Abstract
It remains unclear in adult acute myeloid leukemia (AML) whether leukemic expression of CD33, the target antigen for Gemtuzumab Ozogamicin (GO), add prognostic information on GO effectiveness at different doses. CD33 expression quantified in 1583 patients recruited to UK-NCRI-AML17 (younger adults) and UK-NCRI-AML16 (older adults) trials was correlated with clinical outcomes and benefit from GO including a dose randomisation. CD33 expression associated with genetic subgroups, including lower levels in both adverse karyotype and core-binding factor (CBF)-AML, but was not independently prognostic. When comparing GO versus no GO (n=393, CBF-AMLs excluded) by stratified subgroup-adjusted analysis, patients with lowest quartile (Q1) %CD33-positivity had no benefit from GO (relapse risk, HR 2·41[1·27–4·56], p=0·009 for trend; overall survival, HR 1·52[0·92–2·52]). However from the dose randomisation (NCRI-AML17, n=464, CBF-AMLs included), 6mg/m2 GO only had a relapse benefit without increased early mortality in CD33-low (Q1) patients (relapse risk HR 0·64[0·36–1·12] versus 1.70[0.99-2.92] for CD33-high, p=0·007 for trend). Thus CD33 expression is a predictive factor for GO effect in adult AML; although GO does not appear to benefit the non-CBF AML patients with lowest CD33 expression a higher GO dose may be more effective for CD33-low but not CD33-high younger adults.
Introduction
The modest improvement with conventional cytotoxic therapies in the majority of acute myeloid leukemia (AML) patients provides an opportunity for immunotherapeutic strategies for treating this disease. Expression of CD33 is a feature of most AMLs and has been exploited for immuno-targeting using gemtuzumab ozogamicin (GO), a CD33-directed antibody-drug conjugate (ADC) that has served as a paradigm for antigen-specific immunotherapy of cancer.1 When combined with intensive chemotherapy GO significantly improves outcomes in newly diagnosed adult AML,2–6 and studies demonstrate the importance of appropriately defining patient subgroups that may most benefit from this therapy. A meta-analysis of 3325 adult patients, who did not require to be CD33 positive, in 5 randomised controlled trials of GO combined with intensive chemotherapy, showed that GO significantly reduced relapse risk and improved overall survival.7 The greatest benefit was observed in patients with favourable-risk cytogenetics although significant benefit was also observed for intermediate-risk patients. No benefit was observed from the addition of GO in patients with adverse-risk disease. The meta-analysis appeared to show equivalent outcomes in all genetic subgroups from the lower dosage of GO compared to the higher dose with single dose schedules. This GO-derived reduced relapse risk is also observed when added to intensive chemotherapy in pediatric AML8 though associations with risk group are less clear in these patients.
A key parameter for the potential efficacy of an ADC may be expression levels of the targeted antigen on leukemic cells as this will determine how much of the conjugate will bind. In AML, CD33 blast expression is heterogeneous between patients but there has been uncertainty of the clinical importance of this for GO effectiveness since CD33 expression levels are associated with established prognostic factors including genetic subgroups. Higher CD33 expression is a feature of patients with FLT3-ITD mutation or NPM1 mutation,9–12 while low CD33 expression is characteristic of core-binding factor (CBF) -AML in pediatric patients 9,11 although, perhaps paradoxically, the CBF-AML subgroup derived the most benefit from GO in adult trials. Furthermore CD33 expression may potentially be a prognostic factor independently of these genetic associations as observed in pediatric AML.11
Results from the Children’s Oncology Group (COG) AML trials showed that benefit from GO at a single dose of 3mg/m2 at first induction and then intensification 9 was restricted to pediatric patients with high CD33 blast expression; this was also true for CBF-AMLs. High CD33 also correlated with response to GO in the French ALFA-0701 older adult cohort in which a higher cumulative dose of GO at induction (sequential schedule of 3mg/m2) was administered with standard chemotherapy.10 Notwithstanding these data it remains unclear whether CD33 expression is independently predictive of GO benefit in adults and how this might compare at different doses of GO.
The most recent UK- National Cancer Research Institute (NCRI) -AML trials of younger (NCRI-AML17) and older (NCRI-AML16) adult patients included standard induction chemotherapy randomised with or without a single dose of GO, a GO dose randomisation (NCR-AML17 only) and an assessment of CD33 expression by AML blasts in the pre-treatment sample. We thus performed a retrospective analysis of CD33 expression on the GO treatment effect in a large cohort of these patients
Methods
Study Cohort
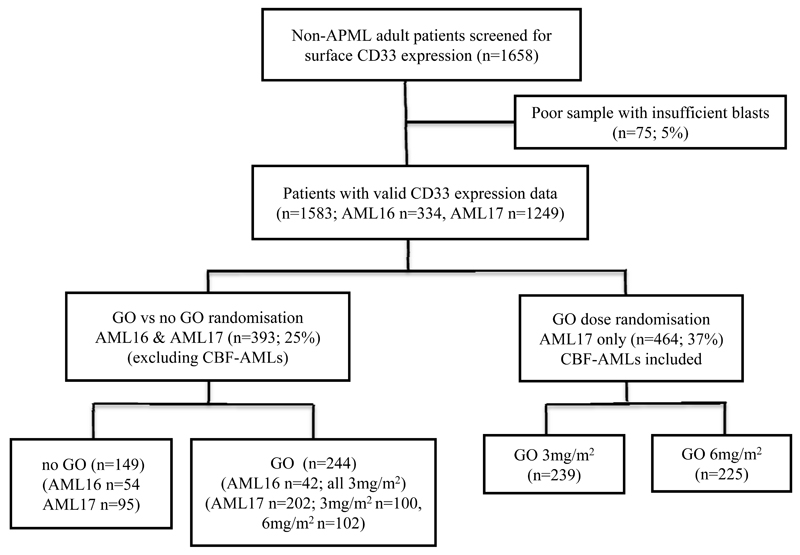
The NCRI-AML16 (ISRCTN11036523) and NCRI-AML17 (ISRCTN55675535) trials enrolled patients with AML (de novo or secondary) or high-risk myelodysplastic syndrome (MDS); patients were mostly aged ≥60 years in NCRI-AML16 and mostly aged <60 years old in NCRI-AML17 (protocols in supplementary information ; Figures S1-S2). In both trials CD33-positivity was not an entry requirement and patients were randomised into intensive chemotherapy arms with or without a single dose of GO in course 1 of induction. In NCRI-AML16 GO was given at 3mg/m2, while in NCRI-AML17 patients were randomised to receive either 3mg/m2 or 6mg/m2 of GO. Trials were conducted in accordance with the Declaration of Helsinki and both institutional and research ethics committee approvals were obtained. Data regarding chemotherapy interventions13 and dose comparisons14 are published separately. Acute promyelocytic leukemia (APML) patients and patients <16 years were excluded from this analysis.
