Abstract
TGFβ1 is thought to be intimately involved in cyclic tissue remodeling and inflammatory events associated with menstruation. Menstruation is initiated by progesterone withdrawal; however, the underlying mechanisms are not well understood. In the present study, we have tested the hypothesis that locally produced TGFβ1 may influence expression of progesterone receptor (PR) or the Wnt antagonist Dickkopf-1 (DKK) with consequential impact on regulation of menstruation. Endometrial stromal cells (ESC) were isolated from endometrial biopsy samples collected from patients undergoing gynecological procedures for benign indications. Treatment of differentiated ESC with TGFβ1 (10 ng/ml) significantly inhibited the expression of mRNAs encoding PR and DKK. TGFβ1 also attenuated the protein expression of PR and secretion of DKK proteins in culture supernatants. Neutralization of endogenous TGFβ1 signaling abolished the TGFβ1-induced effects, significantly increased expression of PR, and increased DKK protein release levels to that of differentiated ESCs, confirming the specificity of the TGFβ1 effect. Additionally, in vitro decidualization of ESCs significantly augmented DKK protein release. Moreover, although TGFβ1 was capable of signaling via the Sma- and mothers against decapentaplegic (MAD)-related protein (SMAD) pathway, the inhibitory effect on DKK was SMAD independent. Conversely, the inhibitory effect of TGFβ1 on PR was dependent on SMAD signal transduction. In conclusion, these results suggest that local TGFβ1 signaling can potentiate progesterone withdrawal by suppressing expression of PR and may coordinate tissue remodeling associated with menstruation by inducing Wnt-signaling via inhibition of DKK, which we found to be up-regulated as a consequence of decidualization of ESCs.
THE PROGESTERONE RECEPTOR (PR) is a member of the superfamily of ligand-activated transcription factors that bind to sequence-specific sites in the promoters of target genes. Two isoforms of the nuclear PR exist, known as PR-A (Mr 94,000) and PR-B (Mr 120,000) (1). A third, truncated form, PR-C (Mr 60,000), has been identified in the breast cancer epithelial cell line T47D and has subsequently been reported in the uterus (2, 3). In the human, expression of PR varies temporally and spatially, in both functional and basal regions and within the epithelial and stromal compartments, across the normal menstrual cycle (4, 5, 6). Decidualization of the stromal cells is associated with rapid down-regulation of PR-B, leaving PR-A as the dominant isoform (7, 8). Expression of the PR-A isoform expression is maintained throughout the cycle in the stromal cells (9). Therefore, the cell, which would have facilitated implantation in the presence of a blastocyst, is the decidualized stromal cell that responds to the withdrawal of progesterone and initiates menstruation.
TGFβ1, a secreted homodimeric protein, is the prototypic member of a family of approximately 40 structurally related proteins known as the TGFβ superfamily. TGFβ1 and its isoforms regulate a plethora of diverse biological functions (10, 11, 12, 13). TGFβ1 has been shown to enhance tissue remodeling and homeostasis in endometrial cells (14, 15), and inactivation of TGFβ1 has been implicated in endometrial carcinogenesis (16).
TGFβ1 is present in its latent form in the endometrium until the late secretory phase when it is activated by plasmin (17). Plasmin is formed from inactive plasminogen by urokinase plasminogen activator, which is itself regulated by plasminogen activator inhibitor (PAI-1) (18). TGFβ1 initiates its diverse cellular responses by stimulating formation of specific heteromeric complexes of type I (ALK 5) and type II serine/threonine kinase transmembrane receptors located at the cell surface. The type II receptor phosphorylates type I in the juxtamembrane region (GS domain, rich in glycine and serine residues), which in turn propagates the signal intracellularly via the phosphorylation of highly conserved members of receptor-regulated Sma- and mothers against decapentaplegic (MAD)-related protein (SMAD) family of transcriptional regulators, SMAD2 and -3 (19, 20, 21).
Protein inhibitors of activated signal transducer and activator of transcription (STAT) (PIAS) are a family of proteins originally identified through interaction with cytokine-induced STAT (22). PIASγ is reported to inhibit STAT1-mediated transcriptional responses (23, 24) and antagonizes Wnt-independent and Wnt-induced transcriptional activation of lymphoid enhancer factor 1 (LEF1) (25). TGFβ1 induces expression of endogenous PIASγ, and in turn, PIASγ interacts with SMAD3 and antagonizes SMAD3-dependent transcriptional activation by TGFβ type 1 receptor, thereby providing a negative feedback mechanism for regulation of TGFβ1 signaling (26) and a potential mechanism for antagonism of downstream transcriptional activity.
Recent studies have shown that a physical association between intracellular components of these two pathways, namely, SMAD3 and lymphoid enhancer factor 1/T-cell-specific factors (27), mediates synergistic activation of Xtwn, a Wnt and TGFβ target gene. It has been shown that the secreted protein Dickkopf (DKK) inhibits Wnt signaling. DKK has been demonstrated to inhibit Wnt signaling by binding to a low-density lipoprotein receptor-related protein, LRP6 and inhibits signaling by disrupting the binding of LRP6 to the Wnt/Fz ligand-receptor complex (28, 29, 30). It has been reported that DKK mRNA expression is significantly up-regulated in the stromal cells in the secretory phase of the cycle, suggesting that progesterone stimulates DKK expression and implying a role for DKK in decidualization of the endometrium (31).
A better understanding of the local mechanisms involved in the regulation of menstruation and implantation is essential to understand the pathophysiology of menstrual bleeding complaints and early pregnancy complications such as spontaneous miscarriage. In the current study, we have therefore investigated the possibility that TGFβ1 may play a role in the initiation of menstruation by modulating PR and/or DKK expression via the SMAD signaling pathway.
RESULTS
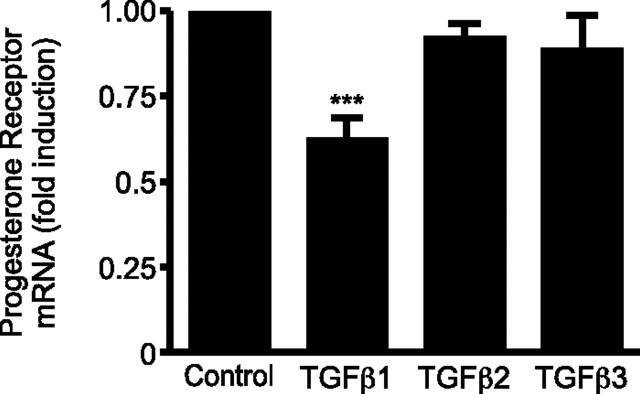
TGFβ1 But Not TGFβ2 or -3 Down-Regulates PR mRNA Expression
TGFβ1, TGFβ2, and TGFβ3 have previously been localized within the endometrium (32, 33). To address the question of whether TGFβ isoforms caused alterations in PR mRNA expression, endometrial stromal cells (ESCs), decidualized in vitro with 8-Br-cAMP (0.5 mm) and medroxyprogesterone acetate (MPA) (1 μm) (decidualizing medium) for 6 d as measured by increase in IGF-binding protein-1 mRNA and protein release (P < 0.05 and P < 0.001, respectively; n = 4 endometrial samples) (supplemental Fig. S1, A and B, respectively, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org), were treated for 72 h with TGFβ1, TGFβ2, or TGFβ3 (10 ng/ml). TGFβ1 alone caused a significant decrease in PR mRNA compared with controls (P < 0.001; n = 6) (Fig. 1). TGFβ2 and TGFβ3 were without significant effect but displayed a trend toward reduction.
Fig. 1.
TGFβ1 Is the Most Effective Isoform Localized within the Endometrium at Suppressing PR mRNA
Cultured ESCs were decidualized in vitro with or without TGFβ1, TGFβ2, or TGFβ3 (all at 10 ng/ml) for 72 h. TGFβ1 reduces expression of mRNA nuclear PR in a significant manner (P < 0.001). TGFβ2 and TGFβ3 show no significant effect. Results are ± sem. n = 6 endometrial samples. ***, P < 0.001.
TGFβ1 Alters PR Expression in Decidualized ESCs in a Time-Dependent Manner
To further address the role of TGFβ1 in regulation of ESC PR expression, ESCs were cultured, decidualized in vitro for 6 d (see above), and then further incubated with decidualizing medium with or without the addition of TGFβ1 for a maximum of 72 h. TGFβ1 up-regulated expression of PR mRNA in decidualized ESCs 2-fold (P < 0.001; n = 6) after 2 h incubation; by 24 and 36 h, levels were not significantly different from those of controls (Fig. 2A). However, by 72 h, expression of PR mRNA was down-regulated 2-fold as compared with unstimulated controls (P < 0.05; n = 6) (Fig. 2A). Similar results were observed in T47D cells (supplemental Fig. S2A, published as supplemental data on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org). Immunoexpression of PR was localized to nuclei in primary ESCs (Fig. 2B) and T47D cells (supplemental Fig. S2B). Comparison of staining intensities was consistent with a decrease of immunoreactive PR in the nuclei of both cell types (compare panels I and II) after 72 h treatment with TGFβ1. Representative examples are shown.
Fig. 2.
