Abstract
Importance
Stimulant use disorder is common, affecting between 0.3 to 1.1% of the population, and costs over $85 billion per year globally. There are currently no licensed treatments. Several lines of evidence implicate the dopamine system in the pathophysiology of substance use disorder. Thus understanding the nature of dopamine dysfunction seen in stimulant users has the potential to aid the development of new therapeutics.
Objective
To comprehensively review the in-vivo imaging evidence for dopaminergic alterations in stimulant (cocaine or amphetamine/methamphetamine) drug abuse or dependence.
Data sources
The entire PubMed, EMBASE and PsycINFO databases were searched for studies from inception date to May 14, 2016.
Study selection
A total of 31 studies were identified that compared dopaminergic measures between 519 stimulant users and 512 controls using positron emission tomography or single-photon emission computed tomography to measure striatal dopamine synthesis or release, or dopamine transporter or receptor availability.
Data extraction and synthesis
Demographic, clinical and imaging measures were extracted from each study and meta-analyses and sensitivity analyses were conducted for stimulants combined and cocaine and amphetamines separately where there were sufficient studies.
Main Outcomes and Measures
We determined the difference in dopamine release (assessed using change in the D2/3 receptor availability following administration of amphetamine or methylphenidate), transporter and receptor availability in cocaine, amphetamine and methamphetamine users and healthy controls.
Results
In majority of the studies the duration of abstinence varied from 5 days to 3 weeks. There was a significant decrease in striatal dopamine release (stimulants combined: Hedge’s g= −0.84; cocaine: −0.87, both p<0.001), dopamine transporter availability (stimulants combined: Hedge’s g= −0.91, p<0.01; amphetamine and methamphetamine: Hedge’s g: −1.47, p<0.001) and D2/3 availability (stimulants combined: Hedge’s g= −0.76; cocaine: −0.73; amphetamine and methamphetamine: −0.81, all p<0.001). We did not find consistent alterations in vesicular monoamine transporter, dopamine synthesis or D1 receptor studies.
Conclusion and relevance
Our data suggest that both pre and post-synaptic aspects of the dopamine system in the striatum are down-regulated in stimulant users. We discuss the commonality and difference between these findings and the discrepancies with the preclinical literature as well as their implications for future drug development.
Introduction
According to World Health Organization estimates amphetamine-like stimulants (predominantly methamphetamine and amphetamine) and cocaine are the second and fourth most common forms of illicit substance abuse respectively (world drug report 2015; https://www.unodc.org). The world-wide prevalence of amphetamine-like stimulant use was estimated at between 0.3-1.1 percent in 2015 (between 13.8 million and 53.8 million users), and for cocaine it was 0.3-0.4 percent of the population aged 15-64 (between 13 million and 20 million users) (world drug report 2015; https://www.unodc.org). Stimulant use is thus a significant burden to society 1. Dopamine dysregulation is hypothesized to underlie addictive behaviour 2–6 and stimulants such as amphetamine and cocaine act on dopamine transporters and increase extracellular dopamine 7–10. Furthermore, preclinical models show that the acute rewarding effects of stimulant drugs are linked to the release of dopamine in the nucleus accumbens measured using micro-dialysis or fast scan cyclic voltammetry 6,11. Positron emission tomography (PET) and single photon emission computed tomography (SPECT) enable us to measure dopaminergic function in-vivo in humans 12. Using these imaging tools, human studies have found that stimulant drugs increase synaptic dopamine levels in the whole striatum (including ventral striatum which includes the nucleus accumbens) and that increases are associated with the subjective perception of drug reward in non-drug abusing controls 13,14. However, determining the dopaminergic effects of stimulants in human stimulant users is essential as the neurobiological mechanisms may be different. A number of studies have investigated dopamine release, dopamine transporter and dopamine receptor levels in stimulant addiction. However, to our knowledge, there has not been a previous meta-analysis of these findings. Thus we aimed to synthesize the PET and SPECT imaging findings on dopaminergic function in cocaine and amphetamine-like (amphetamine and methamphetamine) stimulant addiction and to consider their implications for its treatment. Since both drugs are known to increase extracellular dopamine levels, either by blocking (cocaine) or reversing (amphetamine/methamphetamine) the dopamine transporter, we pooled the data 10. We group findings into studies of dopamine release, dopamine transporter availability, and dopamine receptor availability. We focus on the whole striatum as it is richly innervated with dopaminergic neurons and reliably imaged with PET and SPECT in humans 15.
Methods
Study selection
To be included in the meta-analysis, an article needed to investigate the striatal dopaminergic system in cocaine or amphetamine-like stimulant users (this included amphetamine and methamphetamine) and a control group, including the mean and standard deviations for both groups (see supplementary figures 1 and 2 for the study selection and supplementary methods for further details on the search and inclusion criteria). We focused on amphetamine and methamphetamine as these are the most widely used amphetamine-like drugs.
Data extraction
The main outcome measure was the difference in the dopaminergic imaging index between stimulant users and controls. The following variables were extracted from all the studies: authors, year of publication, subject characteristics of the control and stimulant users group (group size, age, sex, substance use characteristics, comorbid substance abuse, method of abstinence confirmation, duration of abstinence, diagnosis), imaging characteristics (method, radiotracer, scanner type and resolution), route of administration of drug challenge and modelling method.
Data analysis
The main outcome measure was the effect size for the dopaminergic index for the whole striatum in the stimulant users (cocaine and amphetamine-like stimulants studies combined) using a random effects model. Separate secondary meta-analyses were conducted for the studies of dopamine transporter (DAT), dopamine release and dopamine receptor availability in cocaine and amphetamine-like substance users to determine if the effects were consistent across categories of stimulants. Publication bias was assessed using funnel plots as well as regression tests. Heterogeneity was estimated using the I2 value (I2values <50% indicate low to moderate heterogeneity, whereas I2>50% indicate moderate to high heterogeneity). Leave-one-out sensitivity analyses were conducted. A significance level of p<0.05 (2-tailed) was taken as significant (see supplementary materials for further methodological details).
