Abstract
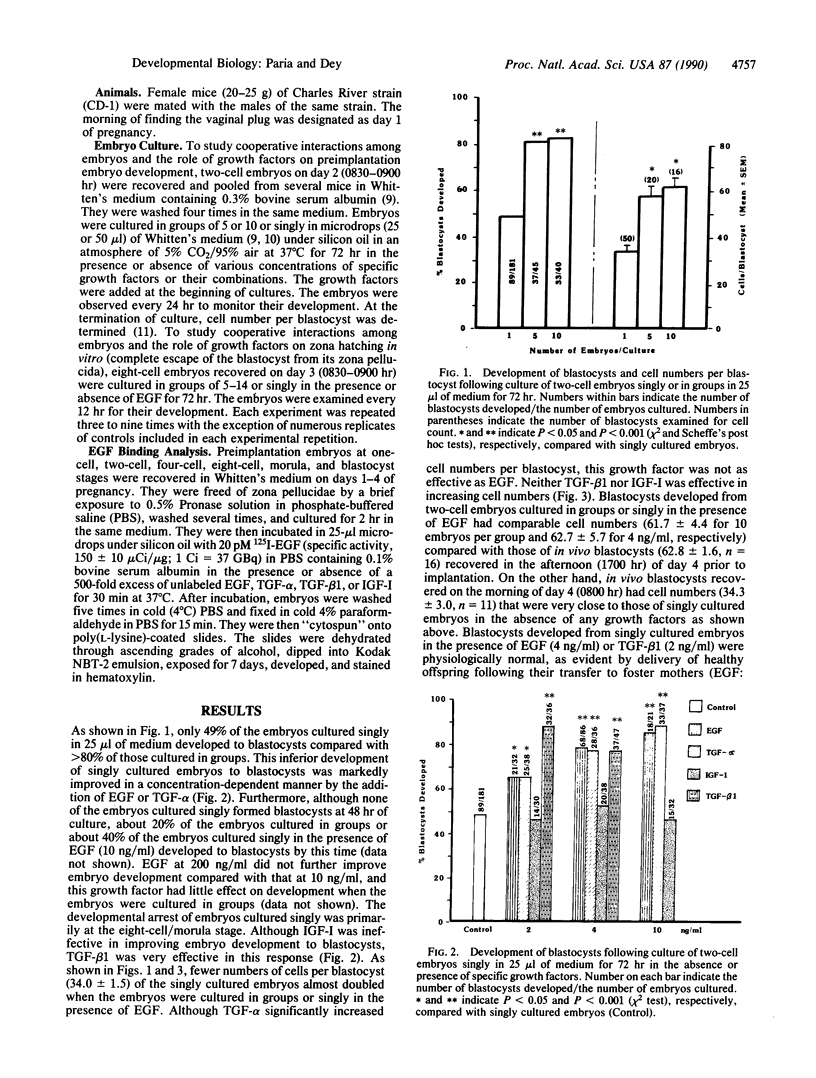
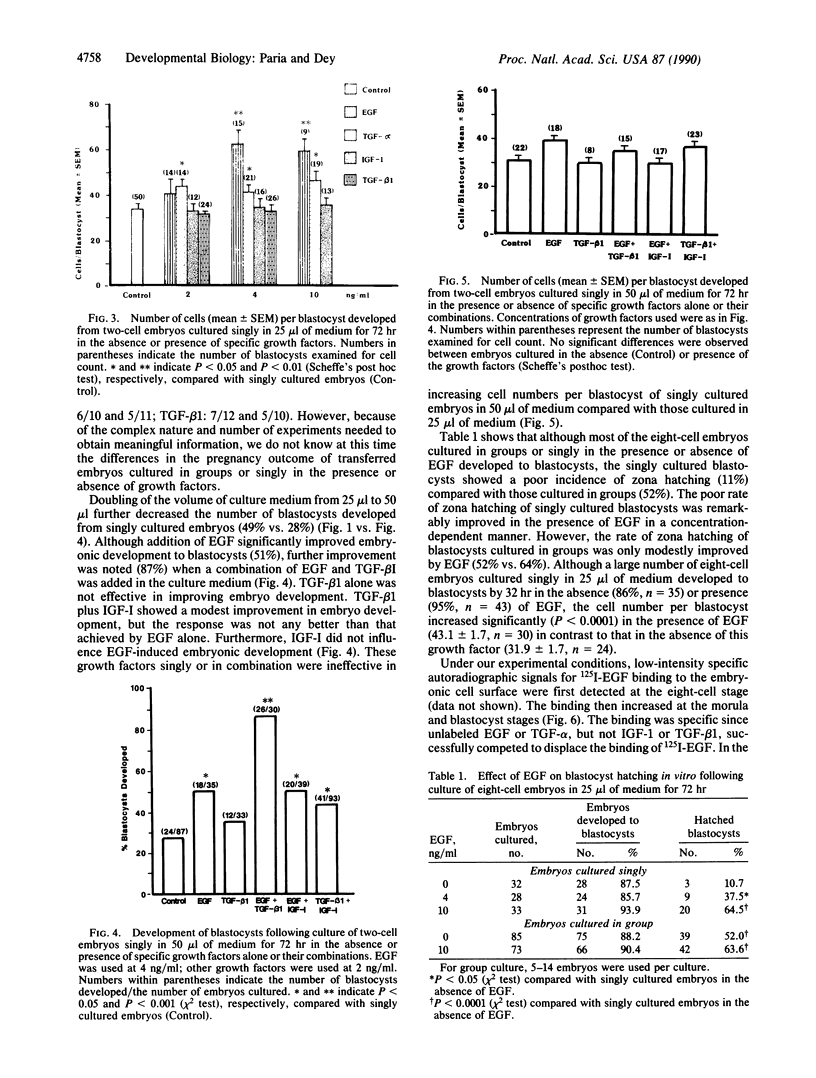
We have established a model that shows cooperative interaction among preimplantation embryos and the role of growth factors on their development and growth. Two-cell mouse embryos cultured singly in 25-microliters microdrops had inferior development to blastocysts and lower cell numbers per blastocyst compared with those cultured in groups of 5 or 10. The inferior development of singly cultured embryos was markedly improved by addition of epidermal growth factor (EGF) or transforming growth factor alpha or beta 1 (TGF-alpha or TGF-beta 1) to the culture medium. The stage of embryonic development, primarily affected by these treatments, was between eight-cell/morula and blastocyst. Furthermore, blastocysts developed from eight-cell embryos cultured in groups or singly in the presence of EGF showed a higher incidence of zona hatching compared with those cultured singly in the absence of EGF. Detection of EGF receptors on the embryonic cell surface at eight-cell/morula and blastocyst stages suggests beneficial effects of EGF or TGF-alpha on preimplantation embryo development and blastocyst functions. Insulin-like growth factor I (IGF-I) had no influence on embryo development. To further document the cooperative interactions among embryos, the volume of the culture medium was doubled to 50 microliters. This increase in culture volume was even more detrimental to the development of singly cultured embryos. However, this detrimental effect was significantly reversed by EGF and reversed even more markedly by a combination of EGF and TGF-beta 1 but not by TGF-beta 1 alone. Although TGF-beta 1 plus IGF-I caused a modest improvement of embryo development, the response was not as great as shown by EGF alone. Furthermore, IGF-I had no additive effect on EGF-induced embryonic development. The study presents clear evidence that specific growth factors of embryonic and/or reproductive tract origin participate in preimplantation embryo development and blastocyst functions in an autocrine/paracrine manner.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adamson E. D., Meek J. The ontogeny of epidermal growth factor receptors during mouse development. Dev Biol. 1984 May;103(1):62–70. doi: 10.1016/0012-1606(84)90007-1. [DOI] [PubMed] [Google Scholar]