Flow cytometric assessment of CD33 expression
CD33 expression of AML blasts from 1583 pre-treatment BM/PB samples of non-APML patients (NCRI-AML16, n=334; NCRI-AML17, n=1249, patient deployment shown in Figure 1) was prospectively determined by multiparameter flow cytometry (MFC). Staining and data acquisition were performed by three national reference flow cytometric laboratories sharing standard operating procedures,14 and then centrally analysed for CD33 blast expression without knowledge of other clinical data for retrospective correlation with clinical characteristics and outcome.
Figure 1.
Outline of AML patient sample flow for CD33 assessment using pre-treatment samples from NCRI-AML16 and NCRI-AML17. CBF, core-binding factor. GO, gemtuzumab ozogamicin.
AML blast CD33 expression was measured both by median fluorescence intensity of CD33 (CD33-MFI) and also as percentage (%) CD33-positivity (gating described in supplemental methods). CD33-MFI was also measured for the immunophenotypically immature CD34+CD38low stem/progenitor cell (SPC) population when present. The CD33-MFI values in each patient were standardized using the CD33-MFI values of lymphocytes (uniformly CD33 negative) present within the same sample. %CD33-positivity was also determined using lymphocytes in each sample; blast cells with CD33 expression equivalent to lymphocytes were classed as CD33− and blasts with higher expression were classed as CD33+(Figure S3). A broad range of CD33-MFI and %CD33-positivity values were observed and so patients were grouped into quartiles (Q1, Q2, Q3, Q4) for both type of measurements.
Statistical methods
Clinical outcome data up to March 2015 for patients enrolled on NCRI-AML16 and NCRI-AML17 were analysed with median follow up of 40.7 months (range 1·2–71·4 months) (AML16 41·8 months (1·3–67·4), AML17 39·7 months (1·2–71·4)). Endpoint definitions are as described by Cheson with the exception that we report here overall response rate (ORR; CR+CRi, i.e. recovery is not required).15 Demographic data were compared using the Wilcoxon rank-sum/Kruskal Wallis test or Spearman’s correlation, or chi-squared/Mantel-Haenszel test for the dichotomous outcome of CD33− or CD33+. Agreement between local and central measurement of CD33 was performed using Bland-Altman plots. Univariate analyses of time to event outcomes were performed using the logrank test; multivariable adjusted analyses were performed using Cox regression. Analysis of the effect of GO treatment was performed stratified by trial as the randomisation was 1:1 in AML16 and 2:1 in AML17, and data displayed using Forest plots. In all cases, estimates of odds/hazard rations (OR/HR) are given with 95% confidence intervals. Analyses were performed using SAS version 9.3. In addition to overall analyses, exploratory analyses were performed stratified by the randomisation stratification parameters and other important variables, with suitable tests for interaction. Because of the well-known dangers of subgroup analysis, these were interpreted cautiously.
Results
CD33 expression and correlations with disease characteristics
Patients from the two trials were divided into quartiles based on CD33-MFI (inter-quartile cut-points; 3·52, 8·71, 19·66) or quartiles based on %CD33-positivity of the total blast population (inter-quartile cut-points; 37·1%, 75·8%, 94·9%). A non-linear correlation between these two parameters was observed and overlap of quartiles (Figure S4). There was poor agreement between our %CD33-positivity data (acquired by the reference laboratories and centrally analysed) and that acquired and entered into trial database by local laboratories (Figure S5).
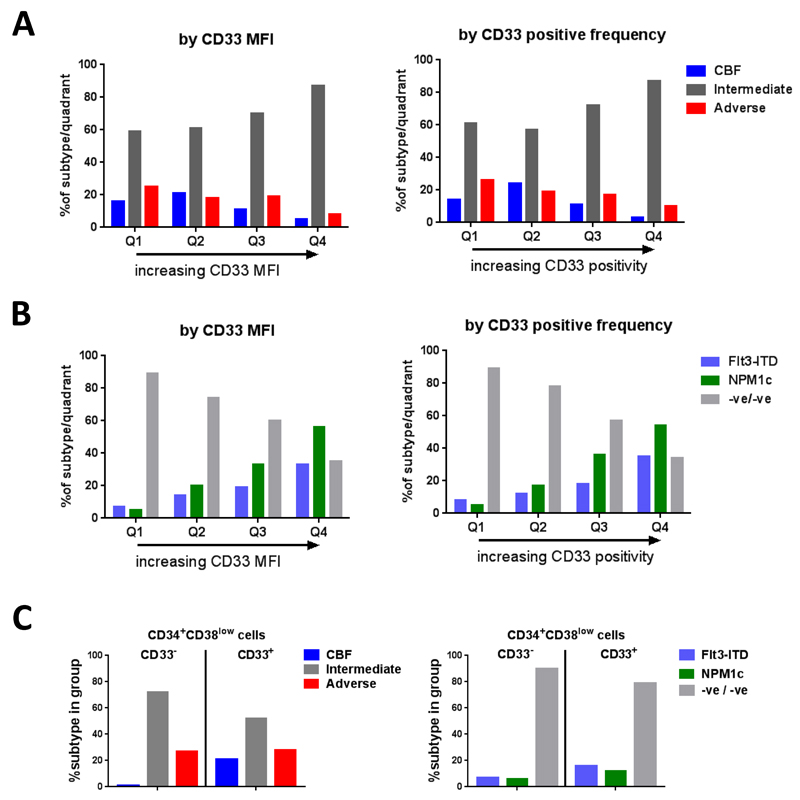
Disease characteristics were then assessed across the CD33 quartiles. Cytogenetic data was available for 1454 of 1583 patients (92%). Corroborating the published data, CBF-AML was found to be inversely correlated with CD33 expression across the quartiles (p<0·0001, Figure 2a-b; Table 1). However, in this adult cohort adverse-risk disease was also associated with lower CD33 expression (p<0·0001, Figure 2a-b). Intermediate-risk cytogenetics significantly increased in prevalence with increasing CD33 quartile (p<0·0001, Figure 2a-b). While FLT3-ITD and NPM1 mutations increased in prevalence with increasing CD33 expression (p<0·0001, Figure 2c-d; Table 1), as already reported,9–11 intermediate-risk patients lacking these mutations were inversely associated with CD33 expression. All the above correlations were observed using either CD33-MFI or %CD33-positivity as the assessment variable.
Figure 2.