Results
Dopamine release
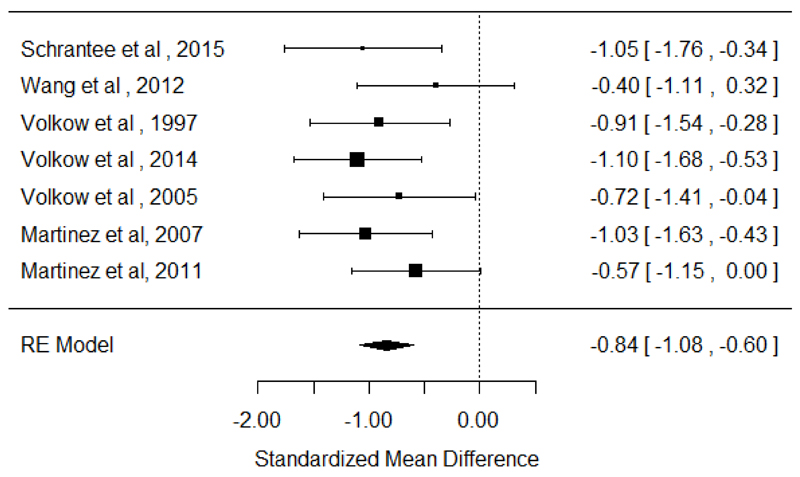
There were 7 studies (5 in cocaine users and 2 in amphetamine-like stimulant users) assessing dopamine release in 164 stimulant users with 139 healthy controls 16–22. The meta-analysis showed a significant reduction in dopamine release in the stimulant users relative to controls with an effect size of −0.84 ([95% confidence interval (CI), −1.08 - −0.60], p <0.001). This was also seen when the meta-analysis was restricted to cocaine users, effect size −0.87 ([95% CI, −1.15 - −0.60], p<0.001). There were too few studies of amphetamine-like stimulant users for a meta-analysis, but the effect sizes in the two studies were in the same direction (standardized mean difference (SMD): −1.05 [95% CI, −1.76 - −0.34] and −0.40 [95% CI, −1.11 - 0.32]) 20,21. The results of heterogeneity and sensitivity analysis are provided in supplementary material (figure 1).
Dopamine transporter
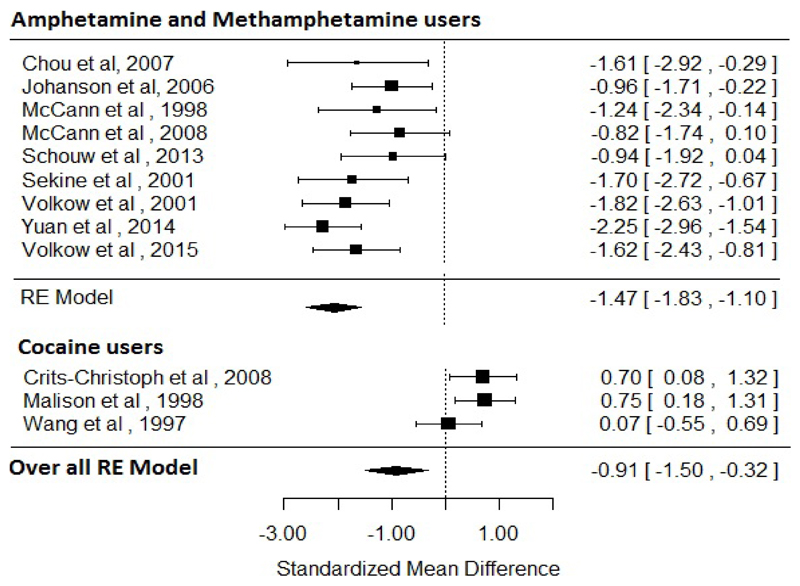
There were 12 studies (3 in cocaine users24–26 and 9 in amphetamine-like stimulant users27–35) assessing dopamine transporter availability in 177 stimulant users and 191 healthy controls. The meta-analysis showed a significant reduction in dopamine transporter availability in the stimulant users relative to controls with an effect size of −0.91 ([95% CI, −1.5 - −0.32], p<0.01). For sub-analysis, there were 9 studies in amphetamine-like stimulant users 27–35 assessing dopamine transporter availability in 108 stimulant users and 126 healthy controls. The meta-analysis showed significantly reduced dopamine transporter availability in amphetamine-like stimulant users (effect size: −1.47 ([95% CI, −1.83 - −1.1], p<0.001). There were 5 studies in cocaine users 24–26,36,37. However, one study was excluded as it included cocaine users with potential CNS co-morbidity (HIV) 36. This left too few studies for a separate meta-analysis in cocaine users. The results of these studies were inconsistent, with the two PET studies (where there was an overlap of samples) showing no significant difference in the DAT availability in cocaine users 26,37, whilst the two SPECT studies reported elevated DAT in cocaine users who were acutely abstinent 24,25. The two SPECT studies in cocaine users had durations of abstinence of a maximum of 4 days25 and mean of 7 days. 24 As such, residual cocaine could block radiotracer binding to DAT, resulting in a slight underestimation of DAT levels in these studies. The results of heterogeneity and sensitivity analysis are provided in supplementary material (figure 2).
Dopamine receptor availability
There were 19 studies assessing dopamine receptor availability in 342 stimulant users and 321 healthy controls (7 studies in amphetamine-like stimulant users 20,21,38–42 and 12 studies in cocaine users 16–19,22,37,43–48).
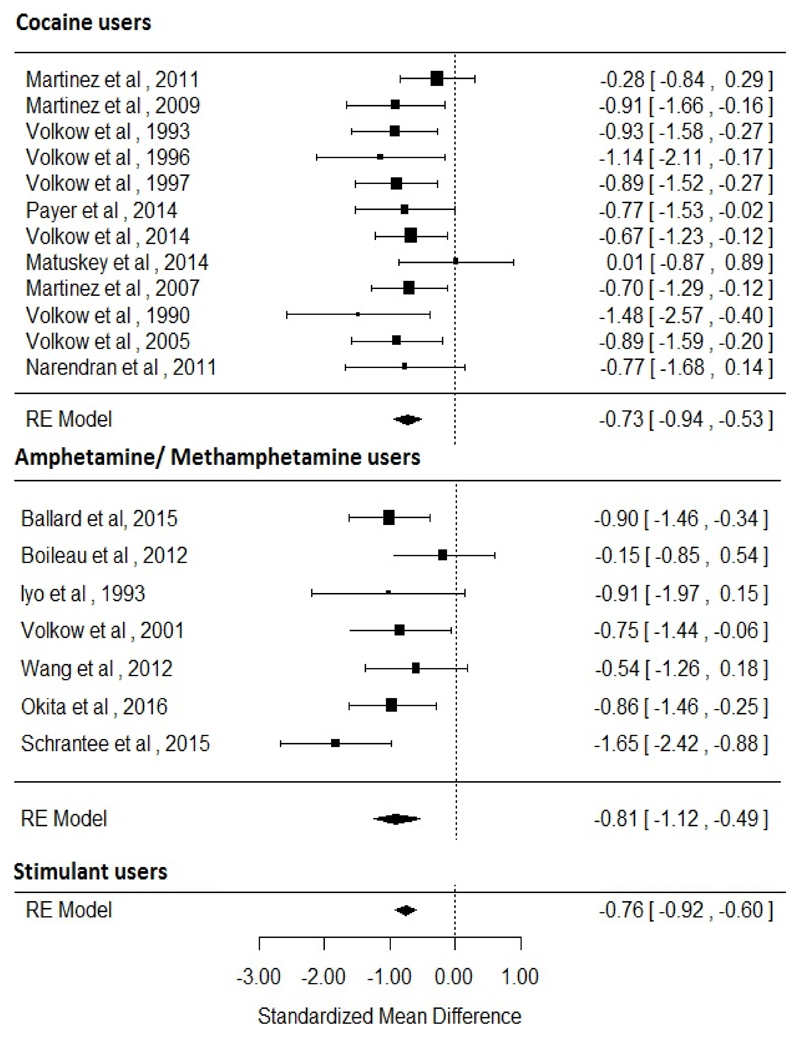
The meta-analysis revealed an overall reduction in D2/3 receptor availability in stimulant users relative to controls with an effect size of −0.76 ([95% CI, −0.92 - −0.6], p<0.001). In the separate analyses a reduction in D2/3 receptor availability was noted in both cocaine users (effect size=−0.73 [95% CI, −0.94 - −0.53], p<0.001) and amphetamine-like stimulant users (effect size=−0.81 [95% CI, −1.12 - −0.49], p<0.001) relative to controls. The results of heterogeneity and sensitivity analysis are provided in supplementary material (figure 3).