- BRINSTER R. L. A METHOD FOR IN VITRO CULTIVATION OF MOUSE OVA FROM TWO-CELL TO BLASTOCYST. Exp Cell Res. 1963 Oct;32:205–208. doi: 10.1016/0014-4827(63)90093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman P., McLaren A. Cleavage rate of mouse embryos in vivo and in vitro. J Embryol Exp Morphol. 1970 Aug;24(1):203–207. [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor-alpha: structure and biological activities. J Cell Biochem. 1986;32(4):293–304. doi: 10.1002/jcb.240320406. [DOI] [PubMed] [Google Scholar]
- Galway A. B., Oikawa M., Ny T., Hsueh A. J. Epidermal growth factor stimulates tissue plasminogen activator activity and messenger ribonucleic acid levels in cultured rat granulosa cells: mediation by pathways independent of protein kinases-A and -C. Endocrinology. 1989 Jul;125(1):126–135. doi: 10.1210/endo-125-1-126. [DOI] [PubMed] [Google Scholar]
- Gan B. S., Hollenberg M. D., MacCannell K. L., Lederis K., Winkler M. E., Derynck R. Distinct vascular actions of epidermal growth factor-urogastrone and transforming growth factor-alpha. J Pharmacol Exp Ther. 1987 Jul;242(1):331–337. [PubMed] [Google Scholar]
- Gandolfi F., Moor R. M. Stimulation of early embryonic development in the sheep by co-culture with oviduct epithelial cells. J Reprod Fertil. 1987 Sep;81(1):23–28. doi: 10.1530/jrf.0.0810023. [DOI] [PubMed] [Google Scholar]
- Heyner S., Rao L. V., Jarett L., Smith R. M. Preimplantation mouse embryos internalize maternal insulin via receptor-mediated endocytosis: pattern of uptake and functional correlations. Dev Biol. 1989 Jul;134(1):48–58. doi: 10.1016/0012-1606(89)90077-8. [DOI] [PubMed] [Google Scholar]
- Huet-Hudson Y. M., Chakraborty C., De S. K., Suzuki Y., Andrews G. K., Dey S. K. Estrogen regulates the synthesis of epidermal growth factor in mouse uterine epithelial cells. Mol Endocrinol. 1990 Mar;4(3):510–523. doi: 10.1210/mend-4-3-510. [DOI] [PubMed] [Google Scholar]
- Ibbotson K. J., Harrod J., Gowen M., D'Souza S., Smith D. D., Winkler M. E., Derynck R., Mundy G. R. Human recombinant transforming growth factor alpha stimulates bone resorption and inhibits formation in vitro. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2228–2232. doi: 10.1073/pnas.83.7.2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimelman D., Kirschner M. Synergistic induction of mesoderm by FGF and TGF-beta and the identification of an mRNA coding for FGF in the early Xenopus embryo. Cell. 1987 Dec 4;51(5):869–877. doi: 10.1016/0092-8674(87)90110-3. [DOI] [PubMed] [Google Scholar]