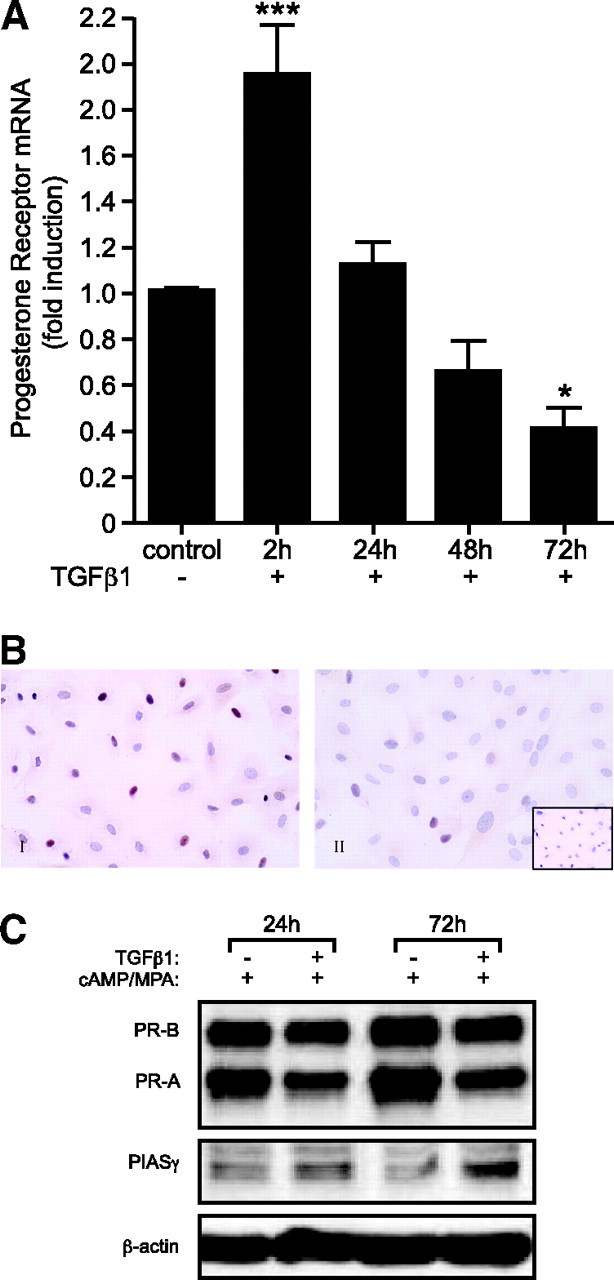
TGFβ1 Inhibits mRNA and Protein Expression of PR and Increases Protein Expression of PIASγ
A, Time course of PR mRNA expression in cultured ESCs decidualized in vitro with or without TGFβ1 measured by Q-RT-PCR. TGFβ1 reduces expression of PR mRNA in a time-dependent manner in primary ESCs. Results are ± sem (P < 0.05); n = 6 endometrial samples. B, Immunohistochemical analysis of PR expression in control and TGFβ1-treated ESC cells; PR immunostaining of ESCs decidualized in vitro (I) and ESCs decidualized in vitro plus TGFβ1 (10 ng/ml) (II). Inset shows negative control (matched isotype control). C, Whole-cell lysates from primary ESC cultures decidualized in vitro for 24 or 72 h, with or without TGFβ1 (10 ng/ml), were subjected to Western blot analysis. TGFβ1 treatment reduced protein expression of both PR-A and PR-B in decidualized ESCs and increased protein expression of PIASγ in decidualized ESC. *, P < 0.05; ***, P < 0.001.
Protein expression of PR in decidualized ESCs was analyzed by Western blotting (Fig. 2C). On Western blots, we observed reduced expression of PR-A and PR-B after treatment of decidualized ESCs with TGFβ1 (Fig. 2C). This response appeared more marked in cells exposed to decidualizing agents for 72 h (Fig. 2C). To determine whether TGFβ1 treatment induced PIASγ expression, thereby stimulating a potential negative feedback loop on its own production or inhibition of its downstream transcriptional activity, protein expression of PIASγ was analyzed by Western blot analysis. TGFβ1 treatment modestly increased protein expression of PIASγ in a time-dependent manner in decidualized cells (Fig. 2C).
TGFβ1 Does Not Affect the Transactivation Potential of Steroid Receptors
To elucidate whether the TGFβ1-mediated-signaling has a direct effect on the progesterone response element (PRE) or the estrogen response element (ERE), a luciferase promoter/reporter assay was conducted. The presence of an appropriate steroid receptor agonist, but not addition of TGFβ1, significantly induced reporter gene expression (P < 0.001 and P < 0.01, Fig. 3, A and B, respectively). Coincubation with steroid agonist and TGFβ1 did not have a significant impact on reporter gene activation as compared with receptor agonist alone but did significantly induce reporter gene expression as compared with control (P < 0.001, Fig. 3, A and B).
Fig. 3.
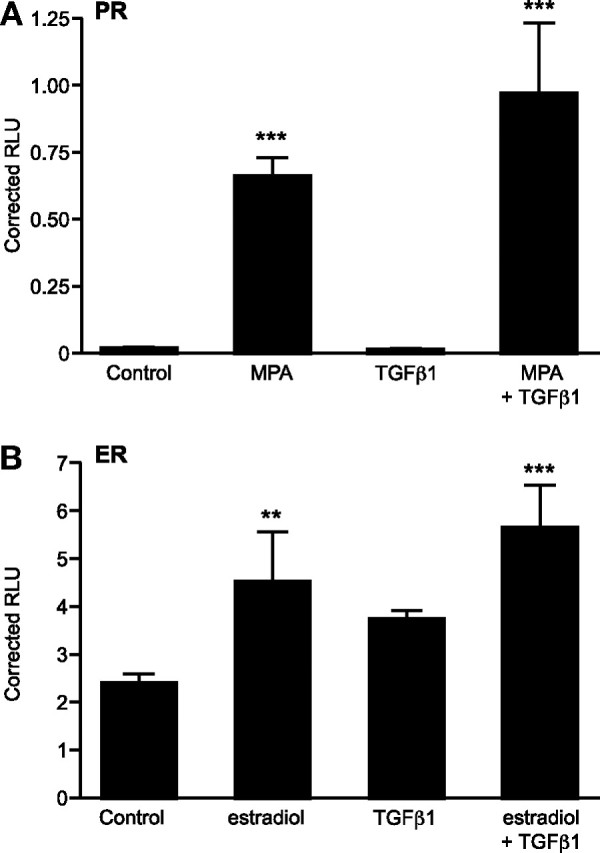
TGFβ1 Does Not Alter the Transactivation Potential of PR and ER
Decidualized ESCs were transiently transfected with either PRE-luc (A) or ERE-luc (B) expression vector, and cells were treated with or without MPA (PRE) or estradiol (ERE) with or without TGFβ1. TGFβ1 was without effect, either alone or in combination with the steroid-specific agonist, on induction of reporter gene activation for both response elements. Results are ± sem; n = 3 endometrial samples. **, P < 0.01; ***, P < 0.001.
TGFβ1 Down-Regulates DKK in Decidualized Stromal Cells
TGFβ1 had a transient but significant impact on DKK mRNA expression at 12 and 24 h (P < 0.05; n = 5) that displayed a return to control levels if cultured for 48 h or more (Fig. 4A). Protein release was also measured in the same samples; reduced release of DKK protein lagged 36 h behind the reduction in mRNA with a significant reduction at 48 and 72 h (both P < 0.001; n = 5) (Fig. 4B). Notably, the concentration of DKK measured in the media was dramatically increased after treatment with decidualizing medium for 72 h (P < 0.001; n = 5) (Fig. 5B).
Fig. 4.
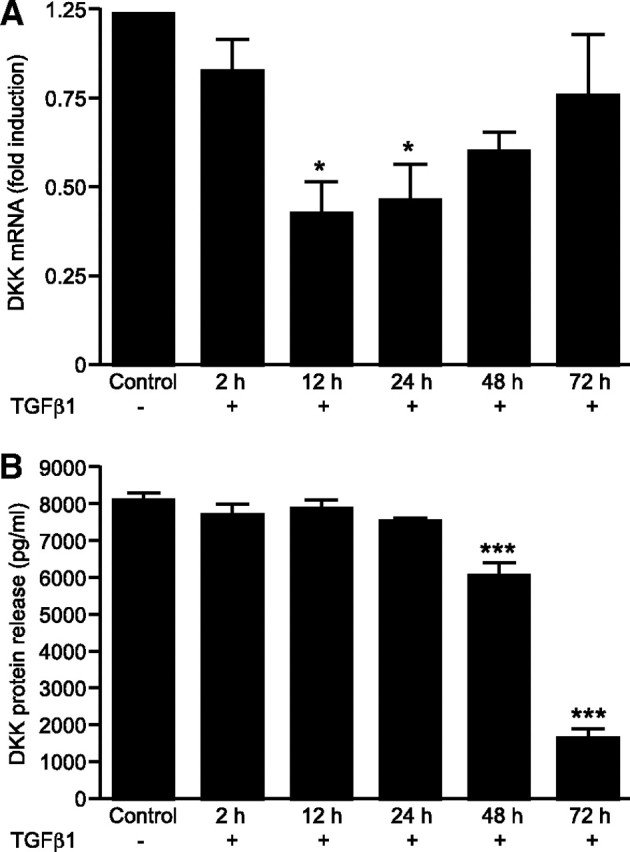
TGFβ1 Treatment Down-Regulates DKK in Decidualized ESCs
A, Time course of DKK mRNA expression in decidualized ESCs with or without TGFβ1 measured by Q-RT-PCR. TGFβ1 reduces expression of DKK mRNA over a period of 24 h but displays a return to control levels if cultured for 48 h or more. B, Time course of DKK protein release in cultured ESCs decidualized in vitro with or without TGFβ1 (10 ng/ml) quantified by ELISA. TGFβ1 significantly reduces protein release of DKK in a time-dependent manner over a period of 48 h (P < 0.001). Down-regulation of DKK is seen at 72 h also (P < 0.001). Results are ± sem; n = 5 endometrial samples. *, P < 0.05; ***, P < 0.001.
Fig. 5.