Other dopaminergic measures
There was only one study in stimulant users using 6-[18F]fluoro-dihydroxy-phenylalanine ([18F]-DOPA) assessing dopamine synthesis capacity 49. This study showed reduced dopamine synthesis capacity in cocaine users and the estimated effect size was found to be 0.46 [95% CI, −0.46 - 1.39]. We could not find any studies on dopamine synthesis capacity in amphetamine-like stimulant users. Four studies assessed vesicular monoamine transporter -2 (VMAT2) availability, with inconsistent findings. Two studies showed significantly reduced VMAT2 availability, one in cocaine users with two weeks of abstinence [Hedge’s g: 1.6, 95% CI: 0.68 - 2.52] 50, and the other in methamphetamine abusers after three months of abstinence [Hedge’s g: 1.68, 95% CI: 0.86 - 2.5] 28. However, two studies in recently abstinent methamphetamine users (mean duration of abstinence: 2.6 days and 19 days) showed elevated VMAT2 levels [Hedge’s g: 1.16, 95% CI: 0.56 - 1.76] 51,52. Moreover, given that metamphetamine interacts with VMAT2 at the same site as the PET tracers and the relatively short duration of abstinence, it is possible that VMAT2 levels were underestimated in some subjects.
There was only one study on stimulant users and D1 receptors, which used [11C] NNC112 to compare cocaine abusers with controls 53. Though there were no differences in D1 receptors between groups, the availability of D1 receptors in cocaine abusers was negatively associated with the choice to self-administer cocaine by the cocaine abusers
Discussion
To our knowledge this is first meta-analysis investigating the nature of dopaminergic dysfunction in stimulant users. Our main findings are that dopamine release, transporter levels and D2/3 receptor availability are all lower in vivo in stimulant users compared to healthy controls with large to very large effect sizes (Hedge’s g: −0.84, −0.91 and −0.76 respectively). This indicates that there is a generalised down-regulation of the dopaminergic system in stimulant users, as summarised in Figure 4. Our sensitivity analyses of the dopamine D2/3 receptor availability and dopamine release findings showed consistent results and we noted low heterogeneity across studies of cocaine and amphetamine-like drugs, and across differing radiotracers and techniques. However, there was a difference between results in amphetamine/methamphetamine and cocaine drug users in dopamine transporter availability. In amphetamine/methamphetamine users there were large and consistent reductions in dopamine transporter availability. In contrast for cocaine users, despite the limited number of studies preventing sub-analysis, there were two studies that showed no difference 26,54 and two other studies that reported elevated dopamine transporter availability, both in acutely abstinent cocaine users 24,25. This may point to a mechanistic difference between the effects of amphetamine-like drugs and cocaine on dopamine transporters, consistent with preclinical findings55, and highlights the need for more studies in cocaine users. Cocaine is known to primarily act by blocking DAT, while amphetamine competitively inhibits dopamine re-uptake at DAT, and increases DAT mediated reverse-transport of dopamine from the cytoplasm into the synaptic cleft independent of action potential evoked vesicular release.7–9 It has also been suggested that amphetamine’s actions depend on its concentration, with it acting primarily as a DAT blocker at low concentrations and reversing dopamine transport at high concentrations.7 In addition, amphetamine-like stimulants are known to trigger internalization of plasmalemmal DAT.56 Finally, cocaine, amphetamine and methamphetamine are also known to act on serotonin and norepinephrine transporters, although their affinities for these are different. 57 Given these pharmacological differences between stimulants, there could be differences in dopaminergic effects between stimulants that are masked by pooling studies.
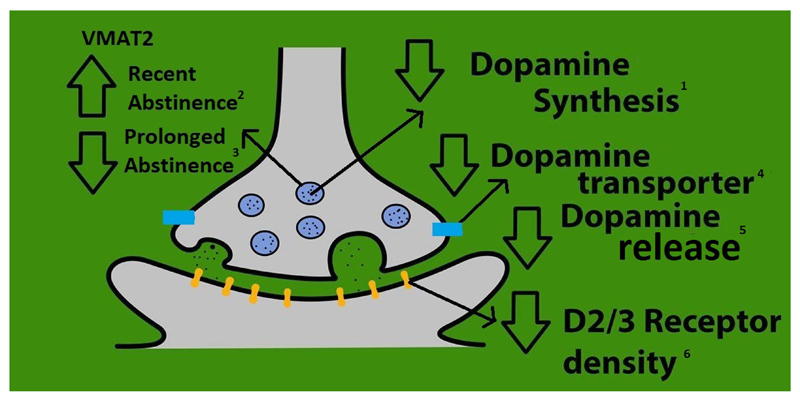
Figure 4. A summary of dopaminergic alterations in stimulant users.
Summary of the synaptic location of the major dopaminergic findings from our meta-analyses and findings from studies of other aspects of the dopamine system.
1. Based on study by Wu et al. 1997
2. Based on study by Boileau et al. 2008 & 2015
3. Based on study by Narendran et al. 2012 and Johanson et al. 2006
4. Meta-analysis finding with effect size of −0.81 [95% CI, −1.34 - −0.29], p<0.01
5. Meta-analysis finding with effect size of −0.91 [95% CI, −1.50 - −0.32], p<0.01
6. Meta-analysis finding with effect size of −0.71 [95% CI, −0.91 - −0.52], p<0.001
There are some specific issues that affect interpretation of the results. For studies of D2/3 receptors, the tracers generally used do not distinguish between D2 and D3 receptors, or between the high and low affinity forms of the D2 receptor, so the reduction could reflect a change in one, or more of these. However, two studies used [11C]-PHNO, which is selective for the D2 high affinity form and shows a higher affinity for D3 receptors over D2 receptors. These studies did not show significant differences between stimulant users and controls in the striatum 44,46. This could suggest that the reduction seen in our meta-analysis reflects a reduction in the low affinity form of the D2 receptor availability. Also, given that the radiotracers used to measure D2/3 receptor availability are sensitive to endogenous dopamine levels 58, one possible interpretation of our finding of reduced D2/3 receptor levels is that this reflects elevated synaptic dopamine levels. However, a dopamine depletion study in cocaine users has shown that baseline synaptic levels are also reduced43. This indicates that the reduction in D2/3 receptor availability represents a reduction in D2/3 receptor levels. Furthermore, when our findings are taken with the observation of reductions in synaptic dopamine levels 43, and dopamine synthesis capacity 49, they suggest there is a generalised reduction in presynaptic dopaminergic activity. Although, with the available data we could not specifically rule out the possibility of upregulation D3 receptor.
