Abstract
CD98 heavy chain (CD98hc) is expressed highly in developing human placental trophoblast. CD98hc is an amino acid transporter and is thought to function in cell fusion, adhesion, and invasion by interacting with integrins. In invasive extravillous trophoblast, αvβ3 integrin is expressed in a temporally and spatially specific manner, which prompted us to investigate the potential role of CD98hc in signal transduction of αvβ3 integrin. Immunocytochemistry of extravillous trophoblast derived from human placenta revealed that CD98hc colocalized with αvβ3 integrin and with αvβ3-associated cytoplasmic proteins including paxillin, vinculin, and focal adhesion kinase. Coimmunoprecipitation of CD98hc and its mutants revealed that the transmembrane domain of CD98hc is necessary for the association of CD98hc with αvβ3 integrin. When CD98hc negative liver cells (FLC4) were stably transfected with CD98hc and the extracellular domain of CD98hc was cross-linked by anti-CD98 antibody, FLC4 cells binding affinity to fibronectin and cell motility increased. The anti-CD98 antibody cross-linking promoted actin stress fiber formation and activation of signal transduction downstream of RhoA GTPase, and elevated the phosphorylation of focal adhesion kinase, paxillin, and protein kinase B. Pretreatment of transfected FLC4 cells with specific inhibitors for αvβ3integrin, phosphatidylinositol 3-kinase, and RhoA diminished these effects caused by anti-CD98 antibody cross-linking. These results suggest that notoriously invasive activity of extravillous trophoblast is mediated by CD98hc, which promotes αvβ3 integrin-dependent signals.
CD98 HEAVY CHAIN (CD98hc, 4F2hc, SLC3A2) is an 85-kDa type II membrane glycoprotein highly expressed in human placenta and in human cancer cells from various origin (1, 2). CD98hc is composed of an uncleavable signal peptide at the N terminus constituting the cytoplasmic domain, followed by a transmembrane (TM), and a C-terminal extracellular domain (3). CD98 heterodimer functions as an amino acid transporter, and CD98/CD147 complex is reported to play a critical role in energy metabolism (4).
Integrins are noncovalently bound heterodimers of type I membrane proteins termed α- and β-subunits. Their activity is controlled by changes in affinity for ligand through a so-called inside-out signaling as well as through an outside-in signaling. Inside-out signaling is largely controlled by intracellular signals to induce affinity for ligand (5). Outside-in signaling involves the binding of integrin cytoplasmic tails to factors such as focal adhesion kinase (FAK)/c-Src complexes, Ras and Rho GTPases, the phosphatidylinositol 3-kinase (PI3-K)/protein kinase B (AKT) complex, and adapters (e.g. paxillin and Cas/Crk), all of which assemble within dynamic adhesion structures, including focal complexes, focal adhesions, and podosomes (6). Consistent with the functional importance of integrin and Rho in the process of human embryo implantation (7, 8, 9), CD98hc is reported to be a major contributor to the integrin-dependent activation of Rac, a member of Rho family GTPases (10), which function as molecular switches in transducing extracellular and intracellular signals in regulation of the cell shape, polarity, and locomotion (11, 12, 13). RhoA not only triggers actin stress fiber formation, but also is activated in concert with Rac1 and Cdc42 to evoke membrane ruffling at the leading edge of migrating cells (14); thus RhoA activates integrins (15, 16), and activated integrins stimulate RhoA (17).
CD98hc is highly expressed in human placenta (18, 19). Although the role of CD98hc in promoting activity of α5β1 integrin has been established in fibroblast and embryonic stem cell cultures (10, 20), we were particularly interested in whether it played a similar role in extravillous trophoblasts (EVTs): previously, we had observed that EVT stimulated with IGF-I exhibited localization of αvβ3 integrin at focal adhesions together with activated paxillin and focal adhesion kinase (22). These findings suggested that αvβ3 activity may be stimulated by CD98hc in EVTs. It is known that down-regulation of αvβ3 in EVTs is correlated with preeclampsia, a pathological condition associated with a failure of EVT invasion of the maternal endovascular system (23). These observations prompted us to investigate the signal transduction of αvβ3 and CD98 leading to invasion activity of EVTs.
We hypothesized that CD98hc could play an important role in invasion of EVTs through stimulation of αvβ3 integrin signals. Here, we show that CD98hc interacts with αvβ3 integrin and that cross-linking of CD98hc by antibody directed to extracellular domain of CD98hc promotes αvβ3 integrin signaling for adhesion and mobility.
RESULTS
Association of CD98hc with αvβ3 Integrin in EVT
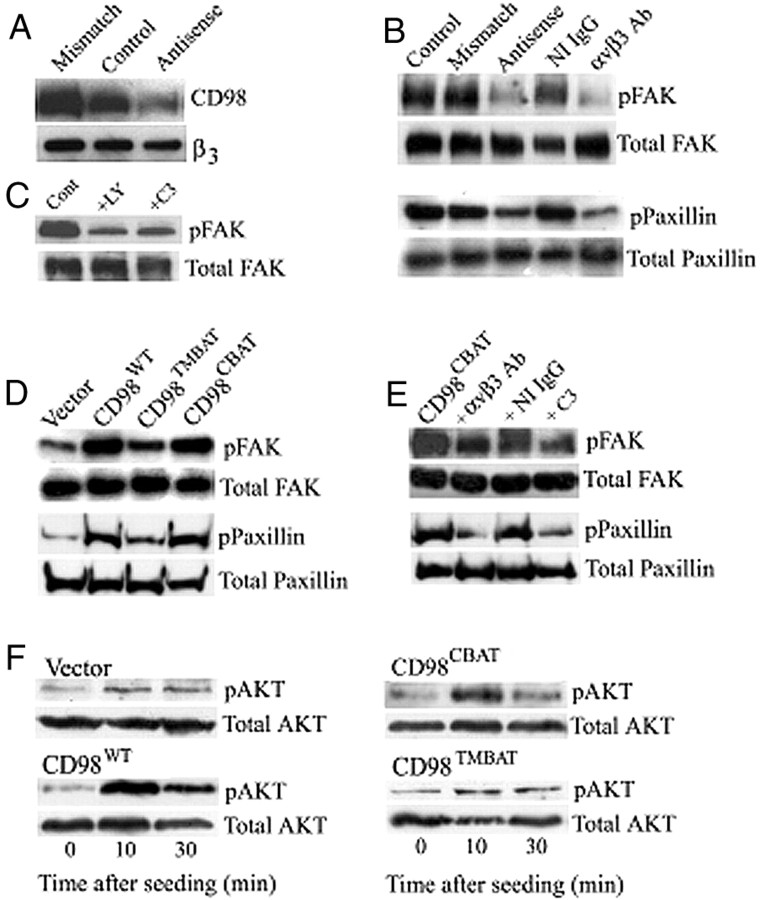
Immunostaining of first-passage human placental EVTs revealed that more than 95% of cells expressed trophoblastic markers: cytokeratin 7 and 8/18, and placental type alkaline phosphatase. EVTs were negative for vimentin, CD9, and factor VIII (data not shown). To investigate a potential interaction between CD98hc and αvβ3 integrin, we undertook coimmunoprecipitation analysis. CD98hc was found in the immunoprecipitated of β3 integrin and β1 integrin (Fig. 1A), whereas CD98 was not detected in the immunoprecipitate of α4 integrin. Immunoprecipitate of β3 integrin from EVT cells transfected with antisense oligonucleotide for CD98hc showed reduced signal for CD98hc (Fig. 1A), verifying the specificity of this assay.
