal Bacteria and MAMPs Are Necessary for Stress-Induced Increases in IL-1b and IL-18 but Not IL-6, IL-10 or MCP-1. PLoS ONE. 2012;7(12):e50636. doi: 10.1371/journal.pone.0050636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Janeway CA., Jr A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature. 1997;388:394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA. An ancient system of host defense. Curr Opin Immunol. 1998 doi: 10.1016/S0952-7915(98)80024-1. [DOI] [PubMed] [Google Scholar]
- Miyauchi E, Morita H, Okuda J, Sashihara T, Shimizu M, Tanabe S. Cell wall fraction of Enterococcus hirae ameliorates TNF-??-induced barrier impairment in the human epithelial tight junction. Lett Appl Microbiol. 2008;46:469–476. doi: 10.1111/j.1472-765X.2008.02332.x. [DOI] [PubMed] [Google Scholar]
- Morath S. Structure-Function Relationship of Cytokine Induction by Lipoteichoic Acid from Staphylococcus aureus. J Exp Med. 2001;193:393–398. doi: 10.1084/jem.193.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher JJ, Brown GC. Neurodegeneration in models of Gram-positive bacterial infections of the central nervous system. Biochem Soc Trans. 2007;35:1166–7. doi: 10.1042/BST0351166. [DOI] [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, André C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–22. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberg H-H, Juricke M, Kabelitz D, Wesch D. Regulation of T cell activation by TLR ligands. Eur J Cell Biol. 2011;90:582–592. doi: 10.1016/j.ejcb.2010.11.012. [DOI] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Holzer P. Evidence from knockout mice that neuropeptide-Y Y2 and Y4 receptor signalling prevents long-term depression-like behaviour caused by immune challenge. J Psychopharmacol. 2010;24:1551–60. doi: 10.1177/0269881109348171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painsipp E, Herzog H, Sperk G, Holzer P. Long-term depression-like effect of a single immune challenge in neuropeptide Y Y2 and Y4 receptor knockout mice. BMC Pharmacol. 2008;8:A39. doi: 10.1186/1471-2210-8-S1-A39. [DOI] [Google Scholar]
- Pandey S, Kawai T, Akira S. notes on Microbial sensing by Toll-like receptors and intracellular nucleic acid sensors 1. 2015 doi: 10.1101/cshperspect.a016246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee JY, Kim SJ, Choi S-Y, Yune TY, Ryu JH. Toll-like receptor-2 deficiency induces schizophrenia-like behaviors in mice. Sci Rep. 2015;5:8502. doi: 10.1038/srep08502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltorak A, He X, Smirnova I, Liu M, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, et al. Defective LPS Signaling in C3H / HeJ and C57BL / 10ScCr Mice: Mutations in Tlr4 Gene. 1998;282:2085–2089. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Qian Z, He X, Liang T, et al. Lipopolysaccharides Upregulate Hepcidin in Neuron via Microglia and the IL-6/STAT3 Signaling Pathway. Mol Neurobiol. 2014;50:811. doi: 10.1007/s12035-014-8671-3. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Knapp DJ, Crews FT, Hill C, Carolina N, Park T. Systemic LPS Causes Chronic Neuroinflammation and Progressive Neurodegeneration. 2007;55:453–462. doi: 10.1002/glia.20467.Systemic. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: Inflammation and the pathogenesis of depression. Trends Immunol. 2006 doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Lieberman L, Vytal K, Ernst M, Grillon C. Anxiety-potentiated amygdala–medial frontal coupling and attentional control. Transl Psychiatry. 2016;6:e833. doi: 10.1038/tp.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson TR, Mazmanian SK. Control of brain development, function, and behavior by the microbiome. Cell Host Microbe. 2015;17:565–576. doi: 10.1016/j.chom.2015.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind O, Missiakas D. Lipoteichoic acids, phosphate-containing polymers in the envelope of gram-positive bacteria. J Bacteriol. 2014;196:1133–1142. doi: 10.1128/JB.01155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder NW, Morath S, Alexander C, Hamann L, Hartung T, Zähringer U, Göbel UB, Weber JR, Schumann RR. Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem. 2003;278(18):15587–94. doi: 10.1074/jbc.M212829200. [DOI] [PubMed] [Google Scholar]
- Sheen TR, Ebrahimi CM, Hiemstra IH, Barlow SB, Doran KS. Penetration of the blood-brain barrier by Staphylococcus aureus: contribution of membrane anchored lipoteichoic acid. 2010;88:633–639. doi: 10.1007/s00109-010-0630-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Jiang Y, Gupta S. Effects of bacterial toxins on endothelial tight junction in vitro: a mechanism-based investigation. Toxicol Mech Methods. 2007;17:331–347. doi: 10.1080/15376510601077029. [DOI] [PubMed] [Google Scholar]
- Spooren A, Kolmus K, Laureys G, Clinckers R, De Keyser J, Heageman G, Gerlo S. Interleukin-6, a mental cytokine. Brain Res Rev. 2011;67(1–2):157–83. doi: 10.1016/j.brainresrev.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Sulakhiya K, Keshavlal GP, Bezbaruah BB, Dwivedi S, Gurjar SS, Munde N, Jangra A, Lahkar M, Gogoi R. Lipopolysaccharide induced anxiety- and depressive-like behaviour in mice are prevented by chronic pre-treatment of esculetin. Neurosci Lett. 2016;611:106–111. doi: 10.1016/j.neulet.2015.11.031. [DOI] [PubMed] [Google Scholar]
- Van Langevelde P, Van Dissel JT, Ravensbergen E, Appelmelk BJ, Schrijver IA, Groeneveld PHP. Antibiotic-induced release of lipoteichoic acid and peptidoglycan from Staphylococcus aureus: Quantitative measurements and biological reactivities. Antimicrob Agents Chemother. 1998;42:3073–3078. doi: 10.1128/aac.42.12.3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogelzangs N, de Jonge P, Smit JH, Bahn S, Penninx BW. Cytokine production capacity in depression and anxiety. Transl Psychiatry. 2016;6:e825. doi: 10.1038/tp.2016.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, Wang PF, Fang H, Chen J, Xiong XY, Yang QW. Toll-like receptor 4 antagonist attenuates intracerebral hemorrhage-induced brain injury. Stroke. 2013;44:2545–2552. doi: 10.1161/STROKEAHA.113.001038. [DOI] [PubMed] [Google Scholar]
- Wardill HR, Mander KA, Van Sebille YZ, Gibson RJ, Logan RM, Bowen JM, Sonis ST. Cytokine-mediated blood brain barrier disruption as a conduit for cancer/chemotherapy-associated neurotoxicity and cognitive dysfunction. Int J cancer. 2016 doi: 10.1002/ijc.30252. [DOI] [PubMed] [Google Scholar]
- Xu WY, Wang L, Wang HM, Wang YQ, Liang YF, Zhao TT, Wu YZ. TLR2 and TLR4 agonists synergistically up-regulate SR-A in RAW264.7 through p38. Mol Immunol. 2007;44:2315–2323. doi: 10.1016/j.molimm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Yao Y, Tsirka SE. Monocyte chemoattractant protein-1 and the blood-brain barrier. Cell Mol Life Sci. 2014;71:683–97. doi: 10.1007/s00018-013-1459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]