Limitations
In common with other meta-analyses of psychiatric imaging studies 59–63, there are variations between studies in terms of both the sample characteristics, such as the inclusion of current or abstinent users, co-morbid use of other substances such as nicotine and alcohol and variations in the durations of abstinence, and methods, in particular in the radiotracer used and delineation of the striatum (see supplementary discussion).
Nevertheless, there was low heterogeneity across the analyses, with the exception of the dopamine transporter, and the random effects model we used allows for variations in effects. Furthermore, if anything, these variations between studies would obscure rather than account for the effects we observed. A general limitation of the literature, apparent in the funnel plots, is that there are few studies with large sample sizes. It should also be noted that there have been a relatively small number of studies on dopamine release and we could not investigate potential differences between oral and intravenous routes of drug challenge to elicit dopamine release. Although, in absolute terms the oral challenge studies showed lower release than those using an intravenous route, both indicated blunted release in stimulant users compared to controls.
Implications for understanding stimulant misuse and dependence
Preclinical studies using in vivo micro-dialysis and chronoamperometry conclusively demonstrate that acute administration of stimulants increases extra-cellular dopamine concentrations in the striatum, and nucleus accumbens. 11 Furthermore, in vivo fast-scan cyclic voltammetry and implantable micro-sensor studies, which are able to quantify the dopamine signalling over a sub-second timescale, have shown that stimulants increase phasic dopamine release 6,11, and human in vivo imaging studies also show evidence consistent with acute exposure of stimulants leading to increased synaptic dopamine either through cue induced dopamine release or blockade of DAT, and that this is linked to subjective ‘high’ 54 and craving66,67. Moreover, change in in vivo dopamine imaging indices, following amphetamine administration, has been shown to be directly related to change in microdialysis measures 68, providing convergence across methods. Thus there is consistency between the preclinical and clinical findings indicating that acute administration of stimulants results in increased extra-cellular dopamine either by stimulating release (amphetamines) or by DAT blockade (cocaine).
Our meta-analysis shows a consistent reduction in dopamine release in people who have been exposed to chronic stimulants. In contrast, preclinical models of chronic use are inconsistent, with some studies showing no change in basal dopamine output after withdrawal of chronic amphetamine 69–72 and cocaine 73–77, whilst others have reported increases in dopamine output after cocaine withdrawal 78–81. The first major implication of our meta-analysis is thus that the findings in many preclinical models of chronic use do not reflect what is seen in the human studies. This suggests caution in extrapolating from preclinical models, and may explain the failure to develop treatments for stimulant addiction based on them. There are a number of potential explanations for this inconsistency, including differences in the dosing regimens and durations used in preclinical models relative to human usage patterns. Nevertheless, this discrepancy suggests we need to develop new preclinical models that reproduce the dopaminergic changes seen in the human condition.
Our findings are also striking in showing reductions in both presynaptic and post-synaptic aspects of the dopaminergic system, suggesting a generalised down-regulation. One potential explanation for the reduction in dopamine release and transporter availability (seen in amphetamine/methamphetamine users only) could be a loss of dopamine neurons per se or damage to the dopaminergic terminals. There is evidence that both cocaine and amphetamines induce apoptosis as indexed by activation of caspases, loss of mitochondrial potential, cytochrome c release and oxidative stress. 82 However, in addition to this, amphetamine and methamphetamine induce dopaminergic neuron damage through the formation of quinones and free radicals. 83–85 Moreover, preclinical models with methamphetamine have shown evidence of dopamine terminal damage that recovers with detoxification 86,87. Furthermore, in humans, dopamine transporters recover with detoxification in methamphetamine abusers (reviewed in 5,35), which was interpreted to indicate that dopamine neurons were not lost. Moreover in the only post-mortem study we could find, which was in methamphetamine abusers, there was evidence of reduction in dopamine transporters but not of dopamine neuronal loss 88. However preliminary evidence from two recent epidemiological studies that methamphetamine abuse might increase the risk for Parkinson’s disease 89,90 suggests that in some cases its abuse might accelerate age-associated dopamine neuronal degeneration 91.
It has been suggested that repeated drug use causes tolerance by various mechanisms including dopamine receptor alterations, changes in second messenger systems and altered regulation of dopamine neuron function.92–94 Thus, it is possible that the dopaminergic differences noted in our meta-analysis could be due to the development of tolerance through one or more of these mechanisms. Pre-clinical studies have also demonstrated that dopaminergic synaptic transmission is modulated by glutamatergic and GABAergic neurons94. Neuroimaging studies investigating these interactions are needed to determine if this is the case in humans.
Two alternative basic models are possible to account for both our pre and post-synaptic findings. The first is that repeated stimulant use results in adaptive changes in the dopamine system that lead to reduced firing of dopamine neurons, potentially similar to the depolarisation blockade that is seen after a period of repeated firing 95, and consequently reduced dopamine synthesis and release. In this context, reduced transporter levels may be compensatory in response to reduced tonic dopamine levels in the synapse. The reduction in D2/3 receptor levels is less easy to understand in the context of the presynaptic reductions. However, D2/3 receptors undergo internalisation following activation by dopamine, and this would reduce radiotracer binding, at least to a number of the tracers used in the studies in our analyses 96,97. Thus the reduction in D2/3 receptor availability could reflect a compensatory increase in internalized D2/3 receptors, which would reduce the number of D2/3 receptors available to bind to dopamine. Repeated exposure may lead to loss of these internalized receptors and long-term transcriptional changes that reduce receptor availability.
The second model is that reductions underlie the pathoetiology of stimulant misuse, and precede its onset. Thus individuals at risk of stimulant misuse may have reductions in dopamine release, transporter levels and D2/3 receptor levels secondary to genetic and/or environmental risk factors. Reductions in D2/3 receptor levels and reduced release of dopamine to stimulants could mean an individual is less sensitive to the effects of taking a stimulant, leading to escalating use. However, it is less easy to see how reduced dopamine transporter levels fit with this model, as they would be anticipated to prolong the effects of stimulants. Longitudinal studies on the effects of stimulant drugs on patients with attention deficit disorder showed downregulation of dopamine release with chronic exposure 98, which indicates that some of the changes are driven by chronic drug exposures.
Finally, a hybrid model may best account for our findings. Evidence suggests that reduced D2/3 receptor levels may precede and predispose to the onset of stimulant misuse but also show further reductions during stimulant use 99, and similar effects may be seen with dopamine release and transporter levels. It is interesting to note in our meta-analysis that dopaminergic alterations are marked even in the studies of several months abstinence, with evidence suggesting that dopamine receptor density and release are down-regulated even after 9 months of abstinence 35. This suggests that effects may persist, with implications for understanding relapse. Our findings also support the opponent process model 100.