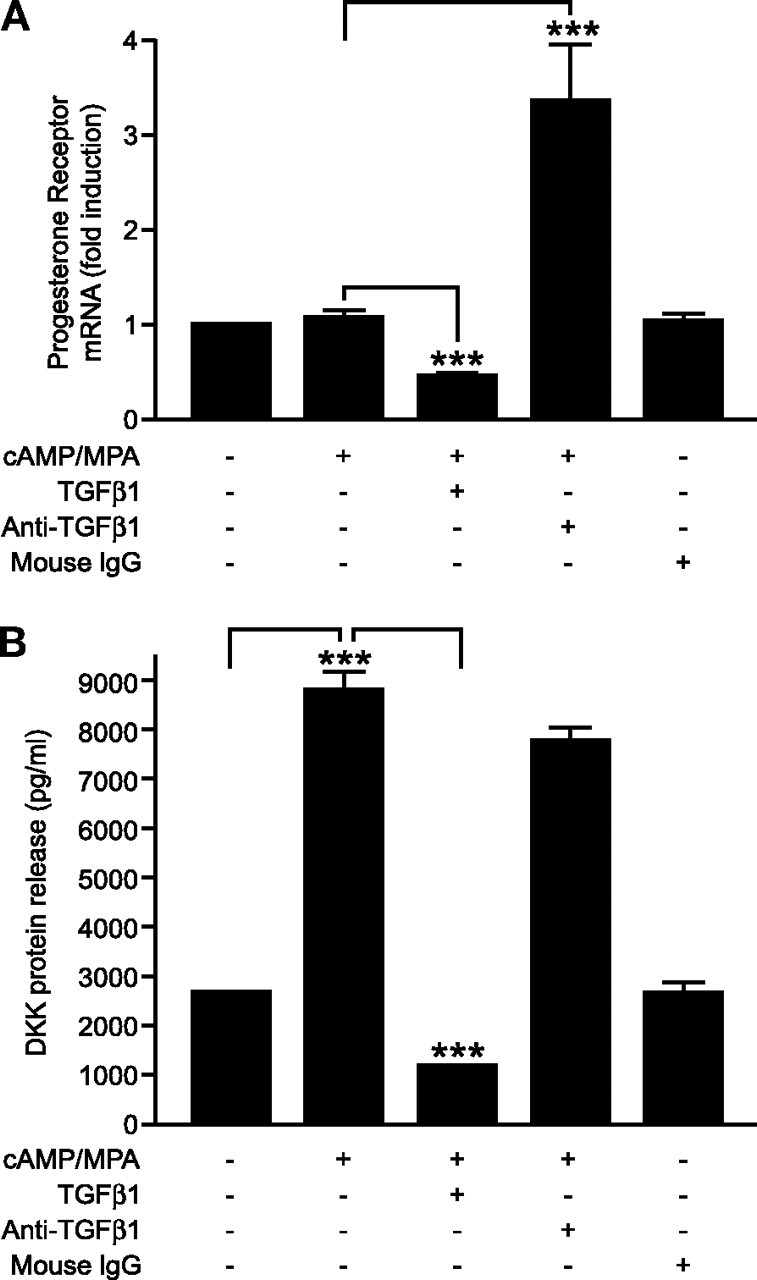
Anti-TGFβ1 Antibody Neutralizes Endogenous TGFβ1 and Potentiates PR Expression but Has No Effect on DKK Protein Release
Cultured ESCs were decidualized in vitro with or without TGFβ1 treatment. To confirm the specificity of the TGFβ1 response, anti-TGFβ1 (1 μg/ml) or mouse IgG control was added, for 72 h. A, TGFβ1 reduces expression of mRNA nuclear PR, whereas anti-TGFβ1 antibody increases nuclear PR. B, TGFβ1 reduces protein release of DKK, whereas anti-TGFβ1 antibody has no effect. Results are ± sem (P < 0.05); n = 5 endometrial samples. ***, P < 0.001.
Blocking Endogenous TGFβ1 Action Negates TGFβ1-Induced PR mRNA Suppression but Has No Significant Effect on DKK Protein Release
We hypothesized that ESCs would be capable of endogenous biosynthesis of TGFβ1 and therefore investigated whether the addition of a blocking antibody could neutralize the activity of both endogenous and exogenous TGFβ1. ESCs were cultured in the presence and absence of decidualizing medium for 6 d and then further cultured with decidualizing medium to maintain the decidualized phenotype in the presence or absence of TGFβ1 (10 ng/ml) or anti-TGFβ1 antibody (1 μg/ml) for 72 h. Consistent with our previous studies herein, addition of TGFβ1 significantly down-regulated expression of PR mRNA in decidualized ESCs (P < 0.001; n = 5) (Fig. 5A). Furthermore, addition of anti-TGFβ1 resulted in a significant increase in PR mRNA as compared with decidualized ESCs in the absence of TGFβ1 treatment, demonstrating that this had been partially down-regulated by endogenous TGFβ1 (P < 0.001; n = 5) (Fig. 5A). Protein release of DKK was also measured; TGFβ1 significantly down-regulated protein release of DKK as compared with decidualized ESC (P < 0.001; n = 5) (Fig. 5B); however, addition of anti-TGFβ1 was without effect on protein release of DKK by the decidualized cells (Fig. 5B). Treatment with the isotype control, mouse IgG1, evoked no response in either PR mRNA expression or DKK protein release (Fig. 5, A and B, respectively). We did not add anti-TGFβ1 to cells exposed to exogenous TGFβ1 because it would be difficult to completely neutralize 10 ng/ml TGFβ1, and therefore any results obtained would be inconclusive.
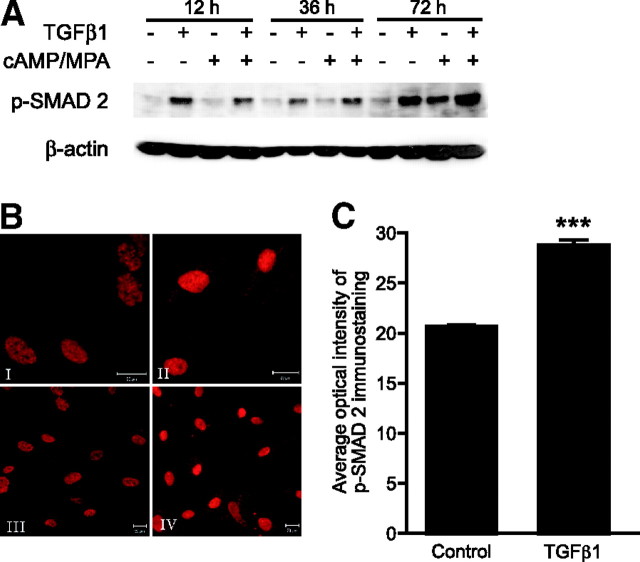
TGFβ1 Signals via the SMAD-Signaling Pathway in Nondecidualized and Decidualized ESCs
To investigate whether TGFβ1 was having an impact on the SMAD-signaling pathway in primary ESCs, cells were cultured as before, and changes in phosphorylated SMAD2 (p-SMAD2) protein expression were investigated. At all time points (12, 36, and 72 h), the amount of p-SMAD2 protein was increased by incubation with TGFβ1 regardless of whether cells were nondecidualized or decidualized (Fig. 6A). Results of Western analysis were confirmed when immunocytofluorescence staining was performed on decidualized cells 72 h after incubation with TGFβ1 (Fig. 6B). Panels I and III depict immunocytofluorescence (ICF) on control cells; panel III is a larger-view field of panel I. Panels II and IV depict ICF on TGFβ1-treated cells; panel IV is a larger-view field of panel II. Deciphering staining intensities with the naked eye can be very subjective; for this reason, the images were quantitatively analyzed (Fig. 6C); 10 ng/ml TGFβ1 significantly up-regulated p-SMAD2 protein expression in decidualized ESCs (P < 0.001).
Fig. 6.
TGFβ1 Induces SMAD2 Phosphorylation in ESC, Both Decidualized and Nondifferentiated
A, Nuclear lysates from untreated primary ESCs or cultures decidualized in vitro for 12, 36, or 72 h, with or without TGFβ1 (10 ng/ml), were subjected to Western blotting analysis. TGFβ1 treatment induced protein expression of p-SMAD2 at each time point. B, ICF analysis of p-SMAD2 expression in control and TGFβ1-treated decidualized ESCs; I–IV, p-SMAD2 immunostaining; I, decidualized ESCs without treatment; II, decidualized ESCs plus TGFβ1 (10 ng/ml); III, decidualized ESCs without treatment, larger-view field; IV, decidualized ESCs plus TGFβ1 (10 ng/ml), larger-view field. C, Quantitative analysis of average optical intensity of p-SMAD2. Fifteen control and 15 TGFβ1-treated decidualized ESCs (10 ng/ml) were analyzed from each endometrial biopsy. All settings were identical for control and treated cells. TGFβ1 significantly up-regulated p-SMAD2 protein expression (P < 0.001); n = 5 endometrial biopsies. ***, P < 0.001.