- Marquardt H., Hunkapiller M. W., Hood L. E., Twardzik D. R., De Larco J. E., Stephenson J. R., Todaro G. J. Transforming growth factors produced by retrovirus-transformed rodent fibroblasts and human melanoma cells: amino acid sequence homology with epidermal growth factor. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4684–4688. doi: 10.1073/pnas.80.15.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson B. A., Rosenblum I. Y., Smith R. M., Heyner S. Autoradiographic evidence for insulin and insulin-like growth factor binding to early mouse embryos. Diabetes. 1988 May;37(5):585–589. doi: 10.2337/diab.37.5.585. [DOI] [PubMed] [Google Scholar]
- McLaren A., Smith R. Functional test of tight junctions in the mouse blastocyst. Nature. 1977 May 26;267(5609):351–353. doi: 10.1038/267351a0. [DOI] [PubMed] [Google Scholar]
- Murphy L. J., Murphy L. C., Friesen H. G. Estrogen induces insulin-like growth factor-I expression in the rat uterus. Mol Endocrinol. 1987 Jul;1(7):445–450. doi: 10.1210/mend-1-7-445. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Marquardt H., Todaro G. J., Gallis B., Casnellie J. E., Bornstein P., Krebs E. G. Transforming growth factor and epidermal growth factor stimulate the phosphorylation of a synthetic, tyrosine-containing peptide in a similar manner. J Biol Chem. 1982 Dec 25;257(24):14628–14631. [PubMed] [Google Scholar]
- Rappolee D. A., Brenner C. A., Schultz R., Mark D., Werb Z. Developmental expression of PDGF, TGF-alpha, and TGF-beta genes in preimplantation mouse embryos. Science. 1988 Sep 30;241(4874):1823–1825. doi: 10.1126/science.3175624. [DOI] [PubMed] [Google Scholar]
- Reuse S., Roger P. P., Vassart G., Dumont J. E. Enhancement of cmyc mRNA concentration in dog thyrocytes initiating DNA synthesis in response to thyrotropin, forskolin, epidermal growth factor and phorbol myristate ester. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1066–1076. doi: 10.1016/s0006-291x(86)80152-8. [DOI] [PubMed] [Google Scholar]