This highlights one fundamental issue raised by our meta-analysis, namely that current findings do not address the temporal relationship between down-regulation in the dopamine system and phase of addiction. Future longitudinal human PET studies as well as preclinical studies that investigate changes in the dopamine system prior to and during stimulant misuse-, and following abstinence are needed to test these models (Box 1; supplementary material). In addition this will help to identify biomarkers to guide treatment and predict outcomes.
Clinical implications of our findings
Our data identify a number of clear targets for treatment interventions. D2/3 receptors stand-out, given our finding of a large effect size reduction. This is further supported by studies in cocaine and in methamphetamine users showing that lower dopamine D2/3 receptor availability at baseline predicts relapse following treatment 19 21. Our data also provide a rationale for the development of drugs that target the presynaptic dopaminergic system to restore tonic striatal dopamine release, which is necessary for the function of the striato-cortical indirect pathway (a key system disrupted in addiction) 5. This is supported by recent preclinical evidence showing that administration of the dopamine precursor L-DOPA restored the aberrant dopaminergic signalling in a cocaine addiction animal model 6,100, and preliminary clinical evidence that inhibiting dopamine reuptake (e.g., with bupropion, modafinil, or mazindol), or inhibiting dopamine metabolism (e.g., with selegiline or disulfiram) might hold some promise in the treatment for stimulant addiction 101. Strategies to upregulate striatal D2 receptors have been shown in animal models to protect against compulsive stimulant drug intake 102 and, thus, interventions that lead to D2 up-regulation, such as physical activity as recently shown in a preliminary study in methamphetamine abusers 103, merit further investigation. A number of dopaminergic treatments have been tried to treat stimulant use disorder with limited success to date.104–106 Our data suggest some potential explanations for the lack of success and identify a number of clear targets for treatment interventions. D2/3 receptors stand-out, given our finding of a large effect size reduction. This is further supported by studies in cocaine and in methamphetamine users showing that lower dopamine D2/3 receptor availability at baseline predicts relapse following treatment 19 21. Our findings may also explain why strategies to block dopamine neurotransmission, for example using dopamine receptor antagonists107, have largely been disappointing to date as dopamine receptor levels are already low, and suggest that strategies to increase dopamine receptor levels or sensitivity could have potential.
Conclusions
There is robust evidence for down-regulated presynaptic and post-synaptic dopamine function in stimulant addiction with large effect sizes. These findings suggest that drug development should target the restoration of dopaminergic function as a target for the treatment of stimulant addiction.
Supplementary Material
Figure 1. Studies of dopamine release in stimulant users. The forest plot shows the effect sizes estimated using a random effects model and 95% confidence intervals of the percentage change in the difference of change in D2/3 binding after challenge. There was an overall decrease in dopamine release in stimulant users relative to controls with a large to very large effect size (−0.84 [95% CI, −1.08 - −0.60], p<0.001).
Schrantee et al. 2015 and Wang et al. 2012 studied amphetamine-like stimulant users. The remaining studies included cocaine users.
Figure 2. Studies of dopamine transporter availability. The forest plot shows the effects sizes estimated using a random effects model and 95% confidence intervals of the difference between amphetamine/methamphetamine users and controls. There was an overall decrease in the dopamine transporter availability in methamphetamine users relative to controls (−0.91 [95% CI, −1.5 - −0.32], p <0.01).
Figure 3. Studies of dopamine receptor availability. The forest plot shows the effect sizes estimated using a random effects model and 95% confidence intervals of D2/3 receptor binding potentials. There was an overall decrease in dopamine receptor availability compared to controls (−0.76 [95% CI, −0.92 - −0.60], p<0.001).
Acknowledgments Statement
Conflict of Interest Disclosure: Dr Ashok conducts research funded by the Medical Research Council (UK) and King’s College London. Dr Mizuno has received manuscript fees or speaker's honoraria from Sumitomo Dainippon Pharma, Astellas, and Yoshitomi Yakuhin, fellowship grants from the Japanese Society of Clinical Neuropsychopharmacology (Eli Lilly Fellowship for Clinical Psychopharmacology) and the Mochida Memorial Foundation for Medical and Pharmaceutical Research, and consultant fees from Bracket within the past three years. Prof Volkow is Director of the National Institute on Drug Abuse (USA) and conducts research as an intramural scientist that is funded by the National Institute of Alcohol Abuse and Alcoholism (USA). Prof Howes conducts research funded by the Medical Research Council (UK), the National Institute of Health Research (UK) and the Maudsley Charity. Prof Howes has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organized by Astra-Zeneca, BMS, Eli Lilly, Jansenn, Lundbeck, Lyden-Delta, Servier, and Roche. Neither Prof Howes or his family have been employed by or have holdings/a financial stake in any biomedical company.
Funding/Support: This study was funded by grants MC-A656-5QD30 from the Medical Research Council-UK, 666 from the Maudsley Charity 094849/Z/10/Z from the Brain and Behavior Research Foundation, and Wellcome Trust to Dr Howes and King’s College London scholarship to Dr Ashok.