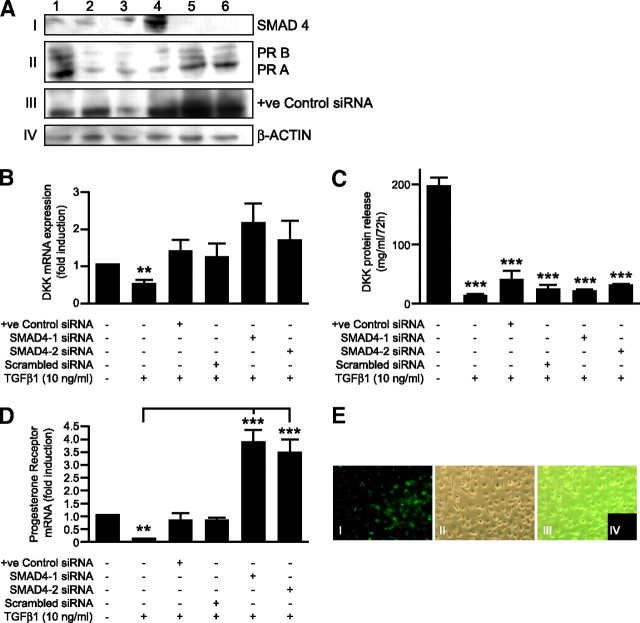
TGFβ1 Inhibits PR But Not DKK in a SMAD-Dependent Manner
Two small interfering RNAs (siRNAs) specific for SMAD4 was used to investigate whether selectively silencing the SMAD signaling pathway would abrogate the TGFβ1-induced responses. Western blotting (Fig. 7A) demonstrated that transfection with both the SMAD4-specific siRNAs resulted in a dramatic reduction in the amount of SMAD4 protein (Fig. 7A, panel I) consistent with efficient transfection (Fig. 7E). Depletion of SMAD4 from decidualized ESCs was not able to abrogate the TGFβ1-dependent reduction in DKK protein release, although there did not appear to be a reduction in mRNA in the presence of the SMAD4 siRNA, so this was not specific or significant (Fig. 7, B and C). In contrast, depletion of SMAD4 had a dramatic and specific impact on the amount of PR mRNA and protein (both A and B) (Fig. 7, A and D). As expected, treatment of mock-transfected cells with TGFβ1 (10 ng/ml) resulted in a reduction in the expression of both PR and DKK mRNA and protein. No significant differences were observed in cells transfected with controls for all siRNAs in the absence of TGFβ1 (data not shown).
Fig. 7.
TGFβ1 Inhibits PR But Not DKK in a SMAD-Dependent Manner
A, Western blot analysis of SMAD4 siRNA-treated ESCs. Samples 1 and 2 represent mock-transfected cells. Sample 3 represents cells transfected with a positive control siRNA. Sample 4 represents cells transfected with a scrambled, nontargeting siRNA. Sample 5 represents cells transfected with SMAD4-1 siRNA. Sample 6 represents cells transfected with SMAD4-2 siRNA. After transfection, samples 2–6 were treated with TGFβ1 (10 ng/ml) for 72 h. Before (36 h) and after transfection (72 h), cells were treated with decidualizing stimuli, 8-Br-cAMP (0.5 mm), and MPA (1 μm). n = 6 endometrial samples. Panel I shows a reduction in SMAD4 protein expression in samples 5 and 6. Panel II demonstrates a reduction in both PR-A and PR-B protein expression in samples 2–4. However, cells treated with SMAD4 siRNA (samples 5 and 6) demonstrate increased protein expression. Panel III demonstrates successful knockdown of the positive control as evidenced by the reduction in protein expression in sample 3, and panel IV shows β-actin protein expression, which served as a loading control. B, TGFβ1 treatment reduces DKK mRNA expression (P < 0.05) in mock-transfected cells but is without significant effect in transfected cells. C, TGFβ1 treatment reduces protein release (P < 0.001) of DKK in the presence and absence of the SMAD signaling pathway. D, TGFβ1 treatment reduces expression of PR mRNA (P < 0.001) but is without effect when SMAD4 is silenced. E, Transfection efficiency of siRNA. Panel I represents cells transfected with 488 nm-labeled AllStars negative control siRNA (5 nm). Panel II shows phase contrast of cells in A. Panel III represents an overlay of both A and B. Panel IV shows cells transfected with the unlabeled AllStars negative control siRNA (5 nm). Cells were transfected for 20 h. **, P < 0.01; ***, P < 0.001.
DISCUSSION
In the present study, we report for the first time evidence demonstrating that TGFβ1 acting via a SMAD-dependent mechanism is able to inhibit expression of PR mRNA and protein. We also report novel findings demonstrating that TGFβ1 may enhance the activity of the Wnt-signaling pathway by inhibiting expression of DKK via a SMAD-independent mechanism.
The postovulatory rise in progesterone, acting via the nuclear PR (34, 35), induces profound phenotypical and morphological remodeling of the estrogen-primed endometrium resulting in decidualization. In the absence of implantation, the withdrawal of progesterone initiates the cascade of events associated with menstruation (36, 37). Several studies provide evidence that progesterone interacts with other factors, e.g. cAMP, to mediate its effect on differentiating endometrial stroma and that these may also participate in the processes leading to menstruation (7, 8, 38, 39, 40). In this study, primary ESCs were treated with 8-Br-cAMP and MPA to induce a decidualized phenotype, and successful in vitro decidualization, as determined by the increase in IGFBP-1 mRNA and protein release, was consistently achieved.
We have demonstrated that after a transient rise, TGFβ1 significantly reduces expression of PR in decidualized endometrial stromal cells in a time-dependent manner. Furthermore, we have shown that this inhibition is not caused by any impact of TGFβ1 on ER-mediated PR gene transcription. Several studies have reported that PR expression is under dual control of estradiol and progesterone acting via their cognate nuclear receptors on EREs and PREs, respectively (41, 42, 43). However, using transient transfections of PRE and ERE into ESC treatment with the appropriate steroid receptor agonist resulted in increased luciferase activity, but addition of TGFβ1 did not activate reporter activity nor did TGFβ1 have any impact on agonist-driven reporter activity. No explanation has been elucidated for the initial TGFβ1-induced up-regulation of PR at 2 h, but we would speculate that this could be due to activation of recently identified nongenomic PRs (44, 45). Because no functional role has been identified for the nongenomic receptors, we were unable to measure any specific downstream target genes to assess the impact of TGFβ1 on these putative membrane-bound receptors.
Previous studies have reported that TGFβ1 inhibited expression of progesterone-induced genes (46). It has been suggested that although TGFβ1 promotes selective progesterone responses, it opposes others, implying that TGFβ1 acts as a PR-independent, gene-specific antiprogestin, although evidence to support such suggestions is scant. In contrast, we have demonstrated that the down-regulation of PR in nondecidualized ESCs (data not shown), decidualized ESCs, and T47D cells suggests that the effect of TGFβ1 is not stromal cell specific but PR specific. Furthermore, we have reported that ESCs contain endogenous TGFβ1, and neutralizing this negated any TGFβ1-induced effects, providing evidence that the down-regulation of PR is TGFβ1 specific. Additional evidence to support a relationship between progesterone and TGFβ1 were provided by Bruner et al. (47), who reported that TGFβ1 and progesterone are intimately involved in the prevention of experimental endometriosis in a nude mouse model, leading to the suggestion that TGFβ1 could be used for treatment of endometriosis (48).
Furthermore, in cases of aberrant endometrial bleeding, the proposed relationship between TGFβ1 and progesterone may be interrupted. In the normal menstrual cycle, progesterone is reported to suppress endometrial-associated bleeding factor (EBAF; a member of the TGFβ family), also known as LEFTY-A, during the secretory phase of the cycle. With subsequent withdrawal of progesterone, EBAF expression is no longer suppressed and can act to stimulate events associated with the shedding of the upper functional layer of endometrium and menstruation (49). The data presented herein would be consistent with TGFβ1 down-regulation of PR expression, augmented progesterone withdrawal, and EBAF and EBAF-mediated tissue shedding. The latter also acts as a profibrogenic cytokine to maintain the integrity of ECM in endometrium (50, 51). Increased expression of TGFβ1 and /or EBAF/LEFTY-A may result in aberrant menstrual bleeding.
In the studies presented herein, TGFβ1 up-regulated protein expression of PIASγ. This is in agreement with previous studies reporting that TGFβ1 induced expression of endogenous PIASγ (26). PIASγ is reported to inhibit SMAD transcriptional activity and other transcriptional responses (26). Interestingly, other studies have demonstrated that conditional overexpression of PIASγ selectively inhibits a subset of endogenous TGFβ-responsive genes, which includes PAI-1 (52). A previous study reported that TGFβ1 stimulates the synthesis of PAI-1, demonstrating a negative feedback loop on its own production. In addition, progesterone has been shown to regulate the release of PAI-1 in stromal cells in vitro (53). Furthermore, urokinase plasminogen activator is expressed in the late secretory phase in coordination with falling progesterone levels before menstruation (32, 54), possibly indicating the existence of a relationship between progesterone and TGFβ1 (55). The story becomes more complex as recent studies have demonstrated that PIASγ is complexed to the PR in human ESCs and that its ability to repress STAT1 signaling is dependent upon activation of PR in response to hormone binding (56). The same study reported that IFNγ and PIASγ synergistically inhibited PR-dependent transcription, demonstrating that the progesterone and IFNγ signaling pathways engage in reciprocal transcriptional antagonism in human endometrium (56). IFNγ has previously been reported to inhibit the TGFβ-induced phosphorylation of SMAD3 and its attendant events to prevent TGFβ-induced gene transcription (57, 58, 59). TGFβ1 may act to up-regulate PIASγ, which is complexed with PR, to interact with IFNγ, present in the late-secretory phase, to inhibit further production or activation of TGFβ1, hence limiting its own biological actions. Furthermore, in our findings, PIASγ protein expression was only modestly up-regulated after 72 h, implying that its regulatory effects on TGFβ1 function are delayed.
In these present studies, incubation of decidualized ESCs with TGFβ1 resulted in a transient but significant reduction in DKK mRNA expression that was followed by a reduction in release of DKK protein. The findings presented herein correlate with published data showing reduced expression of DKK in accordance with increased receptor activity for TGFβ1 in the late secretory phase of the menstrual cycle (54). We did not obtain evidence that TGFβ1-mediated repression of DKK is dependent on PR activity, and further investigations would need to examine the effect of TGFβ1 on DKK expression in cells lacking PR. Notably, in the present studies, we have demonstrated that addition of anti-TGFβ1 antibody potentiates PR mRNA expression as compared with controls but has no effect on DKK protein release. In the first instance, this may suggest that TGFβ1 antagonizes DKK expression, independently of PR. However, an alternative explanation may be that PR serves as a cofactor for another, perhaps rate-limiting, transcription factor responsible for DKK expression. If this was the case, PR knockdown would reduce expression of DKK; however, overexpression of PR might not have the opposite effect.