AML blast CD33 expression in patient subgroups
CD33 expression of pre-treatment AML blasts by normalised CD33-MFI (arbitrary units) and % positivity in cytogenetic risk groups (A) and intermediate-risk patients subdivided based on mutational (FLT3-ITD and NPM1) background (B). Expanded CD34+CD38low blasts (when at least 0·35% of total WBC) classified as CD33− (Q1 CD33-MFI) or CD33+ (Q2-Q4 CD33-MFI) assessed in cytogenetic risk groups and mutational groups (C).
Table 1. Patient demographics and CD33 expression levels by CD33 MFI and %CD33 positivity.
| CD33 MFI normalised blasts | %CD33 positivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Q1 | Q2 | Q3 | Q4 | p-value | Q1 | Q2 | Q3 | Q4 | p-value |
| No of patients | 386 | 386 | 387 | 386 | 395 | 396 | 397 | 395 | ||
| Trial | 0·005* | 0·08* | ||||||||
| AML16 | 100 (26%) | 60 (16%) | 64 (17%) | 75 (19%) | 105 (27%) | 71 (18%) | 78 (20%) | 80 (20%) | ||
| AML17 | 286 (74%) | 326 (84%) | 323 (83%) | 311 (81%) | 290 (73%) | 325 (82%) | 319 (80%) | 315 (80%) | ||
| Randomisation† (AML16/AML17) | ||||||||||
| GO | 39 (42%) | 26 (33%) | 33 (32%) | 51 (43%) | 39 (42%) | 34 (34%) | 36 (40%) | 40 (36%) | ||
| No GO | 53 (58%) | 54 (68%) | 69 (68%) | 67 (57%) | 54 (58%) | 65 (66%) | 55 (60%) | 70 (64%) | ||
| GO dose (AML17) | ||||||||||
| GO 3mg/m2 | 41 (47%) | 54 (50%) | 63 (50%) | 79 (56%) | 54 (54%) | 55 (45%) | 60 (55%) | 70 (53%) | ||
| GO 6mg/m2 | 46 (53%) | 54 (50%) | 62 (50%) | 62 (44%) | 46 (46%) | 68 (55%) | 50 (45%) | 61 (47%) | ||
| Age at diagnosis, y | <·0001** | <·0001** | ||||||||
| 16-29 | 25 (6%) | 44 (11%) | 39 (10%) | 41 (11%) | 25 (6%) | 34 (9%) | 50 (13%) | 41 (10%) | ||
| 30-39 | 25 (6%) | 39 (10%) | 35 (9%) | 30 (8%) | 28 (7%) | 36 (9%) | 36 (9%) | 30 (8%) | ||
| 40-49 | 48 (12%) | 75 (19%) | 67 (17%) | 98 (25%) | 53 (13%) | 73 (18%) | 84 (21%) | 78 (20%) | ||
| 50-59 | 106 (27%) | 107 (28%) | 127 (33%) | 109 (28%) | 106 (27%) | 125 (32%) | 105 (26%) | 115 (29%) | ||
| 60-69 | 139 (36%) | 97 (25%) | 100 (26%) | 82 (21%) | 136 (34%) | 103 (26%) | 95 (24%) | 106 (27%) | ||
| 70+ | 43 (11%) | 24 (6%) | 19 (5%) | 26 (7%) | 47 (12%) | 25 (6%) | 27 (7%) | 25 (7%) | ||
| median (range) | 59 (16-79) | 54 (16-78) | 54 (16-79) | 52 (16-77) | 59 (16-79) | 54 (16-79) | 52 (16-77) | 54 (17-79) | ||
| Sex | ||||||||||
| Female | 154 (40%) | 160 (41%) | 172 (44%) | 201 (52%) | 0·0004* | 149 (39%) | 178 (45%) | 181 (46%) | 192 (49%) | 0·001* |
| Male | 232 (60%) | 226 (59%) | 215 (56%) | 185 (48%) | 246 (62%) | 218 (55%) | 216 (54%) | 203 (51%) | ||
| Diagnosis | 0·0001* | <·0001* | ||||||||
| De Novo | 300 (78%) | 331 (86%) | 320 (83%) | 344 (89%) | 311 (79%) | 322 (81%) | 339 (85%) | 352 (89%) | ||
| Secondary | 49 (13%) | 32 (8%) | 46 (12%) | 31 (8%) | 50 (13%) | 44 (11%) | 39 (10%) | 31 (8%) | ||
| MDS | 37 (10%) | 23 (6%) | 21 (5%) | 11 (3%) | 34 (9%) | 30 (8%) | 19 (5%) | 12 (3%) | ||
| WHO PS | 0·7** | 0·6** | ||||||||
| 0 | 250 (65%) | 265 (69%) | 259 (67%) | 257 (67%) | 264 (67%) | 273 (69%) | 256 (64%) | 267 (68%) | ||
| 1 | 114 (30%) | 104 (27%) | 111 (29%) | 116 (30%) | 112 (28%) | 104 (27%) | 121 (30%) | 115 (29%) | ||
| 2 | 17 (4%) | 12 (3%) | 10 (3%) | 7 (2%) | 14 (4%) | 11 (3%) | 13 (3%) | 10 (3%) | ||
| 3 | 5 (1%) | 4 (1%) | 7 (2%) | 6 (2%) | 5 (1%) | 7 (2%) | 7 (2%) | 3 (1%) | ||
| 4 | 0 | 1 (<.5%) | 0 | 0 | 0 | 1 (<.5%) | 0 | 0 | ||
| WBC count | <·0001** | <·0001** | ||||||||
| 0-9.9 | 257 (67%) | 198 (51%) | 171 (44%) | 155 (40%) | 255 (65%) | 218 (55%) | 183 (46%) | 152 (38%) | ||
| 10-49.9 | 93 (24%) | 121 (31%) | 148 (38%) | 136 (35%) | 94 (24%) | 124 (31%) | 132 (33%) | 155 (39%) | ||
| 50-99.9 | 13 (3%) | 36 (9%) | 40 (11%) | 53 (14%) | 22 (6%) | 26 (7%) | 50 (13%) | 48 (12%) | ||
| 100+ | 23 (6%) | 31 (8%) | 28 (7%) | 42 (11%) | 24 (6%) | 28 (7%) | 32 (8%) | 40 (10%) | ||
| Median (range) | 4·9 | 9·2 | 12·8 | 16·4 | 5·1 | 7·2 | 12·7 | 16·6 | ||
| (0·4-430·0) | (0·4-334·9) | (0·6-249·0) | (0·7-345·0) | (0·4-430·0) | (0·6-334·9) | (0·7-266) | (0·7-345·0) | |||
| Cytogenetics | 0·4** | 0·7** | ||||||||
| Favourable | 54 (16%) | 74 (21%) | 40 (11%) | 18 (5%) | 48 (14%) | 88 (24%) | 41 (11%) | 10 (3%) | ||
| Intermediate | 203 (59%) | 219 (61%) | 254 (70%) | 308 (87%) | 214 (61%) | 211 (57%) | 270 (72%) | 312 (87%) | ||
| Adverse | 87 (25%) | 66 (18%) | 71 (19%) | 28 (8%) | 90 (26%) | 71 (19%) | 62 (17%) | 36 (10%) | ||
| Unknown | 42 | 27 | 21 | 32 | 43 | 25 | 24 | 37 | ||
| FLT3-ITD | <·0001* | <·0001* | ||||||||
| WT | 303 (93%) | 295 (86%) | 289 (81%) | 235 (67%) | 315 (92%) | 315 (88%) | 294 (82%) | 230 (65%) | ||