Fig. 1.

Association of CD98hc with αvβ3 Integrin in Human EVTs
A (Upper row), Immunoblot with anti-CD98 antibody of coimmunoprecipitates with antibodies each for β1, β3, and α4 integrin or CD98 (as a positive control) of the serum-starved EVTs. Cells transfected with CD98hc antisense oligonucleotide (indicated as β3/CM) or with CD98 sense oligonucleotide (indicated as β3/CA). CD98hc/integrin complex was immunoprecipitated by each indicated antibody, resolved in SDS-PAGE and immunoblotted by anti-CD98hc antibody. A (Lower row), samples were immunoprecipitated with anti-α4 integrin and immunoblotted with the same antibody as a control. B, Confocal fluorescence micrographs of cultured EVTs stained for CD98hc and focal adhesion components. EVT cells were doubly stained for CD98hc (green) and αvβ3 integrin or downstream signaling proteins (red). Merged image is shown by yellow. Arrows show overlaps. Scale bar, 10 μm. C, Immunogold electron microscographs of EVTs doubly stained for CD98hc (small particles) and αvβ3 integrin (large particles) (a and b), serum-starved EVTs (c and d), or EVT stained without primary antibodies (e and f). Arrows in b show colocalizations of large and small gold particles. Resolution of cellular structure was slightly altered by mild fixation, which was necessary to maintain antibody reactivity. Scale bar, 0.5 μm. IB, Immunoblotting; IP, immunoprecipitation.
Immunocytochemistry of EVTs revealed that CD98hc colocalizes at focal adhesions with αvβ3 integrin, paxillin, phosphorylated FAK (pFAK), and vinculin (Fig. 1 B). No colocalization was found between CD98hc and α4 integrin (Fig. 1B). Immunogold electron microscopy of EVTs showed colocalization of CD98hc with αvβ3 integrin at the cell membrane, mainly at lamellipodia area (Fig. 1C, a and b). No colocalization was observed in serum-starved EVTs (Fig. 1C, c and d). These results indicate that CD98hc associates with αvβ3 in EVTs. Although EVTs express β1 integrin, studies of CD98hc and β1 integrin, particularly α5β1, have been carried out by others using fibroblast and embryonic stem cells (10). Considering the relevance of αvβ3 integrin expressed by EVTs during endovascular invasion, we focused on αvβ3 in this study.
Involvement of αvβ3 Integrin in CD98-Induced Adhesion and Motility
When EVTs were bound with antibody directed to the extracellular domain of CD98hc, antibody cross-linked cells showed elevated adhesion activity (Fig. 2A). Thus, adhesion assay showed that adhesion activity increased significantly (P < 0.001) in EVTs [A-CD98 (+) in Fig. 2A] on fibronectin (FN)-coated plate. Pretreatment of EVTs with either a CD98-blocking antibody (+B-CD98) or a specific functional blocking antibody against αωβ3 integrin (+αvβ3 Ab in Fig. 2A) showed a significant (P < 0.05) reduction of adherent cell numbers. Transfection of EVTs with antisense oligonucleotide for CD98 (Antisense in Fig. 2A) reduced the numbers of adherent cell numbers. Similar results were obtained by cell mobility assay (Fig. 2B). These results suggest that antibody cross-linking of the extracellular domain of CD98hc transmit a signal to the cytoplasm, which stimulates αvβ3 integrin for cell adhesion and motility.
Fig. 2.
Effect of CD98hc on Trophoblastic and Nontrophoblastic Cell Adhesion and Migration
A, Numbers of adherent EVTs on FN-coated plate were counted. EVTs were serum starved and treated with reagents as indicated. Asterisks indicate a significant increase (P < 0.01) over corresponding controls: a, difference vs. control not significant; b, a significant decrease (P < 0.05) over corresponding controls. B, Numbers of EVTs migrated through transwell. Asterisks indicate a significant increase (P < 0.01) over corresponding controls. C, Schematic presentation of CD98hc and BAT proteins and mutants. C.D., Cytoplasmic domain; extracellular D, extracellular or cell surface domain. D, Coimmunoprecipitation of CD98hc with β3 integrin in the FLC4 cells, which were stably transfected for indicated proteins. Antibodies used for the immunoblot were anti-CD98 antibody (upper panel) and anti-β3 antibody (lower panel). E, Numbers of adherent FLC4 cells transfected for the proteins indicated. Asterisks indicate a significant difference (P < 0.01) over corresponding controls. a, Difference vs. control not significant. F, Numbers of FLC4 cells transfected for each indicated protein that migrated through a transwell. Asterisks indicate a significant difference (P < 0.01) over corresponding controls. Ab, Antibody; IB, immunoblotting.
To investigate further the association of CD98hc with αvβ3 integrin, we prepared CD98hc mutants by replacing TM domain or cytoplasmic domain of CD98hc with each corresponding domain of broad specificity amino acid transporter (BAT), which does not interact with integrins (10) (Fig. 2C). A hepatocyte cell line (FLC4), which does not express endogenous CD98hc, was stably transfected with an expression vector encoding each protein. A coimmunoprecipitation analysis showed that CD98hc interacts with β3 integrin in the CD98WT- and CD98CBAT-transfected cells but not in the CD98TMBAT-, BATWT-, and BATECD98-transfected cells (Fig. 2D), indicating the essential role of TM domain of CD98hc for its association with β3 integrin.