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions:
Dr Ashok and Dr Mizuno had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Ashok, Howes
Acquisition, analysis, or interpretation of data: All authors
Drafting of the manuscript: All authors
Critical revision of the manuscript for important intellectual content: All authors
Statistical analysis: Ashok
Administrative, technical, or material support: Ashok and Mizuno
Study supervision: Howes and Volkow
References
- 1.Degenhardt L, Hall W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet (London, England) 2012;379(9810):55–70. doi: 10.1016/S0140-6736(11)61138-0. [DOI] [PubMed] [Google Scholar]
- 2.Di Chiara G, Bassareo V. Reward system and addiction: what dopamine does and doesn't do. Current opinion in pharmacology. 2007;7(1):69–76. doi: 10.1016/j.coph.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Keiflin R, Janak PH. Dopamine Prediction Errors in Reward Learning and Addiction: From Theory to Neural Circuitry. Neuron. 2015;88(2):247–263. doi: 10.1016/j.neuron.2015.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR. The dopamine theory of addiction: 40 years of highs and lows. Nature reviews Neuroscience. 2015;16(5):305–312. doi: 10.1038/nrn3939. [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162(4):712–725. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Willuhn I, Burgeno LM, Groblewski PA, Phillips PE. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. 2014;17(5):704–709. doi: 10.1038/nn.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calipari ES, Ferris MJ. Amphetamine mechanisms and actions at the dopamine terminal revisited. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(21):8923–8925. doi: 10.1523/JNEUROSCI.1033-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daberkow DP, Brown HD, Bunner KD, et al. Amphetamine paradoxically augments exocytotic dopamine release and phasic dopamine signals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(2):452–463. doi: 10.1523/JNEUROSCI.2136-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annual review of pharmacology and toxicology. 2007;47:681–698. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 10.Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. European journal of pharmacology. 2003;479(1-3):153–158. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 11.Willuhn I, Wanat MJ, Clark JJ, Phillips PE. Dopamine signaling in the nucleus accumbens of animals self-administering drugs of abuse. Current topics in behavioral neurosciences. 2010;3:29–71. doi: 10.1007/7854_2009_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim E, Howes OD, Kapur S. Molecular imaging as a guide for the treatment of central nervous system disorders. Dialogues in clinical neuroscience. 2013;15(3):315–328. doi: 10.31887/DCNS.2013.15.3/ekim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laruelle M, Abi-Dargham A, van Dyck CH, et al. SPECT imaging of striatal dopamine release after amphetamine challenge. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1995;36(7):1182–1190. [PubMed] [Google Scholar]
- 14.Volkow ND, Wang GJ, Fowler JS, et al. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. The Journal of pharmacology and experimental therapeutics. 1999;291(1):409–415. [PubMed] [Google Scholar]
- 15.Egerton A, Demjaha A, McGuire P, Mehta MA, Howes OD. The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage. 2010;50(2):524–531. doi: 10.1016/j.neuroimage.2009.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volkow ND, Wang GJ, Fowler JS, et al. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386(6627):830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- 17.Volkow ND, Wang GJ, Ma Y, et al. Activation of orbital and medial prefrontal cortex by methylphenidate in cocaine-addicted subjects but not in controls: relevance to addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25(15):3932–3939. doi: 10.1523/JNEUROSCI.0433-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martinez D, Narendran R, Foltin RW, et al. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. The American journal of psychiatry. 2007;164(4):622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- 19.Martinez D, Carpenter KM, Liu F, et al. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. The American journal of psychiatry. 2011;168(6):634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrantee A, Vaclavu L, Heijtel DF, et al. Dopaminergic system dysfunction in recreational dexamphetamine users. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015;40(5):1172–1180. doi: 10.1038/npp.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang GJ, Smith L, Volkow ND, et al. Decreased dopamine activity predicts relapse in methamphetamine abusers. Molecular psychiatry. 2012;17(9):918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Volkow ND, Tomasi D, Wang GJ, et al. Stimulant-induced dopamine increases are markedly blunted in active cocaine abusers. Molecular psychiatry. 2014;19(9):1037–1043. doi: 10.1038/mp.2014.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horne MK, Cheng CH, Wooten GF. The cerebral metabolism of L-dihydroxyphenylalanine. An autoradiographic and biochemical study. Pharmacology. 1984;28(1):12–26. doi: 10.1159/000137938. [DOI] [PubMed] [Google Scholar]
- 24.Crits-Christoph P, Newberg A, Wintering N, et al. Dopamine transporter levels in cocaine dependent subjects. Drug and alcohol dependence. 2008;98(1-2):70–76. doi: 10.1016/j.drugalcdep.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malison RT, Best SE, van Dyck CH, et al. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. The American journal of psychiatry. 1998;155(6):832–834. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- 26.Wang GJ, Volkow ND, Fowler JS, et al. Cocaine abusers do not show loss of dopamine transporters with age. Life sciences. 1997;61(11):1059–1065. doi: 10.1016/s0024-3205(97)00614-0. [DOI] [PubMed] [Google Scholar]
- 27.Chou YH, Huang WS, Su TP, Lu RB, Wan FJ, Fu YK. Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: A SPECT study. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2007;17(1):46–52. doi: 10.1016/j.euroneuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Johanson CE, Frey KA, Lundahl LH, et al. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology. 2006;185(3):327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- 29.McCann UD, Kuwabara H, Kumar A, et al. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse (New York, NY) 2008;62(2):91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- 30.McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18(20):8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schouw ML, Caan MW, Geurts HM, et al. Monoaminergic dysfunction in recreational users of dexamphetamine. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2013;23(11):1491–1502. doi: 10.1016/j.euroneuro.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Sekine Y, Iyo M, Ouchi Y, et al. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. The American journal of psychiatry. 2001;158(8):1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- 33.Volkow ND, Chang L, Wang GJ, et al. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(23):9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan J, Lv R, Robert Brasic J, et al. Dopamine transporter dysfunction in Han Chinese people with chronic methamphetamine dependence after a short-term abstinence. Psychiatry research. 2014;221(1):92–96. doi: 10.1016/j.pscychresns.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Volkow ND, Wang GJ, Smith L, et al. Recovery of dopamine transporters with methamphetamine detoxification is not linked to changes in dopamine release. NeuroImage. 2015;121:20–28. doi: 10.1016/j.neuroimage.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Chang L, Wang GJ, Volkow ND, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42(2):869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkow ND, Wang GJ, Fowler JS, et al. Cocaine uptake is decreased in the brain of detoxified cocaine abusers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1996;14(3):159–168. doi: 10.1016/0893-133X(95)00073-M. [DOI] [PubMed] [Google Scholar]