| Mutant | 22 (7%) | 48 (14%) | 66 (19%) | 116 (33%) | 27 (8%) | 43 (12%) | 64 (18%) | 122 (35%) | ||
| Unknown | 61 | 43 | 32 | 35 | 53 | 38 | 39 | 43 | ||
| NPM1c | <·0001* | <·0001* | ||||||||
| WT | 299 (95%) | 272 (80%) | 231 (67%) | 148 (44%) | 316 (95%) | 291 (83%) | 220 (64%) | 155 (46%) | ||
| Mutant | 16 (5%) | 66 (20%) | 112 (33%) | 188 (56%) | 17 (5%) | 61 (17%) | 125 (36%) | 185 (54%) | ||
| Unknown | 71 | 48 | 44 | 50 | 62 | 44 | 52 | 55 | ||
| ITD/NPM1c | <·0001* | <·0001* | ||||||||
| ITD WT, NPM1c WT | 281 (89%) | 248 (74%) | 205 (60%) | 116 (35%) | 295 (89%) | 271 (78%) | 197 (57%) | 115 (34%) | ||
| ITD WT, NPM1c Mut | 11 (4%) | 41 (12%) | 73 (21%) | 110 (33%) | 9 (3%) | 36 (10%) | 85 (25%) | 109 (32%) | ||
| ITD Mut, NPM1c WT | 17 (5%) | 22 (7%) | 26 (8%) | 32 (10%) | 19 (6%) | 17 (5%) | 23 (7%) | 40 (12%) | ||
| ITD Mut, NPM1c Mut | 5 (2%) | 25 (7%) | 39 (11%) | 77 (23%) | 8 (2%) | 25 (7%) | 40 (12%) | 75 (22%) | ||
| Unknown | 72 | 50 | 44 | 51 | 64 | 47 | 52 | 56 | ||
| Post-course 1 risk score (AML17) | 0·04** | 0·2** | ||||||||
| Good | 50 (20%) | 80 (27%) | 44 (15%) | 39 (13%) | 47 (18%) | 86 (28%) | 55 (18%) | 26 (9%) | ||
| Standard | 88 (34%) | 118 (39%) | 147 (49%) | 186 (62%) | 91 (35%) | 111 (36%) | 163 (54%) | 176 (59%) | ||
| Poor | 118 (46%) | 103 (34%) | 112 (37%) | 73 (25%) | 118 (46%) | 108 (35%) | 85 (28%) | 95 (32%) | ||
Wilcoxon-Rank Sum/Kruskal-Wallis test
Spearman correlation
excluding CBF leukaemia (AML16 n=2, AML17 n=46)
Abbreviations: GO=gemtuzumab ozogamicin, WHO PS=World Health Organisation_performance score, WBC=white blood cell, FLT3-ITD=FLT3 internal tandem duplication, WT=wild type; Mut=mutated, MFI=median fluorescence intensity.
In addition to total AML blasts, we also assessed CD33 expression in immunophenotypically immature CD34+CD38low blasts, which are enriched for chemo-resistant leukemic stem-cell (LSC) –like populations in some patients. This analysis was performed on all patients with detectable CD34+CD38low blasts (n=1301), and then focussed on patients with significantly expanded CD34+CD38low blasts (n=779) using a threshold of greater than 0·35% of total WBC (>2SD above mean normal frequency) to exclude patients with immature blasts that may be predominantly non-leukemic. As with total blasts there was considerable variation in CD33 expression on immature blasts across the cohort (Table S1). We classified patients with expanded CD34+CD38low cells into CD33− (Q1) and CD33+ (Q2-Q4), under the supposition that CD33− cells represent a GO-unresponsive subpopulation, and thus may have prognostic value. Comparison between patient sub-groups showed that expanded CD34+CD38low blasts in CBF-AMLs were almost always CD33+ (in Q2-Q4), while in both intermediate-risk and adverse-risk patients the CD34+CD38low blasts were more heterogeneous, containing significant numbers of CD33− cells (Q1) (Figure 2c). Patients with CD33+ CD34+CD38low blasts showed a trend of increased prevalence of FLT3-ITD mutation (16% vs 7%, p=0·03) and NPM1 mutation (12% vs 6%, p=0·1) (Table S1).
CD33 expression and clinical outcomes
In an analysis adjusted for trial, there was no significant difference in outcomes between patients with and without CD33 data (p=0·4). Higher CD33 expression, by either measurement, showed significant positive prognostic value in univariate analyses for both overall survival (OS) and cumulative incidence of relapse (CIR) (Table 2). This did not remain significant, however, after adjustment in multivariable analysis for cytogenetics, age, log-WBC, performance status, FLT3-ITD mutation, NPM1 mutation, secondary disease and trial protocol, (OS; HR 1·01 [0·93–1·09], p=0·8 using CD33-MFI and HR 1·01 [0·94–1·09], p=0·8 using % CD33-positivity, CIR; HR 0·99 [0·91–1·08], p=0·8 using CD33-MFI and HR 1·00 [0·91–1·09], p=0·9 using %CD33-positivity, Table 2). Therefore, in contrast to pediatric AML, CD33 expression on blasts is not independently prognostic for outcomes in our adult cohort. This was also the case when the analysis was limited to the 1077 patients who did not receive GO (Table S2). In NCRI-AML17 all CBF-AML patients received GO during induction. There was no evidence of a significant association between CD33 expression quartiles and outcomes in this subgroup (Figure S6) although this does not exclude that CD33 expression may be prognostic for CBF-AML patients not receiving GO. Perhaps surprisingly patients with expanded CD34+CD38low blasts that were CD33− had improved OS both in the overall cohort (HR 0·61 [0·45–0·84] p=0·002; Table S3a) and when patients receiving GO were excluded (HR 0·73 [0·44-1·01] p=0·05; Table S3b).