The adhesion and migration assays revealed that the adhesion of CD98WT-transfected FLC4 cells increased significantly (P < 0.001) compared with the untransfected control or vector-transfected cells (Fig. 2E). CD98TMBAT failed in promoting adhesion activity. Similarly, CD98WT- or CD98CBAT-transfected FLC4 cells showed significantly increased (P < 0.001) motility in migration assay (Fig. 2F). These results suggest that the TM domain of CD98hc is essential for CD98-induced adhesion and mobility of FLC4 cells.
Antibody Cross-Link of CD98hc Increases Affinity of FN αvβ3 Integrin
To determine further the significance of the TM domain of CD98hc in cell adhesion, the binding kinetics of FN to CD98-expressing cells were measured on a biosensor chip (Fig. 3). A 110-kDa fragment of FN bound to CD98WT-transfected FLC4 cells immobilized to the sensor chip [Fig. 3A, curve (a)]. The affinity of FN to the same cells was increased when FLC4 cells were cross-linked with anti-CD98hc antibody [Fig. 3A, curve (b)]. In a similar assay under the condition of antibody cross-linking, FN bindings showed no difference between CD98WT-transfected and CD98CBAT-transfected FLC4 cells (Fig. 3B). When CD98CBAT-transfected FLC4 cells were cross-linked by the antibody directed to extracellular domain of CD98hc, the binding of FN fragment to FLC4 cells was reduced by EDTA (Fig. 3C) and αvβ3 blocking antibody (Fig. 3D). Summary data described above are provided in Fig. 3E. These results suggest that cross-linking of CD98hc by antibody leads to increase the affinity of αvβ3 integrin to FN in FLC4 cells.
Fig. 3.
FN Binding to CD98hc-Transfected FLC4 Cells Analyzed by Biosensor
A, Association and dissociation of 110-kDa FN fragment to CD98WT-transfected FLC4 cells in the presence (b) or absence (a) of anti-CD98 antibody for cross-linking. B, Binding of FN fragment to CD98CBAT- (a) and CD98WT-transfected (b) FLC4 cells after cross-linking with anti-CD98 antibody. C, Binding of FN fragment to CD98CBAT-transfected cells after antibody cross-linking in the presence (a) or absence (b) of 1 mm EDTA. D, Binding of FN fragment to CD98CBAT-transfected cells, with (a) or without (b) pretreatment with a αvβ3 integrin-blocking antibody. E, Summary of the data shown in panels A–D described above. S, Significant, P < 0.05; NS, nonsignificant, P > 0.05.
Involvement of TM Domain of CD98hc in Activation of Focal Adhesion Proteins
Because αvβ3 integrin is involved in focal adhesion, we examined the role of CD98 in focal adhesion. Western blot analysis showed that human trophoblast cell line 3A-CRL expresses CD98hc and that antisense oligonucleotide for CD98hc reduced the level of endogenous CD98hc protein to 50% (Fig. 4A). The levels of tyrosine-phosphorylated FAK (pFAK) and paxillin (pPaxillin) were reduced in CD98hc-knocked down 3A-CRL cells (Fig. 4B). The levels of pFAK and pPaxillin were also reduced in 3A-CRL cells treated with a αvβ3 integrin-blocking antibody (Fig. 4B). Pretreatment of 3A-CRL cells with the PI3-K inhibitor LY294002 reduced the levels of pFAK (Fig. 4C). Treatment of C3 exozyme, which inhibits Rho GTPase (20), also reduced pFAK (Fig. 4C).
Fig. 4.
Activation of αvβ3 Integrin through Outside-in Signaling by CD98
A, Immunoblot analysis of 3A-CRL cells expressing CD98hc. Lane 1, untransfected; lane 2, transfected with a CD98 mismatch oligonucleotide; lane 3, transfected with a CD98 antisense oligonucleotide. Lower panel shows an internal control by β3 integrin. B, Immunoblot analysis for pFAK and pPaxillin of serum-starved 3A-CRL cells cross-linked with anti-CD98 antibody and allowed to adhere on FN-coated plates for 30 min. C, Immunoblot analysis of pFAK of serum-starved 3A-CRL cells, treated with LY294002 or C3 exozyme. D, Immunoblot analysis for pFAK and pPaxillin of FLC4 cells transfected for each indicated protein. Cells were cross-linked with anti-CD98 antibody and allowed to adhere to FN-coated plates for 30 min. E, Immunoblot analysis for pFAK and pPaxillin of FLC4 cells transfected for CD98CBAT. Cells were treated as indicated and allowed to adhere to FN-coated plates for 30 min. F, Immunoblot analysis for pAKT of FLC4 cells transfected for each indicated protein and cross-linked with anti-CD98 antibody and allowed to adhere to FN-coated plates. Ab, Antibody; Cont, control; NI, nonimmune.
Activation of focal adhesion proteins was determined in FLC4 lines each expressing CD98WT, CD98TMBAT, and CD98CBAT. Anti-CD98 antibody cross-linking increased pFAK and pPaxillin in CD98WT- and CD98CBAT-expressing FLC4 cells, whereas CD98TMBAT-expressing FLC4 cells failed in this activation (Fig. 4D). Also in CD98CBAT-expressing FLC4 cells, αvβ3-blocking antibody and C3 exoenzyme reduced the levels of pFAK and pPaxillin (Fig. 4E). These results indicate the involvement of PI3-K and RhoA activity in CD98hc-mediated αvβ3 integrin-dependent activation of focal adhesion proteins.
We also asked whether cross-linking of CD98 stimulates AKT, a downstream effector of PI3-K, which stimulates β3 integrin outside-in signals. When CD98WT-expressing FLC4 cells were seeded to FN-coated plate, phosphorylated AKT levels increased in 10 min (Fig. 4F). Similar activation of AKT was detected in CD98CBAT-transfected cells, whereas FLC4 cells expressing CD98TMBAT failed in this AKT activation (Fig. 4F). These results suggest that cross-linking of CD98hc stimulates αvβ3 integrin outside-in signaling.