In the present studies, we also demonstrated a marked increase in the release of DKK protein when ESCs were decidualized in vitro. These results would be consistent with the report of Tulac et al. (31) who found a significant up-regulation of DKK in stromal cells during the secretory, compared with the proliferative phase of the menstrual cycle (31, 60). A recent study following up on these findings found that DKK mRNA synthesis and protein expression was up-regulated in human ESCs decidualized in vitro and that the response was progesterone specific and independent of cAMP and estradiol (61). In transfection assays, incubation with TGFβ1 treatment did not alter the activity of either an ERE- or a PRE-driven reporter gene. To date, there has been no evidence for the existence of a PRE (61) in the DKK promoter. In this context, Goldman and Shalev (62) have suggested a possible mechanism for PRE-independent progesterone-mediated responses. In addition to the direct transcriptional activation through binding with its cognate DNA response element, PR is capable of transcriptional activation interacting with other classes of transcription factors on their cognate binding site, e.g. cAMP response element-binding protein-binding protein and/or specificity protein-1 (62). In such cases where PR acts in a PRE-independent manner, it acts as a coactivator or corepressor (62). Although this proposed mechanism has not yet been conclusively proven, it does suggest a potentially novel process by which progesterone could regulate expression of DKK.
Previous studies have reported that Wnt family members are expressed in human endometrium throughout the menstrual cycle (31). Tulac et al. (31) reported no significant menstrual cycle dependence of the Wnt ligands (except Wnt-3), receptors, or downstream effectors. Tulac et al. (31) further reported that Wnt-3 was significantly increased in proliferative compared with secretory-phase endometrium in accordance with data from Hou et al. (63), who reported that canonical Wnt signaling is critical to estrogen-mediated uterine growth. Furthermore, a recent study, using mifeprisone as a model to delineate the molecular response to progesterone withdrawal, has reported coordinated up-regulation of the Wnt receptors and Wnt5A by mifepristone. The authors suggest that in response to progesterone withdrawal, the Wnt signaling cascade may mediate epithelial/mesenchyme interactions during menstruation and endometrial repair (64). Accumulating data would suggest a role for DKK in promoting cellular differentiation in adipocytes (65). Additionally, Wnt signaling in maturing osteoblasts requires down-regulation to enable the formation of a mineralized bone matrix, and this is, in part, due to DKK function (66), suggesting that the normal Wnt-signaling pathway is involved in proliferation and that antagonism of the pathway by DKK promotes differentiation. The present results show that TGFβ1 down-regulates DKK mRNA before it down-regulates PR mRNA. If DKK is indeed involved in decidualization, then this initial inhibition of DKK may induce Wnt signaling and heralds the onset of TGFβ1-induced abrogation of the decidualized phenotype in the ESCs during the normal menstrual cycle.
The present data are consistent with the published literature. During the normal nonpregnant menstrual cycle, the Wnt signaling cascade may mediate uterine proliferation during the proliferative phase (31, 63) and facilitate differentiation of the stromal compartment during the mid-secretory phase in accordance with up-regulation of the Wnt antagonist DKK (31, 60). In response to TGFβ1 activation and subsequent signaling inhibiting DKK during the late-secretory phase, in accordance with the onset of progesterone withdrawal, the Wnt receptors and Wnt 5A are up-regulated to mediate endometrial repair (64).
In the present study, we have demonstrated induction of SMAD2 phosphorylation in response to treatment with TGFβ1. These findings are in agreement with those of others (21, 67, 68, 69) who have reported that SMAD2 and SMAD3 are rapidly phosphorylated in response to TGFβ signaling. Moreover, we have demonstrated that the TGFβ1-specific down-regulation of PR is SMAD dependent. This is in contrast with a study examining disruption of the SMAD signaling pathway in human breast carcinoma (70) in which Xie et al. (70) reported no association between steroid receptor expression and loss of SMAD signaling. We have also demonstrated that the TGFβ1-induced suppression of DKK is not SMAD dependent and would suggest that TGFβ1 is mediating its effects via an alternative pathway. One suggestion is that TGFβ1-specific down-regulation of DKK is SMAD3 dependent and SMAD4 independent. It is possible that PIASγ may down-regulate SMAD3 (26), and we have shown a modest induction of PIASγ in decidualized cells in response to TGFβ1 treatment in agreement with the PIASγ expression profile in decidualized ESCs reported by Jones et al. (71). However, silencing SMAD3 with siRNA may not yield any answer to this phenomenon because other signaling pathways, including SMAD2-mediated SMAD signaling, may compensate for the silencing of SMAD3 and thereby mask any siRNA-induced downstream transcriptional changes. Additionally, because only a modest induction of PIASγ was observed after 72 h, any affect on TGFβ1 signaling and downstream transcriptional activity would likely take place after 72 h. This would mean that the TGFβ1-mediated suppression of DKK mRNA and protein release initially observed after 12 and 48 h, respectively, could not be associated with the induction of PIASγ and the likely subsequent suppression of SMAD3.
In summary, these studies have demonstrated that TGFβ1 activates its receptors in the decidualized ESCs to promote down-regulation of both PR transcript and protein, inhibition of DKK (a Wnt inhibitor), and induction of SMAD2 phosphorylation. Additionally, TGFβ1 may be regulating its own biological actions via induction of PIASγ. Therefore, TGFβ1 may play a key role in regulating processes such as decidualization and menstruation.
MATERIALS AND METHODS
Human Uterine Tissue Collection
Human endometrial tissue specimens (n = 35) were obtained from women undergoing surgery for nonmalignant gynecological conditions. A dedicated research nurse obtained informed patient consent in writing from each patient before tissue collection after local ethical committee approval for the study was granted. Biopsies were collected with an endometrial suction curette (Pipelle; Laboratoire CCD, Paris, France), or alternatively, full-thickness endometrial samples were obtained at hysterectomy. These latter biopsies included superficial and basal endometrium plus the endometrial-myometrial junction. All patients were of reproductive age, had regular menstrual cycles from 25–35 d, and had not received exogenous hormones or used an intrauterine contraceptive device in the 3 months before surgery. All subjects had a serum sample collected at the time of surgery for the determination of circulating estradiol and progesterone levels by RIA. All samples were consistent with the designated cycle stage based on standard histological criteria of Noyes et al. (72) and the patient’s reported last menstrual period (73) and circulating estradiol and progesterone levels at time of biopsy collection.
Endometrial tissue was collected in sterile RPMI 1640 medium (Sigma, Poole, Dorset, UK) and processed in one of two ways, either for histology (fixation in 10% neutral buffered formalin 24 h at 4 C followed by storage in 70% ethanol before wax embedding) or for tissue culture (separation of glandular epithelium and stromal cells, to culture the stromal cells).
Isolation of Stromal Cells
Endometrial specimens (n = 35) were separated into epithelial and stromal cell preparations by enzymatic digestion. Briefly, specimens were washed in Dulbecco’s PBS (Sigma) and minced into 1-mm3 pieces. The minced tissue was digested in collagenase (1 mg/ml; Sigma) and DNase (0.1 mg/ml; Sigma) for 80 min at 37 C. Repeat passage through an 18-gauge needle was used to aid tissue dispersion. Tissue was resuspended in 10 ml RPMI 1640 medium (Sigma). The stromal and glandular epithelium cells were pelleted by centrifugation (1700 rpm, 3 min). The cells were then resuspended in 10 ml RPMI 1640 medium (Sigma) supplemented with 10% fetal calf serum (FCS) (Mycoplex; PAA Laboratories, Kingston-Upon-Thames, UK), penicillin (50 μg/ml; Sigma), streptomycin (50 μg/ml; Sigma), and gentamycin (5 μg/ml; Sigma), and dispersed ESCs separated from endometrial glands by filtration through a 73-μm nylon sieve (Falcon; VWR International Ltd., Leicestershire, UK). The filtrate, containing the stromal cells, was plated in 75-cm3 culture flasks (Corning Inc., Corning, NY) for a minimum period of 5 d and allowed to reach confluence.
In Vitro Primary Cell Culture Experiments
ESCs were maintained at 37 C in 5% (vol/vol) CO2 in RPMI 1640 medium (Sigma) supplemented with 2% FCS (Mycoplex), penicillin (50 μg/ml; Sigma), streptomycin (50 μg/ml; Sigma), and gentamycin (5 μg/ml; Sigma). The cells were seeded in six-well plates at a concentration of 2.5 × 105 cells/ml and allowed to adhere and attain 90% confluence. Supernatant was changed every 3 d. Decidualization of the cells was induced with RPMI 1640 medium containing 2% FCS, 8-Br-cAMP (0.5 mm; Sigma), and 6α-methyl-17α-acetoxyprogesterone (MPA) (1 μm) for 6 d; thereafter, the cells were treated with 2% FCS RPMI 1640 and decidualizing medium containing TGFβ1. Cells were maintained under these conditions for up to 72 h. T47D cells, the breast cancer epithelial cell line, known to constitutively overexpress both isoforms of the PR, PR-A and PR-B (74), and the recently identified PR-C (2), were cultured in RPMI 1640 medium (Sigma) supplemented with 10% FCS (Mycoplex), penicillin (50 μg/ml; Sigma), streptomycin (50 μg/ml; Sigma), and gentamycin (5 μg/ml; Sigma) in the presence of insulin-transferrin-sodium (5 μg/ml) selenite media supplement (5 ng/ml; Sigma). The cells were treated with either serum-free RPMI 1640 medium alone or serum-free RPMI 1640 medium containing TGFβ1 (10 ng/ml; R&D Systems, Abington, UK). Again, cells were maintained under these conditions for up to 72 h.