Table 2. Clinical outcomes and CD33 expression.
| CD33 MFI normalised blasts | %CD33 positivity | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Outcome | Q1 | Q2 | Q3 | Q4 | OR/HR, 95% CI, p-value unadjusted/adjusted | Q1 | Q2 | Q3 | Q4 | OR/HR, 95% CI, p-value unadjusted/adjusted |
| CR/CRi | 79% | 80% | 87% | 89% | 0·75 (0·66–0·85) p<·0001; 0·81 (0·68–0·96) p=0·02 |
76% | 85% | 85% | 87% | 0·78 (0·69–0·88) p<·0001; 0·86 (0·73–1·02) p=0·08 |
| OS | 27% | 36% | 37% | 48% | 0·90 (0.85–0·95) p=0·0005; 1·01 (0·93–1·09) p=0·8 |
27% | 35% | 40% | 45% | 0·90 (0·85–0·96) p=0·0007; 1·01 (0·94–1·09) p=0·8 |
| CIR | 56% | 54% | 49% | 50% | 0·93 (0·86–0·99) p=0·03; 0·99 (0·91–1·08) p=0·8 |
57% | 55% | 50% | 50% | 0·91 (0·85–0·98) p=0·01; 1·00 (0·91–1·09) p=0·9 |
Note: Adjusted OR/HR for age, cytogenetics, trial, log (WBC), secondary disease, ITD, NPM1. OR/HR presented per quartile.
Abbreviations: CR=complete remission, CRi=complete remission with incomplete blood count recovery, OS=overall survival, CIR=cumulative incidence of relapse, MFI=median fluorescence intensity, OR=odds ratio, HR=hazard ratio, CI=confidence interval.
CD33 expression and impact on GO-sensitivity
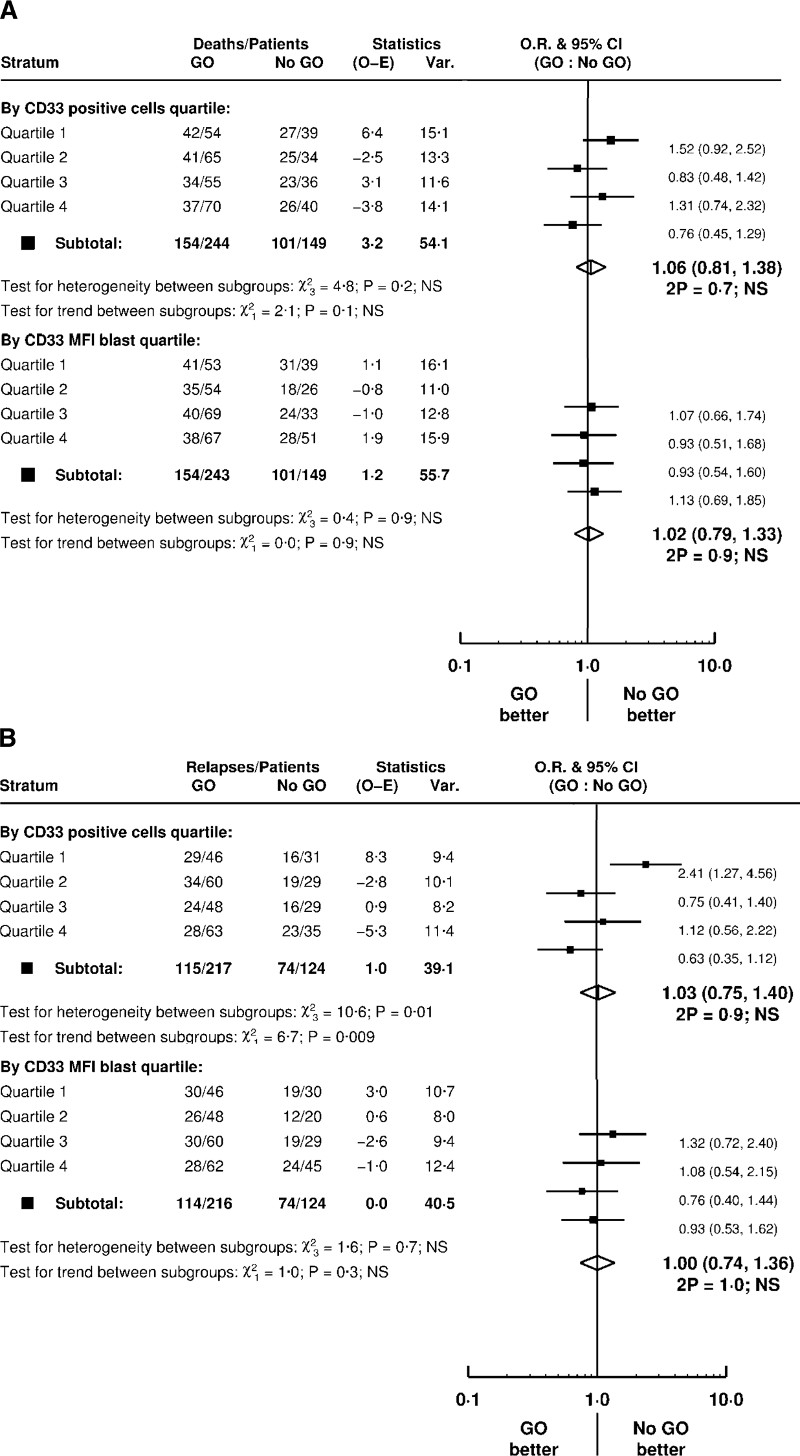
We then asked whether CD33 expression was relevant to benefit in outcomes observed in patients receiving GO with their induction chemotherapy compared with patients receiving chemotherapy alone (GO vs no GO). 393 patients across the two trials were assessable for this GO vs no GO comparison with CBF-AMLs excluded as these were all given GO in AML17 and there were only two CBF-AMLs in AML16. A total of 244 patients received GO (AML16 n=42, all allocated 3mg/m2, AML17 n=202 at either 3mg/m2 (n=100) or 6mg/m2 (n=102); Figure 1) (In AML17, patients receiving DA were not randomised between GO and no GO – all received GO at either 3mg/m2 or 6mg/m2). The results showed no evidence of significant interaction between GO and CD33 quartiles on survival, using either CD33 parameter (Figure 3a). When evaluating relapse, however, there was a significant interaction between GO and %CD33-positive blasts (p=0·009 for trend). Patients with the lowest %CD33-positive blasts (Q1) had a significantly greater relapse risk when given GO (HR 2·41 [1·27–4·56]) while patients with the highest %CD33-positive blasts (Q4) showed reduced relapse risk (HR 0·63 [0·35–1·12]) (Figure 3b). This differential benefit was not observed using blast CD33-MFI (Figure 3b).