The Effect of CD98 on RhoA GTPase
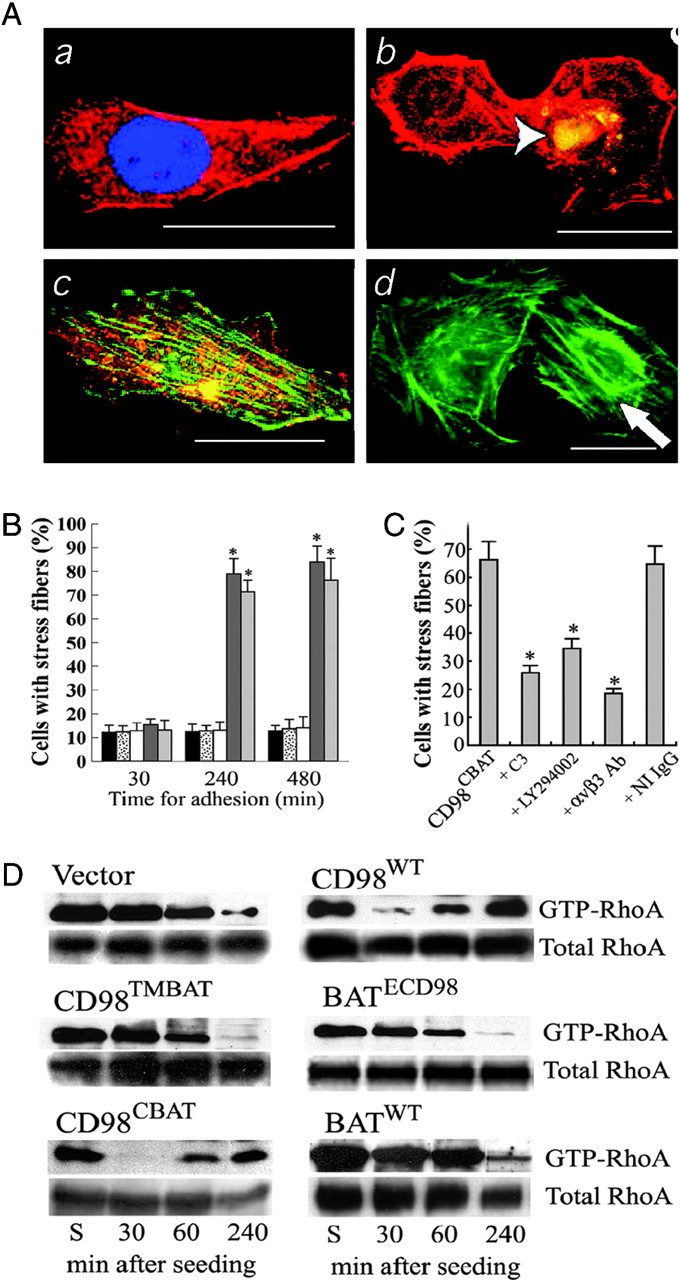
FLC4 cells were transfected with cDNA encoding CD98WT, CD98TMBAT, CD98CBAT, BATECD98, or BATWT. When serum-starved cells were reacted with the CD98-activation antibody, actin stress fibers were formed in the CD98WT- and CD98CBAT-transfected cells (Fig. 5A, c and d) but not in the control and the CD98TMBAT-transfected cells (Fig. 5A, a and b). To examine the role of CD98hc and its TM domain in actin stress fiber formation, transfected cells were allowed to adhere to FN-coated plates for 30, 240, and 480 min, and cell numbers with stress fiber were counted. FLC4 cells transfected with CD98hcWT or CD98CBAT exhibited a significantly (P < 0.001) higher incidence of stress fiber formation compared with those transfected with CD98TMBAT, BATWT, or vector (Fig. 5B). To examine the role of αvβ3 integrin, RhoA, and PI3-K during the CD98-induced actin stress fiber formation, CD98CBAT-transfected cells were allowed to adhere to FN-coated plates with or without pretreatment with a C3 exoenzyme, LY294002, and a specific αvβ3 integrin-blocking antibody and numbers of cells with stress fiber were counted. This analysis showed that the formation of actin stress fibers was significantly (P < 0.05) reduced by these treatments (Fig. 5C).
Fig. 5.
The Effect of CD98 on RhoA GTPase
A, Serum-starved FLC4 cells were cultured for 4 h on FN-coated plates and cross-linked by anti-CD98 antibody. A FLC4 cell transfected with vector and stained with 4′,6-diamidino-2-phenylindole (blue) and Phalloidin (red) for actin fibers (a). FLC4 cells were cotransfected for CD98TMBAT and GFP and stained for actin fibers (red). GFP staining (white, arrowhead) indicates a transfected cell (b). A FLC4 cell transfected for CD98hcWT and stained for CD98hc (red) and actin fibers (green, indicated by an arrow) (c). A FLC4 cell transfected for CD98CBAT and GFP and stained for actin fibers (red) (d). Scale bar: 10 μm. B, Numbers of FLC4 cells with stress fibers. Mock transfected (black), transfected with BATWT (dotted), CD98TMBAT (white), CD98WT (dark gray), or CD98CBAT (light gray). C, Numbers of CD98CBAT expressing FLC4 cells with stress fibers. Cells were pretreated with the indicated inhibitors, followed by cross-linking with anti-CD98 antibody. In B and C, error bars represent the standard deviation from four separate experiments. Asterisk indicates statistical significance (P < 0.01) vs. corresponding controls. D, Immunoblot assay for activated (GTP-bound) RhoA after stimulation with anti-CD98 antibody cross-linking. FLC4 cells mock-transfected or stably transfected with CD98WT, CD98TMBAT, BATECD98, CD98CBAT, or BATWT were suspended in medium (S). Cells were cross-linked with anti-CD98 antibody, plated and cultured for 30, 60, and 240 min. Ab, Antibody.
Because focal adhesion and stress fiber formation are mediated by Rho GTPase, we tested RhoA activation in cells expressing CD98hc and its mutants (Fig. 5D). According to the study by Miao et al. (17), when Chinese hamster ovary cells were kept in suspension, GTP-Rho levels were high, whereas GTP-Rho was down-regulated within 30 min when cells adhered on FN-coated plate. After adherent Chinese hamster ovary cells were cultured for 240 min, the cells formed stress fibers, and GTP-Rho was up-regulated. In all FLC4 cell lines we used, GTP-Rho levels were also high when cells were kept in suspension (Fig. 5D, each far left lane). Upon plating and anti-CD98 antibody cross-linking, CD98WT and CD98CBAT cells adhered within 30 min, spread, and formed stress fibers within 240 min. GTP-Rho was down-regulated during cell adhesion (30 min) and up-regulated during stress fiber formation (240 min) (Fig. 5D, CD98WT and CD98CBAT). On the other hand, CLF4 cells transfected with vector alone (Fig. 5D, Vector) did not adhere to plate in 30 min, did not form stress fibers after culturing for 240 min, but started adhering at 240 min. GTP-Rho levels remained high 30 min after plating, but GTP-Rho was slowly down-regulated when cells started adhering at 240 min. Cells expressing CD98TMBAT, BATECD98, or BATWT showed similar patterns as vector control cells. These results suggest that RhoA GTPase plays a role of molecular switch in CD98hc-triggered activation of αvβ3 integrin.