- 38.Okita K, Ghahremani DG, Payer DE, et al. Emotion dysregulation and amygdala dopamine D2-type receptor availability in methamphetamine users. Drug and alcohol dependence. 2016;161:163–170. doi: 10.1016/j.drugalcdep.2016.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ballard ME, Mandelkern MA, Monterosso JR, et al. Low Dopamine D2/D3 Receptor Availability is Associated with Steep Discounting of Delayed Rewards in Methamphetamine Dependence. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum (CINP) 2015;18(7):pyu119. doi: 10.1093/ijnp/pyu119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boileau I, Payer D, Houle S, et al. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(4):1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volkow ND, Chang L, Wang GJ, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. The American journal of psychiatry. 2001;158(12):2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 42.Iyo M, Nishio M, Itoh T, et al. Dopamine D2 and serotonin S2 receptors in susceptibility to methamphetamine psychosis detected by positron emission tomography. Psychiatry research. 1993;50(4):217–231. doi: 10.1016/0925-4927(93)90002-y. [DOI] [PubMed] [Google Scholar]
- 43.Martinez D, Greene K, Broft A, et al. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. The American journal of psychiatry. 2009;166(10):1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matuskey D, Gallezot JD, Pittman B, et al. Dopamine D(3) receptor alterations in cocaine-dependent humans imaged with [(1)(1)C](+)PHNO. Drug and alcohol dependence. 2014;139:100–105. doi: 10.1016/j.drugalcdep.2014.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narendran R, Martinez D, Mason NS, et al. Imaging of dopamine D2/3 agonist binding in cocaine dependence: a [11C]NPA positron emission tomography study. Synapse (New York, NY) 2011;65(12):1344–1349. doi: 10.1002/syn.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Payer DE, Behzadi A, Kish SJ, et al. Heightened D3 dopamine receptor levels in cocaine dependence and contributions to the addiction behavioral phenotype: a positron emission tomography study with [11C]-+-PHNO. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39(2):311–318. doi: 10.1038/npp.2013.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkow ND, Fowler JS, Wang GJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse (New York, NY) 1993;14(2):169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 48.Volkow ND, Fowler JS, Wolf AP, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. The American journal of psychiatry. 1990;147(6):719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- 49.Wu JC, Bell K, Najafi A, et al. Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;17(6):402–409. doi: 10.1016/S0893-133X(97)00089-4. [DOI] [PubMed] [Google Scholar]
- 50.Narendran R, Lopresti BJ, Martinez D, et al. In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers. The American journal of psychiatry. 2012;169(1):55–63. doi: 10.1176/appi.ajp.2011.11010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boileau I, Rusjan P, Houle S, et al. Increased vesicular monoamine transporter binding during early abstinence in human methamphetamine users: Is VMAT2 a stable dopamine neuron biomarker? The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(39):9850–9856. doi: 10.1523/JNEUROSCI.3008-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boileau I, McCluskey T, Tong J, Furukawa Y, Houle S, Kish SJ. Rapid Recovery of Vesicular Dopamine Levels in Methamphetamine Users in Early Abstinence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez D, Slifstein M, Narendran R, et al. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2009;34(7):1774–1782. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Volkow ND, Wang GJ, Fischman MW, et al. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997;386(6627):827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- 55.Zahniser NR, Sorkin A. Rapid regulation of the dopamine transporter: role in stimulant addiction? Neuropharmacology. 2004;47(Suppl 1):80–91. doi: 10.1016/j.neuropharm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 56.Schmitt KC, Reith ME. Regulation of the dopamine transporter: aspects relevant to psychostimulant drugs of abuse. Annals of the New York Academy of Sciences. 2010;1187:316–340. doi: 10.1111/j.1749-6632.2009.05148.x. [DOI] [PubMed] [Google Scholar]
- 57.Han DD, Gu HH. Comparison of the monoamine transporters from human and mouse in their sensitivities to psychostimulant drugs. BMC pharmacology. 2006;6:6. doi: 10.1186/1471-2210-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Egerton A, Mehta MA, Montgomery AJ, et al. The dopaminergic basis of human behaviors: A review of molecular imaging studies. Neuroscience and biobehavioral reviews. 2009;33(7):1109–1132. doi: 10.1016/j.neubiorev.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Howes OD, Kambeitz J, Kim E, et al. The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Archives of general psychiatry. 2012;69(8):776–786. doi: 10.1001/archgenpsychiatry.2012.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen KC, Yang YK, Howes O, et al. Striatal dopamine transporter availability in drug-naive patients with schizophrenia: a case-control SPECT study with [(99m)Tc]-TRODAT-1 and a meta-analysis. Schizophrenia bulletin. 2013;39(2):378–386. doi: 10.1093/schbul/sbr163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kambeitz J, Abi-Dargham A, Kapur S, Howes OD. Alterations in cortical and extrastriatal subcortical dopamine function in schizophrenia: systematic review and meta-analysis of imaging studies. The British journal of psychiatry : the journal of mental science. 2014;204(6):420–429. doi: 10.1192/bjp.bp.113.132308. [DOI] [PubMed] [Google Scholar]
- 62.Selvaraj S, Arnone D, Cappai A, Howes O. Alterations in the serotonin system in schizophrenia: a systematic review and meta-analysis of postmortem and molecular imaging studies. Neuroscience and biobehavioral reviews. 2014;45:233–245. doi: 10.1016/j.neubiorev.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 63.Kambeitz JP, Howes OD. The serotonin transporter in depression: Meta-analysis of in vivo and post mortem findings and implications for understanding and treating depression. Journal of affective disorders. 2015;186:358–366. doi: 10.1016/j.jad.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 64.Tziortzi AC, Searle GE, Tzimopoulou S, et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: dissection of D3 signal and anatomy. NeuroImage. 2011;54(1):264–277. doi: 10.1016/j.neuroimage.2010.06.044. [DOI] [PubMed] [Google Scholar]
- 65.Gallezot JD, Beaver JD, Gunn RN, et al. Affinity and selectivity of [(1)(1)C]-(+)-PHNO for the D3 and D2 receptors in the rhesus monkey brain in vivo. Synapse (New York, NY) 2012;66(6):489–500. doi: 10.1002/syn.21535. [DOI] [PubMed] [Google Scholar]
- 66.Wong DF, Kuwabara H, Schretlen DJ, et al. Increased occupancy of dopamine receptors in human striatum during cue-elicited cocaine craving. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(12):2716–2727. doi: 10.1038/sj.npp.1301194. [DOI] [PubMed] [Google Scholar]
- 67.Volkow ND, Wang GJ, Telang F, et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(24):6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schiffer WK, Volkow ND, Fowler JS, Alexoff DL, Logan J, Dewey SL. Therapeutic doses of amphetamine or methylphenidate differentially increase synaptic and extracellular dopamine. Synapse (New York, NY) 2006;59(4):243–251. doi: 10.1002/syn.20235. [DOI] [PubMed] [Google Scholar]
- 69.Crippens D, Camp DM, Robinson TE. Basal extracellular dopamine in the nucleus accumbens during amphetamine withdrawal: a 'no net flux' microdialysis study. Neuroscience letters. 1993;164(1–2):145–148. doi: 10.1016/0304-3940(93)90878-o. [DOI] [PubMed] [Google Scholar]
- 70.Crippens D, Robinson TE. Withdrawal from morphine or amphetamine: different effects on dopamine in the ventral-medial striatum studied with microdialysis. Brain research. 1994;650(1):56–62. doi: 10.1016/0006-8993(94)90206-2. [DOI] [PubMed] [Google Scholar]
- 71.Paulson PE, Robinson TE. Regional differences in the effects of amphetamine withdrawal on dopamine dynamics in the striatum. Analysis of circadian patterns using automated on-line microdialysis. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1996;14(5):325–337. doi: 10.1016/0893-133X(95)00141-Y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Segal DS, Kuczenski R. Repeated cocaine administration induces behavioral sensitization and corresponding decreased extracellular dopamine responses in caudate and accumbens. Brain research. 1992;577(2):351–355. doi: 10.1016/0006-8993(92)90297-m. [DOI] [PubMed] [Google Scholar]