Figure 3.
Effect of CD33 expression levels on (A) overall survival and (B) relapse in GO versus no GO randomised AML patients
Forest plot analysis of 393 non-CBF patients assessable for GO vs no GO comparison. Patients were stratified into CD33 expression quartile using CD33-MFI and %CD33-positivity.
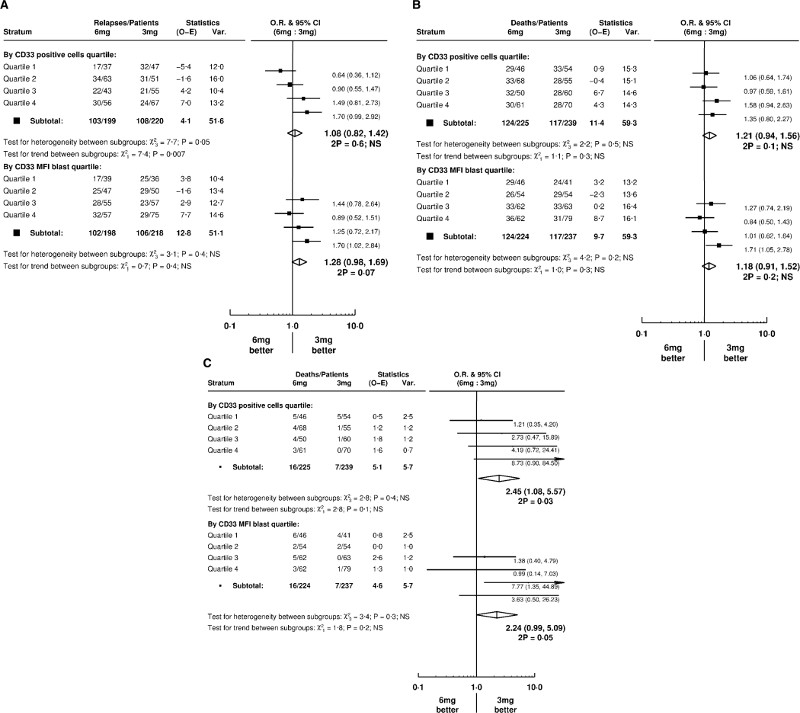
Having established CD33 expression was relevant to effect of GO on relapse, we then assessed for difference in outcomes by CD33 expression in 464 patients entering the AML17 GO dose randomisation (3mg/m2, n=239; 6mg/m2, n=225; Figure 1). Stratification of patients by CD33 expression quartiles showed a differential benefit by GO dose for relapse (Figure 4a) but not for OS (Figure 4b). Using %CD33-positivity, patients with lowest CD33 expression (Q1) had most benefit from the higher 6mg/m2 dose of GO (p=0·007 for trend) (Figure 4a). Importantly, there was no excess early (60-day) mortality from the 6mg/m2 dose in these patients (Figure 4c). Patients with the highest %CD33-positive blast levels (Q4) did not benefit from the higher dose (relapse, HR 1·70 [0·99–2·92]) (Figure 4a).
Figure 4.
Effect of CD33 expression levels on (A) relapse, (B) overall survival and (C) early mortality (60 days) in patients randomised to receive 6mg/m2 or 3mg/m2 GO dose
Forest plot analysis of 464 younger patients (NCRI-AML17 trial) assessable for GO vs no GO comparison. Patients were stratified into CD33 expression quartile using CD33-MFI and %CD33-positivity.
As expanded CD34+CD38low blasts in CBF-AMLs were almost always CD33+, we hypothesized this might contribute to greater GO efficacy in CBF-AMLs as clearance of potential LSCs in the CD34+CD38low subset by GO would not be limited by their low CD33 expression. An exploratory subgroup analysis of non-CBF AML patients in the GO versus no GO and GO dose randomisations did not show a significant interaction between GO treatments and CD33+ versus CD33− expanded CD34+CD38low blasts (Figure S7).
Discussion
In this report, we assessed the importance of CD33 expression in a large cohort of adult AML patients that included randomisations to receive standard chemotherapy alone or in combination with a single dose of GO at 3mg/m2 or 6mg/m2.
Greater efficacy of GO in patients with higher expression of the target antigen is logical and supported by in vitro data showing a direct relationship between CD33 expression and GO-sensitivity,16 and clinical data from GO monotherapy in relapsed AML patients17 and older patients deemed unfit for intensive chemotherapy.18 Very recent data has emerged from the COG and French ALFA trials that pediatric and older (50-70 years) AML patients with lower CD33 expression do not benefit from the addition of GO to standard chemotherapy (3mg/m2 single dose at induction I and intensification II in COG trial, 3mg/m2 fractionated doses at induction I plus single dose at consolidation for ALFA-0701).9–10 In these studies CD33 expression was measured using % positivity and MFI respectively. We assessed CD33 using both types of measurement sub-divided by quartiles rather than a single threshold value in order to evaluate prognostic and response correlations for the range of blast CD33 expression. CD33 expression data for this study were acquired by reference laboratories and analysed centrally. The discrepancy between these data and analyses from local laboratories primarily classifying AML blasts as CD33 positive or negative 2,3 highlights the value of standardised analysis for flow cytometric biomarkers that input into trial data. Interestingly our non-linear concordance profile of these measurements (Figure S2) is similar to that of the ALFA group10 despite the inevitable differences of instrumentation as well as reagents and blast gating between studies. This further validates these CD33 biomarker assays as reproducible and practical in different centers but also shows that CD33MFI and %CD33-positivity are not equivalent for some patients since higher %CD33 values are included in CD33-MFI lower quartiles. Notwithstanding we observed similar associations for both expression parameters with patient disease characteristics such as cytogenetics and molecular aberrations (FLT3-ITD and NPM1 mutations). From our adult cohort adverse karyotype, wild type FLT3 / NPM1 as well as CBF-AML are all associated with lower CD33 expression. We also demonstrate an independent correlation between %CD33-positivity and GO benefit for younger and older adults with non-CBF AML.