Expression Vectors, siRNA Duplexes, and Transient Transfection
The reporter vectors PRE/-32/luc3 and ERE-tk/Luc3 were obtained from Dr. Jan Brosens (Imperial College, London, UK). PCH110, β-galactosidase expression vector, was purchased from Pharmacia Biotech (Piscataway, NJ). Nontargeting siRNA and siRNA specific for SMAD4 was purchased from Dharmacon (Perbio Science, Erembodegem Belgium). Two HP GenomeWide siRNA duplexes to SMAD4 (Genbank accession no. NM_005359) were purchased from QIAGEN (Crawley, UK). A positive control siRNA (QIAGEN), 5′-AATGCTGACTCCAAAGCTCTG, was obtained to monitor that the experimental set-up for transfection and knockdown analysis was working optimally. A nonsilencing control (5′-AATTCTCCGAACGTGTCACGT) was used in all experiments (QIAGEN). In addition, a negative control duplex (5′-AATTCTCCGAACGTGTCACGT) labeled with Alexa Fluor 488 (QIAGEN) was used to monitor transfection efficiency.
Cells were transfected with siRNA duplexes using HiPerfect transfection reagent (QIAGEN) according to the manufacturer’s instructions. All experiments were performed in duplicate. Briefly, 48 h before the transfection, cells were seeded onto 6-well culture dishes at a confluence of 70%. Cells were decidualized in vitro as before for 36 h. Cells were washed twice with PBS, and duplexes were added to wells at 5 nm in RPMI supplemented with 10% FCS for 24 h at 37 C and 5% CO2. Thereafter, cells were treated with decidualizing stimulus and TGFβ1 (10 ng/ml) for 72 h. After treatment, conditioned medium was removed and analyzed for DKK protein levels by ELISA (R&D Systems). In parallel, mRNA and whole-cell lysates were prepared as described previously and analyzed by quantitative real-time PCR (Q-RT-PCR) and Western blotting, respectively.
Decidualized primary ESC cultures were transiently transfected with a PRE or ERE, linked to a luciferase reporter gene (125 ng/well) in 24-well plates using calcium phosphate precipitation in medium supplemented with 2% dextran-coated charcoal (DCC)-FCS, as previously described (75, 76). Luciferase activity was measured after 20 h of treatments with a luciferase reagent kit (Promega Corp., Madison, WI) and expressed as relative light units. Cotransfection of β-galactosidase expression vector was used to control for transfection efficiency. Transfections were performed in triplicate, using DCC media, a 1:1 mixture of DMEM and Ham’s F-12 containing 5% FCS that had been depleted of steroids by treatment with DCC, 100 U/ml penicillin, and 100 μg/ml streptomycin and supplemented with 10−9 m 17β-estradiol and 1 μg/ml insulin.
Six hours after transfection, the medium was replaced with 2% DCC-FCS. The cells were treated with or without the appropriate steroid agonist with or without TGFβ1 (10 ng/ml). The cells were harvested 48 h after treatment for analysis with a luciferase assay. Transfection efficiency was analyzed with a β-galactosidase assay. The samples were normalized by dividing each sample’s luciferase reading with the corresponding β-galactosidase reading. This numerical reading was used as a comparison against other samples.
TaqMan Q-RT-PCR
RNA was extracted from cells in Tri reagent (ABgene House, Surrey, UK) as detailed in the manufacturer’s protocol. RNA samples were reverse transcribed using random primers. Gene-specific primers and probes were designed using Primer Express software (PerkinElmer/Applied Biosystems, Cheshire, UK): PR-A+B forward, 5′-CAGTGGGCGTTCCAAATGA-3′; PR-A+B reverse; 5′-GGTGGAATCAACTGTATGTCTTGA-3′; PR A+B probe, 5′-AGCCAAGCCCTAAGCCAGAGATTCACTTT-3′; DKK forward, 5′-GGAATAAGTACCAGACCATTGACAAC-3′; DKK reverse, 5′-GGGACTAGCGCAGTACTCATCA-3′; DKK probe, 5′-ACCAGCCGTACCCGTGCGCA-3′. Primers were diluted to 250 μm and probes to 50 μm in Tris-EDTA buffer (10 mm Tris, 1 mm EDTA in diethylpyrocarbonate H2O). PCR mixtures contained TaqMan Universal PCR Master Mix, No AmpErase UNG (Applied Biosystems) (7.2 mm MgCl2, 1.6 mm Stratagene dNTP mix, 1.6 mm Boehringer dNTP mix, 0.05 U/μl Taq polymerase, 2× PCR buffer, and 0.06% reference dye diluted in diethylpyrocarbonate H2O) and specific forward and reverse primers (250 nm; Biosource, Nivelles, Belgium) and probe (50 nm; Biosource) in a final volume of 25 μl/well. Ribosomal 18S primers and probe (PE Biosystems, Warrington, UK) were added at a final concentration of 50 nm. PCR were run on ABI Prism 7900 (Applied Biosystems). Samples were measured in duplicate, and mean values were used in subsequent analyses. Relative quantification was achieved using the formula 2−ΔΔCt, which relates the amount of cDNA of the specific amplicon to the 18S internal control and the control cDNA, which was the average of all time-matched control samples across the experiment.
ELISA
Culture supernatants were stored at −20 C until assayed. The DKK assay used matched antibody pairs (R&D, Abingdon, Oxford, UK) and was conducted according to manufacturer’s protocols. Samples were assayed for DKK protein release in duplicate. DKK concentration was determined by interpolation from a standard curve using known concentrations of DKK standards. All samples from each experiment were analyzed in the same assay to preclude interassay variability.
SDS-PAGE and Western Blotting
Protein was harvested using a lysis buffer and protease inhibitor cocktail and quantified using the DC Protein Microassay (Bio-Rad Laboratories Ltd., Hemel Hampstead, UK) as per the manufacturer’s instructions. Forty milligrams of total protein from each sample were loaded onto a precast 4–20% gradient Tris-glycine gel (Novex; Invitrogen, Paisley, UK), resolved at 100 V for 1 h, and then electrotransferred onto polyvinylidene difluoride membranes. Blots were blocked for 1 h at 25 C in Tris-buffered saline (TBS) plus Tween 20 (TBST) (50 mm Tris-HCl, 150 mm NaCl, and 0.05% Tween 20) plus 3% milk. The primary antibody, mouse monoclonal anti-human PR-A+B (1:25; Novocastra Laboratories Ltd., Newcastle-Upon-Tyne, UK), SMAD4 (1:200; Santa Cruz Biotechnology, Inc., Heidelberg, Germany), actin (1:750; Santa Cruz), or rabbit polyclonal antihuman ERK (1:1000; Santa Cruz) was added in TBST (plus 3% milk) for 18 h at 4 C. After washing three times with TBST, membranes were subsequently incubated for 1 h with the relevant secondary antibody, rabbit polyclonal antimouse IgG (1:5000; Sigma), or goat polyclonal antirabbit IgG (1:5000) (Sigma) conjugated to horseradish peroxidase. Blots were visualized using the enhanced chemiluminescence method (ECL plus kit; Amersham Biosciences, Little Chalfont, UK) following the manufacturer’s instructions. Membranes were reprobed for β-actin to correct for protein loading.
Immunocytochemistry
Immunocytochemistry used TBS and ICF used PBS. Cultured cells were washed with cold TBS/PBS (Sigma) and then fixed in 4% neutral buffered formalin for 2 h. Thereafter, cells were washed with TBS/PBS and then permeabilized by incubating the slides for 20 min at room temperature with 0.2% Nonidet P-40 (Sigma), 1% BSA (Sigma), and 10% nonimmune goat serum (NGS). Nonspecific binding sites of the primary antibody were blocked by incubating the slides in a 1:5 dilution of NGS in TBS/PBS containing 5% BSA (NGS/TBS or PBS/BSA). Endogenous avidin/biotin was blocked using a commercially available avidin-biotin blocking kit (Vector Laboratories Ltd., Peterborough, UK) and then washed twice with PBS. Slides were incubated at 4 C overnight in a 1:40 dilution of mouse monoclonal antihuman PR-A+B (Novocastra) or a 1:100 dilution of mouse monoclonal antihuman p-SMAD2 (Abcam, Cambridge, UK) made up in NGS/TBS or PBS/BSA. After washing once with TBS/PBS Tween and once with TBS/PBS for 5 min each, PR antibody binding was detected by applying a 1:500 dilution of biotinylated goat antimouse antibody (Dako UK Ltd., Ely, UK) in NGS/TBS/BSA, followed by an avidin/biotin horseradish peroxidase complex (Dako) for 60 and 30 min, respectively, at room temperature. Finally, antigenic sites were visualized by 3,3′-diaminobenzidine (Dako) before counterstaining in Harris’s hematoxylin, dehydrating, and mounting with Pertex mountant. The p-SMAD2 binding was detected by applying a 1:500 dilution of biotinylated goat antimouse antibody (Dako) in PBS followed by streptavidin 546 (Molecular Probes, Invitrogen) for 60 min each at room temperature. After washing the slides as described above, cells were counterstained with 4′,6-diamidino-2-phenylindole (1:200 in PBS) for 10 min at room temperature. The slides were mounted under a glass coverslip using Permafluor mounting medium. Negative controls were performed by incubating with a matched IgG control antibody (mouse IgG; Sigma) at the same antibody concentration as the primary antibody. Images captured on the LSM 510 laser scanning confocal microscope (Zeiss, Hertfordshire, UK) were analyzed to measure the mean OD of the fluorescence in the cell nucleus and compare with other images using the program Image Pro Plus (Media Cybernetics, Marlow, Buckinghamshire, UK).
Statistical Analysis
Before any statistical analysis, data were tested for and shown to exhibit Gaussian distribution. Gaussian distribution was determined by applying the Shapiro-Wilk normality test to the data. Where appropriate, values were presented as means ± sem. Comparison of the different parameters for the various treatment groups was determined by repeated-measures ANOVA. Significant differences were assigned using Kruskal-Wallis post hoc test. The criterion for significance for all tests was set at P < 0.05. Specific software was used to assist in the data analysis (GraphPad Prism version 4.0b for Macintosh; GraphPad Software, San Diego, CA).