- Ristimäki A. Transforming growth factor alpha stimulates prostacyclin production by cultured human vascular endothelial cells more potently than epidermal growth factor. Biochem Biophys Res Commun. 1989 May 15;160(3):1100–1105. doi: 10.1016/s0006-291x(89)80116-0. [DOI] [PubMed] [Google Scholar]
- Rizzino A. Appearance of high affinity receptors for type beta transforming growth factor during differentiation of murine embryonal carcinoma cells. Cancer Res. 1987 Aug 15;47(16):4386–4390. [PubMed] [Google Scholar]
- Roblero L. S., Garavagno A. C. Effect of oestradiol-17 beta and progesterone on oviductal transport and early development of mouse embryos. J Reprod Fertil. 1979 Sep;57(1):91–95. doi: 10.1530/jrf.0.0570091. [DOI] [PubMed] [Google Scholar]
- Roblero L. Effect of progesterone in vivo upon the rate of cleavage of mouse embryos. J Reprod Fertil. 1973 Oct;35(1):153–155. doi: 10.1530/jrf.0.0350153. [DOI] [PubMed] [Google Scholar]
- Rosa F., Roberts A. B., Danielpour D., Dart L. L., Sporn M. B., Dawid I. B. Mesoderm induction in amphibians: the role of TGF-beta 2-like factors. Science. 1988 Feb 12;239(4841 Pt 1):783–785. doi: 10.1126/science.3422517. [DOI] [PubMed] [Google Scholar]
- Schreiber A. B., Winkler M. E., Derynck R. Transforming growth factor-alpha: a more potent angiogenic mediator than epidermal growth factor. Science. 1986 Jun 6;232(4755):1250–1253. doi: 10.1126/science.2422759. [DOI] [PubMed] [Google Scholar]
- Stern P. H., Krieger N. S., Nissenson R. A., Williams R. D., Winkler M. E., Derynck R., Strewler G. J. Human transforming growth factor-alpha stimulates bone resorption in vitro. J Clin Invest. 1985 Nov;76(5):2016–2019. doi: 10.1172/JCI112202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. L., Hehnke K., Rickords L. F., Southern L. L., Thompson D. L., Jr, Wood T. C. Early embryonic development in vitro by coculture with oviductal epithelial cells in pigs. Biol Reprod. 1989 Sep;41(3):425–430. doi: 10.1095/biolreprod41.3.425. [DOI] [PubMed] [Google Scholar]
- Winkler M. E., O'Connor L., Winget M., Fendly B. Epidermal growth factor and transforming growth factor alpha bind differently to the epidermal growth factor receptor. Biochemistry. 1989 Jul 25;28(15):6373–6378. doi: 10.1021/bi00441a033. [DOI] [PubMed] [Google Scholar]
- Wood S. A., Kaye P. L. Effects of epidermal growth factor on preimplantation mouse embryos. J Reprod Fertil. 1989 Mar;85(2):575–582. doi: 10.1530/jrf.0.0850575. [DOI] [PubMed] [Google Scholar]