The recent COG data similarly describes an association between CD33 expression (by a different CD33-MFI assay) and GO response in their pediatric AAML0531 cohort 9 that included ~25% CBF AMLs. It appears that there was a relatively higher frequency of CBF-AMLs with low CD33 expression (~45% of CBFs in Q1) enrolled in their trial than in our adult cohort (~29% of CBFs in Q1, Table 1). Since CD33-low patients derive the least benefit from GO, this may plausibly contribute to why the significant association of GO benefit with CBF-AML reported from adult studies has not been demonstrated for this COG cohort.8
In this study all CBF-AML patients included in the analysis received GO (3mg/m2 or 6mg/m2) at induction, thus excluding an analysis of GO versus no GO stratified by CD33 expression quartiles. There was however no significant correlation between CBF CD33 expression and outcome suggesting that other factors could be important for the relative GO sensitivity of this subgroup in adults. Alternatively GO exposure may differentially counteract any negative prognostic impact from for example higher CD33 expression (as suggested by COG data 9,11) in CBF-AMLs.
Our analysis also defined CD33 expression in the immunophenotypically immature CD34+CD38low blast population, which is often expanded in AML and reported as clinically and experimentally relevant for treatment responses.19–21 Previous data have shown that high CD33 expression by such cells enhances their GO sensitivity.22 Interestingly, expanded immature blasts in CBF-AMLs were almost exclusively CD33+ despite lower CD33 expression of the global blast population. Conversely, there was variable CD33 expression on expanded CD34+CD38low blasts in intermediate-risk and adverse-risk patients. CD33-positivity of this candidate LSC- enriched population may allow effective antigen-specific targeting and clearance of potentially more chemo-resistant subpopulations in CBF-AMLs. Our results however did not show a significant interaction between CD33 status of expanded CD34+CD38low blasts in non-CBF AML patients and GO response. This is not unexpected due to the confounding variables of heterogeneous CD33 expression in the main blast population between patients and other biological factors for GO resistance.
The clinical trials of combined chemotherapy with GO, mentioned earlier, used different doses and schedules of GO, however the meta-analysis of the individual patient data from these trials suggested a single dose of 3mg/m2 was as effective at preventing relapse as a 6mg/m2 dose, while having less toxicity. The NCRI-AML17 trial included a 6mg/m2 vs 3mg/m2 randomisation to ascertain whether efficacy was enhanced by the higher dose. Results overall showed no significant benefit and a higher rate of veno-occlusive disease with the higher dose although there was a trend for improved outcomes in the adverse karyotype patients.23 Our analysis using CD33 as a stratification variable showed a significant interaction between dose and %CD33-positivity in NCRI-AML17 patients (younger adults); the higher 6mg/m2 dose of GO most improved relapse risk and was well tolerated by patients with the lowest CD33 expression. Conversely, patients with higher CD33 levels independently of risk group do not appear to derive any additional benefit from increasing the dose from 3mg/m2 to 6mg/m2 as single induction dose. This is the first demonstration of a pre-treatment biomarker that could inform appropriate use of a higher GO dose (and potentially other CD33-targeted antibody conjugates) at induction and suggests that the 6mg/m2 dose benefit for adverse-risk AML outcomes may be specific to patients with Q1-CD33 expression. These findings have to be interpreted however within the recognised limitations of potential false-positives from subgroup analysis. Moreover it is as yet uncertain whether the higher dose improves GO chemosensitivity for patients with co-association of the multidrug resistance phenotype and CD33-low expression 17 or HFE mutations.24
Further optimisation of treatment schedules in ongoing trials includes a single GO dose versus fractionated GO dose comparison (NCRI-AML18/19). Interestingly from the ALFA-0701 data the fractionated GO schedule (3mg/m2 on day 1, maximum dose: 5mg) did not improve outcome in older adults with lower CD33 expression. Although this may imply that a single higher 6mg/m2dose is more effective than a cumulative higher dose at reducing relapse in the CD33-lower subgroup, our data is restricted to younger adults and therefore may not be relevant to the older age group.
Next-generation CD33-directed ADC including SGN-CD33A are reported to be more potent than GO, without liver toxicity25 and may also be more active in multidrug resistance.26 The results of our study suggest that assessment of CD33 expression in trials using these next-generation CD33-directed ADCs will be important to inform future optimal dosing. Ultimately, this could lead to a more personalized mode of GO treatment based on patient AML blast CD33 expression.
Supplementary Material
Supplementary information accompanies this paper on the Leukemia website
Acknowledgements
We are grateful to NIHR and Cancer Research UK for research funding for this trial, to Pfizer Japan for supplying Gemtuzumab Ozogamicin at a discounted price, to the staff of the Haematology Trials Office and Cardiff Experimental Cancer Medicine Centre for supporting the trial and to our co-investigators and research nurses who provided diagnostic samples from the NCRI AML Trial centres (listed in Supplementary Appendix).
Footnotes
Declaration of interests
AKB has served on advisory boards for Wyeth/Pfizer during the study. The remaining authors declare no conflict of interest
References
- 1.Rashidi A, Walter RB. Antigen-specific immunotherapy for acute myeloid leukemia. Expert Rev Hematol. 2016;9:335–350. doi: 10.1586/17474086.2016.1142868. [DOI] [PubMed] [Google Scholar]
- 2.Burnett AK, Hills RK, Milligan D, Kjeldsen L, Kell J, Russell NH, et al. Identification of patients with acute myeloblastic leukemia who benefit from the addition of gemtuzumab ozogamicin: results of the MRC AML15 trial. J Clin Oncol. 2011;29:369–377. doi: 10.1200/JCO.2010.31.4310. [DOI] [PubMed] [Google Scholar]
- 3.Burnett AK, Russell NH, Hills RK, Kell J, Freeman S, Kjeldsen L, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy improves survival in older patients with acute myeloid leukemia. J Clin Oncol. 2012;30:3924–3931. doi: 10.1200/JCO.2012.42.2964. [DOI] [PubMed] [Google Scholar]