DISCUSSION
Placental EVTs are extremely invasive and are often compared with malignant cancer cells (26). Because both placenta and cancer cells highly express CD98, we asked whether EVTs might employ CD98 to achieve this extraordinarily invasive behavior at the embryo-maternal interface. Here, we showed roles of CD98 in activating αvβ3 integrin signals in both inside-out and outside-in mechanisms. In particular, we report here that the TM domain of CD98hc plays an essential role in the CD98-induced αvβ3 integrin activation.
Previous studies showed high expression of CD98hc in cytotrophoblasts of human placenta and indicated its involvement in cytotrophoblast fusion and in the secretion of human chorionic gonadotropin (8, 27). Given the remarkable invasive activity exhibited by EVTs and expression of αvβ3 as the core integrin of their focal adhesions (24), the potential association of CD98 with αvβ3 integrin may have physiological relevance to fertility. The association of activated CD98 with αvβ3 integrin on the EVTs surface suggests the possibility of CD98-induced αvβ3 integrin activation through its internalization, which merits further investigation. Colocalization of CD98 with αvβ3 integrin at EVTs focal adhesion indicates its potential contribution to promote the αvβ3 integrin-dependent adhesion and motility, the mechanism of which is mainly discussed in this study. It is known that αvβ3 integrin is down-regulated in preeclampsia (23). However, we found no significant difference in levels of CD98hc in EVTs from preeclampsia and normal placentas (data not shown), indicating that CD98hc activities do not underlie this pathological condition.
Our findings of CD98 in EVT (Fig. 2, A and B) agree with previous reports demonstrating a positive role for CD98 in adhesion and motility of other cell types (1, 10, 28, 29, 30). However, because heterodimers of CD98hc and human L amino acid transporter1 (hLAT1) form a complex in human trophoblasts (31) and a CD147-CD98 cell surface supercomplex or large cluster has been shown to play a critical role in energy metabolism (4), the adhesion- and migration-inhibitory effects of CD98 knockdown cells could be considered as its nutrition-related effects. To exclude the possibility of the effects of CD98hc as a amino acid transporter, we constructed several expression vectors encoding different domains of CD98hc (Fig. 2C) and generated stable cell lines from FLC4, which does not express endogenous CD98hc. Using transfected FLC4 cells, we were able to demonstrate that the TM domain of CD98hc plays an essential role in activating αvβ3 integrin in cell adhesion and motility (Fig. 2, E and F).
The kinetic analysis of FN binding to transfected FLC4 cells shown in this study revealed that cross-linking of CD98 by antibody to extracellular domain of CD98hc enhances affinity of transfected cells to a 110-kDa fragment of FN and that the CD98-induced affinity for FN is αvβ3 integrin dependent (Fig. 3). Based on these findings, we conclude that cross-linking of CD98hc on cell surface leads to activation of the αvβ3 integrin by an inside-out signaling pathway.
In this study, we asked whether cross-linking of CD98 promotes the phosphorylation of AKT, FAK, and paxillin, which are part of downstream targets of αvβ3 integrin outside-in signaling. We showed here that cross-linking of CD98 increased AKT phosphorylation and tyrosine phosphorylation of FAK and paxillin in trophoblastic and transfected FLC4 cells (Fig. 4). These results are consistent with a previous study reporting that β1 integrin-dependent FAK phosphorylation is impaired in CD98hc−/− cells (10). Additionally, our results indicate an interaction between the CD98hc TM domain and αvβ3 integrin as well as the functional importance of RhoA and PI3-K activity in stimulating FAK phosphorylation.
Actin filament dynamics are crucial to cell motility. A recent study reports that actin can switch between active and inactive conformations in response to external signals (32). The present study showed the active role of CD98hc but excludes the possibility that the cytoplasmic and/or extracellular domain of CD98hc played a role in this process (Fig. 5A). Moreover, the functional importance of αvβ3 integrin, PI3-K, and RhoA during the CD98-induced actin stress fiber formation was demonstrated using CD98CBAT-transfected cells (Fig. 5, B and C). Considering that RhoA directly stimulates actin polymerization and given the link between the expression of the CD98hc TM domain and actin stress fiber formation, we asked whether the TM domain of CD98hc was required for RhoA activation during CD98-induced actin stress fiber formation. The present study demonstrated that RhoA was activated by cross-linking of CD98 in both CD98WT- and CD98CBAT-transfected FLC4 cells, but not in BATWT- or CD98TMBAT-transfected cells (Fig. 5D).
In conclusion, results obtained by this study reveal an essential role of the TM domain of CD98hc in the stimulation of αvβ3 integrin for cell adhesion and motility. The essential role of TM domain of CD98hc represents a novel role of CD98hc during the complex process of placenta formation after embryo implantation and provides an additional mechanism by which EVTs achieve extraordinarily high invasive activity. Because cellular control of integrin activation plays important roles in health and disease in humans, clarification of mechanisms underlying interactions between the CD98hc TM domain and integrins should suggest novel strategies to control human diseases.
MATERIALS AND METHODS
Plasmids, Reagents, and Antibodies
Expression vectors encoding full-length human CD98hc (GenBank accession no. AB018010) and BAT cDNA (GenBank accession no. AB033549) and affinity-purified polyclonal rabbit anti-BAT were prepared by Yoshikatsu Kanai. CD98-activating antibody was purified by protein A affinity chromatography from conditioned medium of hybridoma cell (Y. Kanai, unpublished data). The FLC4 cell line was generated in the Department of General Medicine of Kyorin University lines (Japan). The antisense oligonucleotide to human CD98 (5′-CCTGGCTCATGGTGCCTG-3′) and an irrelevant control oligonucleotide (5′-GGTCCCTCATCCTGGGTG-3′) were purchased from Sawaday Technology (Tokyo, Japan). Cell culture reagents were from Life Technologies, Inc. (Gaithersburg, MD).
Trophoblastic Cell Lines and Primary Culture of Placental Trophoblasts
The 3A-SUB-E CRL trophoblastic cell line from normal placenta was obtained from the American Type Culture Collection (Manassas, VA). Primary cultures of EVTs were established as described elsewhere (33) using placental tissues from 20 legal abortions of 7- to 9-wk pregnancies. Patients gave informed consent, and the use of human materials was approved by the Institutional Review Board of Kyorin University, School of Medicine, Tokyo, Japan. First-passage cells were used routinely.