Footnotes
This work was supported by the Medical Research Council.
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 21, 2007
Abbreviations: DCC, Dextran-coated charcoal; DKK, Dickkopf; EBAF, endometrial-associated bleeding factor; ERE, estrogen response element; ESC, endometrial stromal cell; FCS, fetal calf serum; ICF, immunocytofluorescence; MPA, medroxyprogesterone acetate; NGS, nonimmune goat serum; PAI-1, plasminogen activator inhibitor; PIAS, protein inhibitors of activated signal transducer and activator of transcription; PR, progesterone receptor; PRE, progesterone response element; p-SMAD2, phosphorylated SMAD2; siRNA, small interfering RNA; SMAD, Sma- and mothers against decapentaplegic (MAD)-related protein; STAT, signal transducer and activator of transcription; TBS, Tris-buffered saline; TBST, TBS plus Tween 20.
References
- 1.Kastner P, Krust A, Turcotte B, Stropp U, Tora L, Gronemeyer H, Chambon P 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J 9:1603–1614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei LL, Gonzalez-Aller C, Wood WM, Miller LA, Horwitz KB 1990. 5′-Heterogeneity in human progesterone receptor transcripts predicts a new amino-terminal truncated “C”-receptor and unique A-receptor messages. Mol Endocrinol 4:1833–1840 [DOI] [PubMed] [Google Scholar]
- 3.Wei LL, Hawkins P, Baker C, Norris B, Sheridan PL, Quinn PG 1996. An amino-terminal truncated progesterone receptor isoform, PRc, enhances progestin-induced transcriptional activity. Mol Endocrinol 10:1379–1387 [DOI] [PubMed] [Google Scholar]
- 4.Lessey BA, Killam AP, Metzger DA, Haney AF, Greene GL, McCarty Jr KS 1988. Immunohistochemical analysis of human uterine estrogen and progesterone receptors throughout the menstrual cycle. J Clin Endocrinol Metab 67:334–340 [DOI] [PubMed] [Google Scholar]
- 5.Garcia E, Bouchard P, De Brux J, Berdah J, Frydman R, Schaison G, Milgrom E, Perrot-Applanat M 1988. Use of immunocytochemistry of progesterone and estrogen receptors for endometrial dating. J Clin Endocrinol Metab 67:80–87 [DOI] [PubMed] [Google Scholar]
- 6.Snijders M, de Goeij A, Koudstaal J, Bosman F 1996. Oestrogen and progestogen receptor content in human endometrium. Eur J Obstet Gynecol Reprod Biol 70:9–10 [DOI] [PubMed] [Google Scholar]
- 7.Brosens JJ, Hayashi N, White JO 1999. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 140:4809–4820 [DOI] [PubMed] [Google Scholar]
- 8.Mote PA, Balleine RL, McGowan EM, Clarke CL 2000. Heterogeneity of progesterone receptors A and B expression in human endometrial glands and stroma. Hum Reprod 15(Suppl 3):48–56 [DOI] [PubMed]
- 9.Wang H, Critchley HOD, Kelly RW, Shen D, Baird DT 1998. Progesterone receptor subtype B is differentially regulated in human endometrial stroma. Mol Hum Reprod 4:407–412 [DOI] [PubMed] [Google Scholar]
- 10.Ignotz RA, Massague J 1986. Transforming growth factor-β stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J Biol Chem 261:4337–4345 [PubMed] [Google Scholar]
- 11.Desmouliere A, Geinoz A, Gabbiani F, Gabbiani G 1993. Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 122:103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lewis MP, Lygoe KA, Nystrom ML, Anderson WP, Speight PM, Marshall JF, Thomas GJ 2004. Tumour-derived TGF-β1 modulates myofibroblast differentiation and promotes HGF/SF-dependent invasion of squamous carcinoma cells. Br J Cancer 90:822–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shephard P, Martin G, Smola-Hess S, Brunner G, Krieg T, Smola H 2004. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-β and interleukin-1. Am J Pathol 164:2055–2066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bruner KL, Rodgers WH, Gold LI, Korc M, Hargrove JT, Matrisian LM, Osteen KG 1995. Transforming growth factor-β mediates the progesterone suppression of an epithelial metalloproteinase by adjacent stroma in the human endometrium. Proc Natl Acad Sci USA 92:7362–7366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulloa L, Creemers JW, Roy S, Liu S, Mason J, Tabibzadeh S 2001. Lefty proteins exhibit unique processing and activate the MAPK pathway. J Biol Chem 276:21387–21396 [DOI] [PubMed] [Google Scholar]
- 16.Parekh TV, Gama P, Wen X, Demopoulos R, Munger JS, Carcangiu ML, Reiss M, Gold LI 2002. Transforming growth factor β signaling is disabled early in human endometrial carcinogenesis concomitant with loss of growth inhibition. Cancer Res 62:2778–2790 [PubMed] [Google Scholar]
- 17.Lyons RM, Gentry LE, Purchio AF, Moses HL 1990. Mechanism of activation of latent recombinant transforming growth factor β1 by plasmin. J Cell Biol 110:1361–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loskutoff DJ, Sawdey M, Keeton M, Schneiderman J 1993. Regulation of PAI-1 gene expression in vivo. Thromb Haemost 70:135–137 [PubMed] [Google Scholar]
- 19.Massague J, Wotton D 2000. Transcriptional control by the TGF-β/Smad signaling system. EMBO J 19:1745–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y, Massague J 2003. Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell 113:685–700 [DOI] [PubMed] [Google Scholar]
- 21.Graff JM, Bansal A, Melton DA 1996. Xenopus Mad proteins transduce distinct subsets of signals for the TGFβ superfamily. Cell 85:479–487 [DOI] [PubMed] [Google Scholar]
- 22.Chung CD, Liao J, Liu B, Rao X, Jay P, Berta P, Shuai K 1997. Specific inhibition of Stat3 signal transduction by PIAS3. Science 278:1803–1805 [DOI] [PubMed] [Google Scholar]
- 23.Liu B, Gross M, ten Hoeve J, Shuai K 2001. A transcriptional corepressor of Stat1 with an essential LXXLL signature motif. Proc Natl Acad Sci USA 98:3203–3207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gross M, Liu B, Tan J, French FS, Carey M, Shuai K 2001. Distinct effects of PIAS proteins on androgen-mediated gene activation in prostate cancer cells. Oncogene 20:3880–3887 [DOI] [PubMed] [Google Scholar]
- 25.Sachdev S, Bruhn L, Sieber H, Pichler A, Melchior F, Grosschedl R 2001. PIASy, a nuclear matrix-associated SUMO E3 ligase, represses LEF1 activity by sequestration into nuclear bodies. Genes Dev 15:3088–3103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imoto S, Sugiyama K, Muromoto R, Sato N, Yamamoto T, Matsuda T 2003. Regulation of transforming growth factor-β signaling by protein inhibitor of activated STAT, PIASy through Smad3. J Biol Chem 278:34253–34258 [DOI] [PubMed] [Google Scholar]
- 27.Letamendia A, Labbe E, Attisano L 2001. Transcriptional regulation by Smads: crosstalk between the TGF-β and Wnt pathways. J Bone Joint Surg Am 83-A(Suppl 1):S31–S39 [PubMed]
- 28.Mao J, Wang J, Liu B, Pan W, Farr GH, 3rd, Flynn C, Yuan H, Takada S, Kimelman D, Li L, Wu D 2001. Low-density lipoprotein receptor-related protein-5 binds to Axin and regulates the canonical Wnt signaling pathway. Mol Cell 7:801–809 [DOI] [PubMed] [Google Scholar]
- 29.Wodarz A, Nusse R 1998. Mechanisms of Wnt signaling in development. Annu Rev Cell Dev Biol 14:59–88 [DOI] [PubMed] [Google Scholar]
- 30.Tamai K, Semenov M, Kato Y, Spokony R, Liu C, Katsuyama Y, Hess F, Saint-Jeannet JP, He X 2000. LDL-receptor-related proteins in Wnt signal transduction. Nature 407:530–535 [DOI] [PubMed] [Google Scholar]
- 31.Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek E, Giudice LC 2003. Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. J Clin Endocrinol Metab 88:3860–3866 [DOI] [PubMed] [Google Scholar]
- 32.Casslen B, Sandberg T, Gustavsson B, Willen R, Nilbert M 1998. Transforming growth factor β1 in the human endometrium. Cyclic variation, increased expression by estradiol and progesterone, and regulation of plasminogen activators and plasminogen activator inhibitor-1. Biol Reprod 58:1343–1350 [DOI] [PubMed] [Google Scholar]
- 33.Arici A, MacDonald PC, Casey ML 1996. Modulation of the levels of transforming growth factor β messenger ribonucleic acids in human endometrial stromal cells. Biol Reprod 54:463–469 [DOI] [PubMed] [Google Scholar]
- 34.Conneely OM, Lydon JP, De Mayo F, O’Malley BW 2000. Reproductive functions of the progesterone receptor. J Soc Gynecol Investig 7:S25–S32 [DOI] [PubMed]
- 35.Mulac-Jericevic B, Mullinax RA, DeMayo FJ, Lydon JP, Conneely OM 2000. Subgroup of reproductive functions of progesterone mediated by progesterone receptor-B isoform. Science 289:1751–1754 [DOI] [PubMed] [Google Scholar]
- 36.Jabbour HN, Kelly RW, Fraser HM, Critchley HO 2006. Endocrine regulation of menstruation. Endocr Rev 27:17–46 [DOI] [PubMed] [Google Scholar]
- 37.Critchley HO, Kelly RW, Brenner RM, Baird DT 2001. The endocrinology of menstruation: a role for the immune system. Clin Endocrinol (Oxf) 55:701–710 [DOI] [PubMed] [Google Scholar]
- 38.Brar AK, Frank GR, Kessler CA, Cedars MI, Handwerger S 1997. Progesterone-dependent decidualization of the human endometrium is mediated by cAMP. Endocrine 6:301–307 [DOI] [PubMed] [Google Scholar]