- 4.Petersdorf SH, Kopecky KJ, Slovak M, Willman C, Nevill T, Brandwein J, et al. A phase 3 study of gemtuzumab ozogamicin during induction and postconsolidation therapy in younger patients with acute myeloid leukemia. Blood. 2013;121:4854–4860. doi: 10.1182/blood-2013-01-466706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delaunay J, Recher C, Pigneux A, Witz F, Vey N, Blanchet O, et al. Addition of gemtuzumab ozogamycin to chemotherapy improves event-free survival but not overall survival of AML patients with intermediate cytogenetics not eligible for allogeneic transplantation: results of the GOELAMS AML 2006 IR study. Blood (ASH annual meeting abstracts) 2011;118 abstr 79. [Google Scholar]
- 6.Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, Bastie JN, et al. Acute Leukemia French Association Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet. 2012;379:1508–1516. doi: 10.1016/S0140-6736(12)60485-1. [DOI] [PubMed] [Google Scholar]
- 7.Hills RK, Castaigne S, Appelbaum FR, Delaunay J, Petersdorf SH, Othus M, et al. Addition of gemtuzumab ozogamicin to induction chemotherapy in adult patients with acute myeloid leukaemia: a meta-analysis of individual patient data from randomised controlled trials. Lancet Oncology. 2014;15:986–996. doi: 10.1016/S1470-2045(14)70281-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gamis AS, Alonzo TA, Meshinchi S, Sung L, Gerbing RB, Raimondi SC, et al. Gemtuzumab ozogamicin in children and adolescents with de novo acute myeloid leukemia improves event-free survival by reducing relapse risk: results from the randomized phase III Children’s Oncology Group trial AAML0531. J Clin Oncol. 2014;32:3021–3032. doi: 10.1200/JCO.2014.55.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pollard JA, Loken M, Gerbing RB, Raimondi SC, Hirsch BA, Aplenc R, et al. CD33 Expression and Its Association With Gemtuzumab Ozogamicin Response: Results From the Randomized Phase III Children's Oncology Group Trial AAML0531. J Clin Oncol. 2016;34:747–755. doi: 10.1200/JCO.2015.62.6846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olombel G, Guerin E, Guy J, Perrot JY, Dumezy F, de Labarthe A, et al. Impact of blast CD33 expression on the effect of gemtuzumab ozogamicin (GO) in adult acute myeloid leukemia (AML): an ALFA-0701 study. Blood. 2016;127:2157–2160. doi: 10.1182/blood-2016-01-689976. [DOI] [PubMed] [Google Scholar]
- 11.Pollard J, Alonzo TA, Loken M, Gerbing RB, Ho PA, Bernstein ID, et al. Correlation of CD33 expression level with disease characteristics and response to gemtuzumab ozogamicin containing chemotherapy in childhood AML. Blood. 2012;119:3705–3711. doi: 10.1182/blood-2011-12-398370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ehninger A, Kramer M, Röllig C, Thiede C, Bornhauser M, von Bonin M, et al. Distribution and levels of cell surface expression of CD33 and CD123 in acute myeloid leukemia. Blood Cancer J. 2014;4:e218. doi: 10.1038/bcj.2014.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burnett AK, Russell NH, Hills RK, Kell J, Cavenagh J, Kjeldsen L, et al. A randomized comparison of daunorubicin 90 mg/m2 vs 60mg/m2 in AML induction: results from the UK NCRI AML 17 trial in 1206 patients. Blood. 2015;125:3878–3885. doi: 10.1182/blood-2015-01-623447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freeman SD, Virgo P, Couzens S, Grimwade D, Russell N, Hills RK, et al. Prognostic relevance of treatment response measured by flow cytometric residual disease detection in older patients with acute myeloid leukemia. J Clin Oncol. 2013;31:4123–4131. doi: 10.1200/JCO.2013.49.1753. [DOI] [PubMed] [Google Scholar]
- 15.Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 16.Walter RB, Raden BW, Kamikura DM, Cooper JA, Bernstein ID. Influence of CD33 expression levels and ITIM-dependent internalization on gemtuzumab ozogamicin-induced cytotoxicity. Blood. 2005;105:1295–1302. doi: 10.1182/blood-2004-07-2784. [DOI] [PubMed] [Google Scholar]
- 17.Walter RB, Gooley TA, van der Velden VH, Loken MR, van Dongen JJ, Flowers DA, et al. CD33 expression and P-glycoprotein-mediated drug efflux inversely correlate and predict clinical outcome in patients with acute myeloid leukemia treated with gemtuzumab ozogamicin monotherapy. Blood. 2007;109:4168–4170. doi: 10.1182/blood-2006-09-047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amadori S, Suciu S, Selleslag D, Aversa F, Gaidano G, Musso M, et al. Gemtuzumab Ozogamicin Versus Best Supportive Care in Older Patients With Newly Diagnosed Acute Myeloid Leukemia Unsuitable for Intensive Chemotherapy: Results of the Randomized Phase III EORTC-GIMEMA AML-19 Trial. J Clin Oncol. 2016;34:972–979. doi: 10.1200/JCO.2015.64.0060. [DOI] [PubMed] [Google Scholar]
- 19.Saito Y, Uchida N, Tanaka S, Suzuki N, Tomizawa-Murasawa M, Sone A, et al. Induction of cell cycle entry eliminates human leukemia stem cells in a mouse model of AML. Nature Biotechnol. 2010;28:275–280. doi: 10.1038/nbt.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rhenen A, Feller N, Kelder A, Westra AH, Rombouts E, Zweegman S, et al. High stem cell frequency in acute myeloid leukemia at diagnosis predicts high minimal residual disease and poor survival. Clin Cancer Res. 2005;11:6520–6527. doi: 10.1158/1078-0432.CCR-05-0468. [DOI] [PubMed] [Google Scholar]
- 21.Walter RB, Appelbaum FR, Estey EH, Berstein ID. Acute myeloid leukemia stem cells and CD33-targeted immunotherapy. Blood. 2012;119:6198–6208. doi: 10.1182/blood-2011-11-325050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jawad M, Seedhouse C, Mony U, Grundy M, Russell N, Pallis M. Analysis of factors that affect in vitro chemosensitivity of leukemic stem and progenitor cells to gemtuzumab ozogamicin (Mylotarg) in acute myeloid leukemia. Leukemia. 2010;24:74–80. doi: 10.1038/leu.2009.199. [DOI] [PubMed] [Google Scholar]
- 23.Burnett AK, Cavenagh J, Russell NH, Hills R, Kell J, Jones G, et al. on behalf of the UK NCRI AML Study Group Defining the Dose Gemtuzumab Ozogamicin in combination with Induction Chemotherapy in Acute Myeloid Leukaemia: A Comparison of 3mg/m2 with 6mg/m2 in the NCRI AML17 Trial. Haematologica. 2016;101:724–731. doi: 10.3324/haematol.2016.141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paubelle E, Marceau A, Zylbersztejn F, Dussiot M, Moura IC, Cornillet-Lefebvre P, et al. HFE Gene Mutation Status Predicts Response to Gemtuzumab Ozogamicin in AML. Blood (ASH annual meeting abstracts) 2015;126 abstr 1307. [Google Scholar]
- 25.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood. 2016;127:71–78. doi: 10.1182/blood-2015-07-604538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung Sutherland MS, Walter RB, Jeffrey SC, Burke PJ, Yu C, Kostner H, et al. SGN-CD33A: A novel CD33-targeting antibody-drug conjugate using a pyrrolobenzodiazepine dimer is active in models of drug-resistant AML. Blood. 2013;122:1455–1463. doi: 10.1182/blood-2013-03-491506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.