- 73.Hooks MS, Duffy P, Striplin C, Kalivas PW. Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology. 1994;115(1–2):265–272. doi: 10.1007/BF02244782. [DOI] [PubMed] [Google Scholar]
- 74.Kalivas PW, Duffy P. Time course of extracellular dopamine and behavioral sensitization to cocaine. I. Dopamine axon terminals. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1993;13(1):266–275. doi: 10.1523/JNEUROSCI.13-01-00266.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kuczenski R, Segal DS, Todd PK. Behavioral sensitization and extracellular dopamine responses to amphetamine after various treatments. Psychopharmacology. 1997;134(3):221–229. doi: 10.1007/s002130050445. [DOI] [PubMed] [Google Scholar]
- 76.Meil WM, Roll JM, Grimm JW, Lynch AM, See RE. Tolerance-like attenuation to contingent and noncontingent cocaine-induced elevation of extracellular dopamine in the ventral striatum following 7 days of withdrawal from chronic treatment. Psychopharmacology. 1995;118(3):338–346. doi: 10.1007/BF02245964. [DOI] [PubMed] [Google Scholar]
- 77.Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain research. 1988;462(2):211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- 78.Heidbreder CA, Thompson AC, Shippenberg TS. Role of extracellular dopamine in the initiation and long-term expression of behavioral sensitization to cocaine. The Journal of pharmacology and experimental therapeutics. 1996;278(2):490–502. [PubMed] [Google Scholar]
- 79.Imperato A, Mele A, Scrocco MG, Puglisi-Allegra S. Chronic cocaine alters limbic extracellular dopamine. Neurochemical basis for addiction. European journal of pharmacology. 1992;212(2–3):299–300. doi: 10.1016/0014-2999(92)90349-9. [DOI] [PubMed] [Google Scholar]
- 80.Johnson DW, Glick SD. Dopamine release and metabolism in nucleus accumbens and striatum of morphine-tolerant and nontolerant rats. Pharmacology, biochemistry, and behavior. 1993;46(2):341–347. doi: 10.1016/0091-3057(93)90362-w. [DOI] [PubMed] [Google Scholar]
- 81.Weiss F, Paulus MP, Lorang MT, Koob GF. Increases in extracellular dopamine in the nucleus accumbens by cocaine are inversely related to basal levels: effects of acute and repeated administration. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1992;12(11):4372–4380. doi: 10.1523/JNEUROSCI.12-11-04372.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cunha-Oliveira T, Rego AC, Oliveira CR. Cellular and molecular mechanisms involved in the neurotoxicity of opioid and psychostimulant drugs. Brain research reviews. 2008;58(1):192–208. doi: 10.1016/j.brainresrev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 83.LaVoie MJ, Hastings TG. Dopamine quinone formation and protein modification associated with the striatal neurotoxicity of methamphetamine: evidence against a role for extracellular dopamine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1999;19(4):1484–1491. doi: 10.1523/JNEUROSCI.19-04-01484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tata DA, Yamamoto BK. Interactions between methamphetamine and environmental stress: role of oxidative stress, glutamate and mitochondrial dysfunction. Addiction (Abingdon, England) 2007;102(Suppl 1):49–60. doi: 10.1111/j.1360-0443.2007.01770.x. [DOI] [PubMed] [Google Scholar]
- 85.De Vito MJ, Wagner GC. Methamphetamine-induced neuronal damage: a possible role for free radicals. Neuropharmacology. 1989;28(10):1145–1150. doi: 10.1016/0028-3908(89)90130-5. [DOI] [PubMed] [Google Scholar]
- 86.Harvey DC, Lacan G, Tanious SP, Melega WP. Recovery from methamphetamine induced long-term nigrostriatal dopaminergic deficits without substantia nigra cell loss. Brain research. 2000;871(2):259–270. doi: 10.1016/s0006-8993(00)02439-2. [DOI] [PubMed] [Google Scholar]
- 87.Melega WP, Jorgensen MJ, Lacan G, et al. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(6):1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- 88.Wilson JM, Kalasinsky KS, Levey AI, et al. Striatal dopamine nerve terminal markers in human, chronic methamphetamine users. Nature medicine. 1996;2(6):699–703. doi: 10.1038/nm0696-699. [DOI] [PubMed] [Google Scholar]
- 89.Callaghan RC, Cunningham JK, Sykes J, Kish SJ. Increased risk of Parkinson's disease in individuals hospitalized with conditions related to the use of methamphetamine or other amphetamine-type drugs. Drug and alcohol dependence. 2012;120(1–3):35–40. doi: 10.1016/j.drugalcdep.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 90.Curtin K, Fleckenstein AE, Robison RJ, Crookston MJ, Smith KR, Hanson GR. Methamphetamine/amphetamine abuse and risk of Parkinson's disease in Utah: a population-based assessment. Drug and alcohol dependence. 2015;146:30–38. doi: 10.1016/j.drugalcdep.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Collier TJ, Kanaan NM, Kordower JH. Ageing as a primary risk factor for Parkinson's disease: evidence from studies of non-human primates. Nature reviews Neuroscience. 2011;12(6):359–366. doi: 10.1038/nrn3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nestler EJ. Epigenetic mechanisms of drug addiction. Neuropharmacology. 2014;76 Pt B:259–268. doi: 10.1016/j.neuropharm.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nestler EJ. Reflections on: “A general role for adaptations in G-Proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function”. Brain research. 2016;1645:71–74. doi: 10.1016/j.brainres.2015.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. The New England journal of medicine. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Grace AA. The tonic/phasic model of dopamine system regulation: its relevance for understanding how stimulant abuse can alter basal ganglia function. Drug and alcohol dependence. 1995;37(2):111–129. doi: 10.1016/0376-8716(94)01066-t. [DOI] [PubMed] [Google Scholar]
- 96.Sun W, Ginovart N, Ko F, Seeman P, Kapur S. In vivo evidence for dopamine-mediated internalization of D2-receptors after amphetamine: differential findings with [3H]raclopride versus [3H]spiperone. Molecular pharmacology. 2003;63(2):456–462. doi: 10.1124/mol.63.2.456. [DOI] [PubMed] [Google Scholar]
- 97.Guo N, Guo W, Kralikova M, et al. Impact of D2 receptor internalization on binding affinity of neuroimaging radiotracers. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35(3):806–817. doi: 10.1038/npp.2009.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Volkow ND, Wang GJ, Tomasi D, et al. Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(3):841–849. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nader MA, Morgan D, Gage HD, et al. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nature neuroscience. 2006;9(8):1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- 100.Caprioli D, Calu D, Shaham Y. Loss of phasic dopamine: a new addiction marker? Nature neuroscience. 2014;17(5):644–646. doi: 10.1038/nn.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Perez-Mana C, Castells X, Vidal X, Casas M, Capella D. Efficacy of indirect dopamine agonists for psychostimulant dependence: a systematic review and meta-analysis of randomized controlled trials. Journal of substance abuse treatment. 2011;40(2):109–122. doi: 10.1016/j.jsat.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 102.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse (New York, NY) 2008;62(7):481–486. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Robertson CL, Ishibashi K, Chudzynski J, et al. Effect of Exercise Training on Striatal Dopamine D2/D3 Receptors in Methamphetamine Users during Behavioral Treatment. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41(6):1629–1636. doi: 10.1038/npp.2015.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Molecular psychiatry. 2004;9(6):557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- 105.Minozzi S, Amato L, Pani PP, et al. Dopamine agonists for the treatment of cocaine dependence. The Cochrane database of systematic reviews. 2015;(5):Cd003352. doi: 10.1002/14651858.CD003352.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Castells X, Cunill R, Perez-Mana C, Vidal X, Capella D. Psychostimulant drugs for cocaine dependence. The Cochrane database of systematic reviews. 2016;9:Cd007380. doi: 10.1002/14651858.CD007380.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Indave BI, Minozzi S, Pani PP, Amato L. Antipsychotic medications for cocaine dependence. The Cochrane database of systematic reviews. 2016;3:Cd006306. doi: 10.1002/14651858.CD006306.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.