Transfection of CD98 Antisense Oligonucleotide
3A-CRL cells were cultured for 2–3 d to 50% confluency. Antisense oligonucleotides to human CD98 or a control mismatched oligonucleotide (27) (3 μg per plate) were introduced into cells using FuGENE 6 transfection reagent (Roche Diagnostics, Lewes, UK), according to the manufacturer’s protocol.
Construction of CD98TMBAT, CD98CBAT, and BATECD98 Mutants
Human CD98hc cDNA (GenBank accession no. AB018010) and human BAT cDNA (GenBank accession no. AB033549) (21) were subcloned into the mammalian expression vector pcDNA3.1 (Invitrogen, Carlsbad, CA) at the HindIII and BamHI sites and at the HindIII and XhoI sites, respectively. To generate CD98TMBAT, CD98 cDNA [nucleotide (nt) 1–353] encoding the N-terminal cytoplasmic domain was amplified by PCR using forward primer 5′-GCGCGGTACCGCGCGGAGCCACAGAGGCCGG-3′ (KpnI site underlined) and reverse primer 5′-GCGCGCTAGCGCGGGTGCGTACCCA-GCC-3′ (NheI site underlined) and digested by KpnI and NheI. CD98 cDNA (nt 421,3′ end) encoding the extracellular domain was amplified by PCR using forward primer 5′-GCGCGCTAGCGTGCGAGCGCCGCGTTGTCGC-3′ (NheI site underlined) and reverse primer 5′-GCGCGCGGCCGCCAGTCGAGGCTGATCAGCGGG-3′ (NotI site underlined) and digested by NheI and NotI. These DNA fragments were ligated into pcDNA3.1 at the KpnI and NotI sites to make truncated CD98 without the TM domain. BAT cDNA (nt 321–385) encoding the TM domain was amplified by PCR using forward primer 5′-GCGCGCTAGCATCCTCTTCTGGCTCACAG-3′ (NheI site underlined) and reverse primer 5′-GCGCGCTAGCAATGATGGCTATGGTGGCCGC-3′ (NheI site underlined), digested by NheI and ligated at the NheI site of truncated CD98 in pCDNA3.1. To generate CD98CBAT, BAT cDNA (nt 1–339) encoding the cytoplasmic domain was amplified by PCR using forward primer 5′-GCGCGGTACCGCCACTCTTCCACCTCCCTTAC-3′ (KpnI site underlined) and reverse primer 5′-GCGCGCTAGCATCCTCTTCTGGCTCACAG-3′ (NheI site underlined), and the amplicon was digested by KpnI and NheI. CD98 cDNA (nt 421,3′ end) encoding the extracellular domain was amplified by PCR using forward primer 5′-GCGCGCTAGCGTGCGAGCGCCGCGTTGTCGC-3′ (NheI site underlined) and reverse primer 5′-GCG-CGCGGCCGCCAGTCGAGGCTGATCAGCGGG-3′ (NotI site underlined) and digested by NheI and NotI. These DNA fragments were ligated into pcDNA3.1 at the KpnI and NotI sites to make truncated CD98/BAT without the TM domain. CD98 cDNA (nt 353–421) encoding the TM domain was amplified by PCR using forward primer 5′-GCGCGCTAGCTGGGCACTGCTGCTGCTC-3′ (NheI site underlined) and reverse primer 5′-GCGCGCTAGCGATTATGACCACGGCACCAGC-3′ (NheI site underlined), digested by NheI and ligated at the NheI site of the truncated CD98/BAT in pCDNA3.1. To generate BATECD98, CD98 cDNA (nt 1–353) encoding the N-terminal cytoplasmic domain was amplified by PCR using forward primer and reverse primer (as mentioned above) and digested by KpnI and NheI. BAT cDNA (nt 384–467 end) encoding the extracellular domain was amplified by PCR using forward primer and reverse primer (as mentioned above) and digested by NheI and NotI. These two DNA fragments were ligated into pcDNA3.1 at the KpnI and NotI sites to make truncated BAT/CD98 without the TM domain. BAT cDNA (nt 321–385) encoding TM domain was amplified by PCR using forward primer and reverse primer (as mentioned above), digested by NheI, and ligated at the NheI site of truncated CD98 in pCDNA3.1. All constructs were sequenced using a sequencing kit (Applied Biosystems, Foster City, CA).
Generation of Stably Transfected Cells
FLC4 cells were cultured with DMEM (Invitrogen) supplemented with 10% fetal bovine serum and antibiotics (34) and transfected using LipofectAMINE 2000 (Invitrogen). Forty-eight hours after transfection, Geneticin (Invitrogen) at a concentration of 100 μg/ml was added to the medium. Expression of transfected genes was confirmed by immunocytochemistry and immunoblotting.
Immunocytochemistry and Electron Microscopy
EVTs were grown on FN 110-kDa fragment-coated (FN-coated) chamber slides, fixed with 4% paraformaldehyde, permeabilized with 0.5% Triton X-100, and blocked with 5% normal serum. Cells were doubly stained for CD98hc (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), for paxillin (Chemicon, Temecula, CA), for pFAK (Chemicon), for α4 or αvβ3 integrin (monoclonal mouse IgG2a, Chemicon), and for vinculin (monoclonal mouse IgG, Sigma Chemical Co., St. Louis, MO). After washing, cells were incubated with fluorescent-labeled secondary antibodies at room temperature. Experiments were undertaken four times using EVTs prepared from four independent human placentas.
Transfected serum-starved FLC4 cells were plated onto FN-coated plates in serum-free medium. Some cells were pretreated with a specific monoclonal αvβ3 integrin function-blocking antibody (10 μg/ml) (Chemicon), with LY294002 (5 μm) (Sigma Chemical Co.) (Chemicon), or with Botulinum C3 exoenzyme (10 ng/ml) (BIOMOL Research Laboratories, Inc., Plymouth Meeting, PA). Cells were fixed and permeabilized and then were incubated with fluorescein isothiocyanate- and Alexa 568-conjugated phalloidin (Sigma) at room temperature for 45 min. The percentage of cells with central stress fibers was determined in 20 fields per test for four replicates and expressed as the mean ± sem.
Immunogold transmission electron microscopy was performed as previously described (33).