- 39.Gellersen B, Kempf R, Telgmann R, DiMattia GE 1994. Nonpituitary human prolactin gene transcription is independent of Pit-1 and differentially controlled in lymphocytes and in endometrial stroma. Mol Endocrinol 8:356–373 [DOI] [PubMed] [Google Scholar]
- 40.Mak IY, Brosens JJ, Christian M, Hills FA, Chamley L, Regan L, White JO 2002. Regulated expression of signal transducer and activator of transcription, Stat5, and its enhancement of PRL expression in human endometrial stromal cells in vitro J Clin Endocrinol Metab 87:2581–2588 [DOI] [PubMed] [Google Scholar]
- 41.Savouret JF, Bailly A, Misrahi M, Rauch C, Redeuilh G, Chauchereau A, Milgrom E 1991. Characterization of the hormone responsive element involved in the regulation of the progesterone receptor gene. EMBO J 10:1875–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng L, Zhu HH 1997. Regulation of progesterone receptor messenger ribonucleic acid by progestin in human endometrial stromal cells. Biol Reprod 57:1360–1366 [DOI] [PubMed] [Google Scholar]
- 43.Tang M, Mazella J, Gao J, Tseng L 2002. Progesterone receptor activates its promoter activity in human endometrial stromal cells. Mol Cell Endocrinol 192:45–53 [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Bond J, Thomas P 2003. Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor. Proc Natl Acad Sci USA 100:2237–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Losel R, Wehling M 2003. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol 4:46–56 [DOI] [PubMed] [Google Scholar]
- 46.Casey ML, MacDonald PC 1996. Transforming growth factor-β inhibits progesterone-induced enkephalinase expression in human endometrial stromal cells. J Clin Endocrinol Metab 81:4022–4027 [DOI] [PubMed] [Google Scholar]
- 47.Bruner KL, Eisenberg E, Gorstein F, Osteen KG 1999. Progesterone and transforming growth factor-β coordinately regulate suppression of endometrial matrix metalloproteinases in a model of experimental endometriosis. Steroids 64:648–653 [DOI] [PubMed] [Google Scholar]
- 48.Dou Q, Williams RS, Chegini N 1997. Inhibition of transforming growth factor-beta 1 alters the growth, anchor-dependent cell aggregation and integrin mRNA expression in human promonocytes: implications for endometriosis and peritoneal adhesion formation. Mol Hum Reprod 3:383–391 [DOI] [PubMed] [Google Scholar]
- 49.Cornet PB, Picquet C, Lemoine P, Osteen KG, Bruner-Tran KL, Tabibzadeh S, Courtoy PJ, Eeckhout Y, Marbaix E, Henriet P 2002. Regulation and function of LEFTY-A/EBAF in the human endometrium. mRNA expression during the menstrual cycle, control by progesterone, and effect on matrix metalloproteinases. J Biol Chem 277:42496–42504 [DOI] [PubMed] [Google Scholar]
- 50.Tabibzadeh S 2002. Decoding implantation and menstruation: the tale of two opposing signals. Front Biosci 7:d1475–d1486 [DOI] [PubMed]
- 51.Tabibzadeh S 2002. Homeostasis of extracellular matrix by TGF-β and lefty. Front Biosci 7:d1231–d1246 [DOI] [PubMed]
- 52.Long J, Matsuura I, He D, Wang G, Shuai K, Liu F 2003. Repression of Smad transcriptional activity by PIASy, an inhibitor of activated STAT. Proc Natl Acad Sci USA 100:9791–9796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Casslen B, Andersson A, Nilsson IM, Astedt B 1986. Hormonal regulation of the release of plasminogen activators and of a specific activator inhibitor from endometrial tissue in culture. Proc Soc Exp Biol Med 182:419–424 [DOI] [PubMed] [Google Scholar]
- 54.Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC 2006. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 147:1097–1121 [DOI] [PubMed] [Google Scholar]
- 55.Sandberg T, Eriksson P, Gustavsson B, Casslen B 1997. Differential regulation of the plasminogen activator inhibitor-1 (PAI-1) gene expression by growth factors and progesterone in human endometrial stromal cells. Mol Hum Reprod 3:781–787 [DOI] [PubMed] [Google Scholar]
- 56.Zoumpoulidou G, Jones MC, Fernandez de Mattos S, Francis JM, Fusi L, Lee YS, Christian M, Varshochi R, Lam EW, Brosens JJ 2004. Convergence of interferon-γ and progesterone signaling pathways in human endometrium: role of PIASy (protein inhibitor of activated signal transducer and activator of transcription-y). Mol Endocrinol 18:1988–1999 [DOI] [PubMed] [Google Scholar]
- 57.Ulloa L, Doody J, Massague J 1999. Inhibition of transforming growth factor-beta/SMAD signalling by the interferon-γ/STAT pathway. Nature 397:710–713 [DOI] [PubMed] [Google Scholar]
- 58.Eickelberg O, Pansky A, Koehler E, Bihl M, Tamm M, Hildebrand P, Perruchoud AP, Kashgarian M, Roth M 2001. Molecular mechanisms of TGF-β antagonism by interferon-γ and cyclosporine A in lung fibroblasts. FASEB J 15:797–806 [DOI] [PubMed] [Google Scholar]
- 59.Higashi K, Inagaki Y, Fujimori K, Nakao A, Kaneko H, Nakatsuka I 2003. Interferon-γ interferes with transforming growth factor-β signaling through direct interaction of YB-1 with Smad3. J Biol Chem 278:43470–43479 [DOI] [PubMed] [Google Scholar]
- 60.Giudice LC 2004. Microarray expression profiling reveals candidate genes for human uterine receptivity. Am J Pharmacogenomics 4:299–312 [DOI] [PubMed] [Google Scholar]
- 61.Tulac S, Overgaard MT, Hamilton AE, Jumbe NL, Suchanek E, Giudice LC 2006. Dickkopf-1, an inhibitor of Wnt signaling, is regulated by progesterone in human endometrial stromal cells. J Clin Endocrinol Metab 91:1453–1461 [DOI] [PubMed] [Google Scholar]
- 62.Goldman S, Shalev E 2006. A proposed mechanism for progesterone regulation of trophoblast MMP2 transcription independent of classical progesterone response elements on its promoter. J Exp Clin Assist Reprod 3:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou X, Tan Y, Li M, Dey SK, Das SK 2004. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18:3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Catalano RD, Critchley HO, Heikinheimo O, Baird DT, Hapangama D, Sherwin JR, Charnock-Jones DS, Smith SK, Sharkey AM 2007. Mifepristone induced progesterone withdrawal reveals novel regulatory pathways in human endometrium. Mol Hum Reprod 13:641–654 [DOI] [PubMed] [Google Scholar]
- 65.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O’Rahilly S, Sethi JK, Vidal-Puig A 2006. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J Cell Sci 119:2613–2620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Horst G, van der Werf SM, Farih-Sips H, van Bezooijen RL, Lowik CW, Karperien M 2005. Downregulation of Wnt signaling by increased expression of Dickkopf-1 and -2 is a prerequisite for late-stage osteoblast differentiation of KS483 cells. J Bone Miner Res 20:1867–1877 [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Sun Y, Constantinescu SN, Karam E, Weinberg RA, Lodish HF 1997. Transforming growth factor β-induced phosphorylation of Smad3 is required for growth inhibition and transcriptional induction in epithelial cells. Proc Natl Acad Sci USA 94:10669–10674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakao A, Roijer E, Imamura T, Souchelnytskyi S, Stenman G, Heldin CH, ten Dijke P 1997. Identification of Smad2, a human Mad-related protein in the transforming growth factor β signaling pathway. J Biol Chem 272:2896–2900 [DOI] [PubMed] [Google Scholar]
- 69.Zhang Y, Feng X, We R, Derynck R 1996. Receptor-associated Mad homologues synergize as effectors of the TGF-β response. Nature 383:168–172 [DOI] [PubMed] [Google Scholar]
- 70.Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp RL, Haffty BG, Reiss M 2002. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res 62:497–505 [PubMed] [Google Scholar]
- 71.Jones MC, Fusi L, Higham JH, Abdel-Hafiz H, Horwitz KB, Lam EW, Brosens JJ 2006. Regulation of the SUMO pathway sensitizes differentiating human endometrial stromal cells to progesterone. Proc Natl Acad Sci USA 103:16272–16277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Noyes RW, Hertig AT, Rock J 1950. Dating the endometrial biopsy. Fertil Steril 1:3–25 [DOI] [PubMed] [Google Scholar]
- 73.Critchley HO, Henderson TA, Kelly RW, Scobie GS, Evans LR, Groome NP, Saunders PT 2002. Wild-type estrogen receptor (ERβ1) and the splice variant (ERβcx/β2) are both expressed within the human endometrium throughout the normal menstrual cycle. J Clin Endocrinol Metab 87:5265–5273 [DOI] [PubMed] [Google Scholar]
- 74.Horwitz KB, Mockus MB, Lessey BA 1982. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell 28:633–642 [DOI] [PubMed] [Google Scholar]
- 75.Christian M, Marangos P, Mak I, McVey J, Barker F, White J, Brosens JJ 2001. Interferon-γ modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology 142:3142–3151 [DOI] [PubMed] [Google Scholar]
- 76.Christian M, Zhang X, Schneider-Merck T, Unterman TG, Gellersen B, White JO, Brosens JJ 2002. Cyclic AMP-induced forkhead transcription factor, FKHR, cooperates with CCAAT/enhancer-binding protein β in differentiating human endometrial stromal cells. J Biol Chem 277:20825–20832 [DOI] [PubMed] [Google Scholar]