Immunoprecipitation and Immunoblotting
Transfected 3A-CRL and FLC4 cells were cultured on FN-coated plates in serum-free medium and harvested by trypsinization. Some cells were kept in suspension at 37 C for 30 min, and others were cultured for 30 min on FN-coated plates in serum-free medium containing CD98 activating antibody (5 μg/ml). Cells were lysed with lysis buffer (1% Triton X-100; 1 mm EGTA; 150 mm NaCl; 25 mm HEPES, pH 7.5; 5 mm MgCl2; supplemented with protease inhibitor mixture) on ice for 15 min. Lysate proteins (200 μg) were incubated with protein A beads precoated with 2 μg of the appropriate primary antibody at 4 C overnight, and bound proteins were eluted by boiling in sample buffer. Proteins (20 μg /lane) were resolved by SDS-PAGE and transferred to polyvinylidinedifluoride membranes, blocked in Tris-buffered saline-Tween 20 containing 3% BSA and incubated with primary antibodies at 4 C for 20 h, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were identified using enhanced chemiluminescence.
Transwell Migration Assays
Migration assays were performed using 24-well plates with transwell membranes (Millipore Corp., Bedford, MA) coated with FN as described elsewhere (24). 3A-CRL cells or transfected FLC4 cells were preincubated for 30 min with serum-free medium containing 0.01% BSA in the presence or the absence of CD98-activation antibody (5 μg/ml). After incubation, filters were fixed and stained, and the numbers of cells in the lower surface were determined under a microscope. The number of migrated cells was expressed as the mean ± sem of four independent assays performed in triplicate.
Cell Adhesion Assay
Cells were plated on FN-coated plates; some cells were pretreated as described above or with nonimmune control IgG (10 μg/ml). Adhesion was assayed as described elsewhere (33). After plating for 1 h, cells were fixed and stained using a Diff Quick kit (International Reagents Corp., Kobe, Japan), and adherent cells were counted under a phase-contrast microscope. Data were acquired by counting the mean of 10 fields per test, and four independent experiments were performed in triplicate. Statistical analysis was performed as described above.
Kinetic Analysis
The IAsys plus biosensor (Affinity Sensors, Cambridge, UK) was used to analyze FN binding affinity of transfected cells on the surface of an aminosilane-coated cuvette, with or without activation by CD98 antibody (5 μg/ml), or pretreatment with EDTA (1 mm) or the αvβ3 integrin function-blocking antibody (10 μg/ml). FLC4 cells (2 × 105 cells) were immobilized on the cuvette and cross-linked to the aminosilane surface using BS3 (Bis[sulfosuccinimidyl] substrate (Pierce Chemical Co., Rockford, IL). Binding affinity of the 110-kDa FN fragment (10 μg/ml of PBS) was recorded for 30 min. Dissociation of FN was initiated by adding 0.05% Tween 20 in PBS (washing buffer) to the cuvette, and the arc second was recorded for 5 min. Experimental groups were assayed in triplicate.
Rho Activity Assay
Rho activity was determined by an affinity-precipitation assay (30) using a Rho activation kit (Pierce Chemical Co.). Briefly, serum-starved transfected FLC4 cells were plated under serum-free conditions supplemented with CD98-activated antibody, lysed in high-salt radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.2; 1% Triton X-100; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 500 mm NaCl; 10 mm MgCl2; 10 μg/ml leupeptin; 1 mm Na3VO4; and 1 mm phenylmethylsulfonyfluoride), and centrifuged. Lysates were incubated with 20 μg glutathione-S-transferase-Rhotekin-Rho binding domain beads for 45 min at 4 C, and activated Rho was detected by immunoblotting using a monoclonal antibody. Experimental groups were assayed in triplicate.
Acknowledgments
We thank Dr. Y. Kudo (Department of Obstetrics and Gynecology, Graduate School of Biomedical Sciences, Hiroshima University) and Dr. Y. Iribe (Department of Pharmacology and Toxicology, Kyorin University School of Medicine, Tokyo, Japan) for their help in preparing CD98-activation antibody. We also thank Mr. M. Fukuda (Department of Anatomy, Kyorin University School of Medicine, Tokyo, Japan) for technical assistance in electron microscopy.
Footnotes
This work was supported by a (C) 16591689 (to S.S.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; a Japan Society for the Promotion of Science postdoctoral fellowship (to M.K-S.) for foreign researchers; and Department of Defence Grant W81XWH-04-1-0917 (to M.N.F.).
Disclosure Statement: The authors have nothing to disclose.
First Published Online November 21, 2007
Abbreviations: AKT, Protein kinase B; BAT, broad-specificity amino acid transporter; CD98hc, CD98 heavy chain; EVT, extravillous trophoblast; FN, fibronectin; nt, nucleotide; pFAK, phosphorylated FAK; PI3-K, phosphatidylinositol 3-kinase; pPaxillin, phosphorylated Paxillin; TM, transmembrane.
References
- 1.Henderson NC, Collis EA, Mackinnon AC, Simpson KJ, Haslett C, Zent R, Ginsberg M, Sethi T 2004. CD98hc (SLC3A2) interaction with β 1 integrins is required for transformation. J Biol Chem 279:54731–54741 [DOI] [PubMed] [Google Scholar]
- 2.Yanagida O, Kanai Y, Chairoungdua A, Kim DK, Segawa H, Nii T, Cha SH, Matsuo H, Fukushima J, Fukasawa Y, Tani Y, Taketani Y, Uchino H, Kim JY, Inatomi J, Okayasu I, Miyamoto K, Takeda E, Goya T, Endou H 2001. Human L-type amino acid transporter 1 (LAT1): characterization of function and expression in tumor cell lines. Biochim Biophys Acta 1514:291–302 [DOI] [PubMed] [Google Scholar]
- 3.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y 2004. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 447:532–542 [DOI] [PubMed] [Google Scholar]
- 4.Xu D, Hemler ME 2005. Metabolic activation-related CD147-CD98 complex. Mol Cell Proteomics 4:1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderwood DA 2004. Talin controls integrin activation. Biochem Soc Trans 32:434–437 [DOI] [PubMed] [Google Scholar]
- 6.Zaidel-Bar R, Cohen M, Addadi L, Geiger B 2004. Hierarchical assembly of cell-matrix adhesion complexes. Biochem Soc Trans 32:416–420 [DOI] [PubMed] [Google Scholar]
- 7.Shiokawa S, Iwashita M, Akimoto Y, Nagamatsu S, Sakai K, Hanashi H, Kabir-Salmani M, Nakamura Y, Uehata M, Yoshimura Y 2002. Small guanosine triphospatase RhoA and Rho-associated kinase as regulators of trophoblast migration. J Clin Endocrinol Metab 87:5808–5816 [DOI] [PubMed] [Google Scholar]
- 8.Ayuk PT, Sibley CP, Donnai P, D’Souza S, Glazier JD 2000. Development and polarization of cationic amino acid transporters and regulators in the human placenta. Am J Physiol Cell Physiol 278:C1162–C1171 [DOI] [PubMed]
- 9.Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Iwashita M 2004. The role of α(5)β(1)-integrin in the IGF-I-induced migration of extravillous trophoblast cells during the process of implantation. Mol Hum Reprod 10:91–97 [DOI] [PubMed] [Google Scholar]
- 10.Feral CC, Nishiya N, Fenczik CA, Stuhlmann H, Slepak M, Ginsberg MH 2005. CD98hc (SLC3A2) mediates integrin signaling. Proc Natl Acad Sci USA 102:355–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narumiya S 1996. The small GTPase Rho: cellular functions and signal transduction. J Biochem 120:215–228 [DOI] [PubMed] [Google Scholar]
- 12.Ridley AJ, Hall A 1994. Signal transduction pathways regulating Rho-mediated stress fibre formation: requirement for a tyrosine kinase. EMBO J 13:2600–2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Etienne-Manneville S, Hall A 2002. Rho GTPases in cell biology. Nature 420:629–635 [DOI] [PubMed] [Google Scholar]
- 14.Kurokawa K, Matsuda M 2005. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol Biol Cell 16:4294–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flinn HM, Ridley AJ 1996. Rho stimulates tyrosine phosphorylation of focal adhesion kinase, p130 and paxillin. J Cell Sci 109:1133–1141 [DOI] [PubMed] [Google Scholar]
- 16.Vielkind S, Gallagher-Gambarelli M, Gomez M, Hinton HJ, Cantrell DA 2005. Integrin regulation by RhoA in thymocytes. J Immunol 175:350–357 [DOI] [PubMed] [Google Scholar]
- 17.Miao H, Li S, Hu YL, Yuan S, Zhao Y, Chen BP, Puzon-McLaughlin W, Tarui T, Shyy JY, Takada Y, Usami S, Chien S 2002. Differential regulation of Rho GTPases by β1 and β3 integrins: the role of an extracellular domain of integrin in intracellular signaling. J Cell Sci 115:2199–2206 [DOI] [PubMed] [Google Scholar]
- 18.Dalton P, Christian HC, Redman CW, Sargent IL, Boyd CA 2007. Membrane trafficking of CD98 and its ligand galectin 3 in BeWo cells—implication for placental cell fusion. FEBS J 274:2715–2727 [DOI] [PubMed] [Google Scholar]
- 19.Kudo Y, Boyd CA 2000. Heterodimeric amino acid transporters: expression of heavy but not light chains of CD98 correlates with induction of amino acid transport systems in human placental trophoblast. J Physiol 523:13–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu C, Keivens VM, O’Toole TE, McDonald JA, Ginsberg MH 1995. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell 83:715–724 [DOI] [PubMed] [Google Scholar]
- 21.Ren X.D, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA 2000. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci 113:3673–3678 [DOI] [PubMed] [Google Scholar]
- 22.Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Nagamatsu S, Sakai K, Nakamura Y, Lotfi A, Kawakami H, Iwashita M 2003. αvβ3 integrin signaling pathway is involved in insulin-like growth factor I-stimulated human extravillous trophoblast cell migration. Endocrinology 144:1620–1630 [DOI] [PubMed] [Google Scholar]
- 23.Zhou Y, Damsky CH, Fisher SJ 1997. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype. One cause of defective endovascular invasion in this syndrome? J Clin Invest 99:2152–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumagai N, Morii N, Fujisawa K, Nemoto Y, Narumiya S 1993. ADP-ribosylation of rho p21 inhibits lysophosphatidic acid-induced protein tyrosine phosphorylation and phosphatidylinositol 3-kinase activation in cultured Swiss 3T3 cells. J Biol Chem 268:24535–24538 [PubMed] [Google Scholar]
- 25.Soundararajan R, Rao AJ 2004. Trophoblast ‘pseudo-tumorigenesis’: significance and contributory factors. Reprod Biol Endocrinol 2:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kudo Y, Boyd CA 2004. RNA interference-induced reduction in CD98 expression suppresses cell fusion during syncytialization of human placental BeWo cells. FEBS Lett 577:473–477 [DOI] [PubMed] [Google Scholar]
- 27.Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, Zent R 2005. CD98 modulates integrin β1 function in polarized epithelial cells. J Cell Sci 118:889–899 [DOI] [PubMed] [Google Scholar]
- 28.Fenczik CA, Sethi T, Ramos JW, Hughes PE, Ginsberg MH 1997. Complementation of dominant suppression implicates CD98 in integrin activation. Nature 390:81–85 [DOI] [PubMed] [Google Scholar]
- 29.Chandrasekaran S, Guo NH, Rodrigues RG, Kaiser J, Roberts DD 1999. Pro-adhesive and chemotactic activities of thrombospondin-1 for breast carcinoma cells are mediated by α3β1 integrin and regulated by insulin-like growth factor-1 and CD98. J Biol Chem 274:11408–11416 [DOI] [PubMed] [Google Scholar]
- 30.Okamoto Y, Sakata M, Ogura K, Yamamoto T, Yamaguchi M, Tasaka K, Kurachi H, Tsurudome M, Murata Y 2002. Expression and regulation of 4F2hc and hLAT1 in human trophoblasts. Am J Physiol Cell Physiol 282:C196–C204 [DOI] [PubMed]
- 31.Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman S, Arnaout, MA 2002. Crystal structure of the extracellular segment of integrin α Vβ3 in complex with an Arg-Gly-Asp ligand. Science 296:151–155 [DOI] [PubMed] [Google Scholar]
- 32.Kabir-Salmani M, Shiokawa S, Akimoto Y, Sakai K, Sakai K, Iwashita M 2005. Tissue transglutaminase at embryo-maternal interface. J Clin Endocrinol Metab 90:4694–4702 [DOI] [PubMed] [Google Scholar]
- 33.Mizoguchi, K, Cha, SH, Chairoungdua, A, Kim, DK, Shigeta, Y, Matsuo, H, Fukushima J, Awa Y, Akakura K, Goya T, Ito H, Endou H, Kanai Y 2001. Human cystinuria-related transporter: localization and functional characterization. Kidney Int 59:1821–1833 [DOI] [PubMed] [Google Scholar]