Abstract
Previous study showed that mice lacking modulator recognition factor-2 (Mrf-2) were lean, with significant decreases in white adipose tissue. One postulated mechanism for the lean phenotype in Mrf-2 knockout mice is a defect in adipogenesis. In order to investigate this further, we examined the effects of Mrf-2 deficiency on adipogenesis in vitro. In mouse fibroblasts (MEFs) derived from Mrf-2−/− embryos, and in 3T3-L1 cells after knockdown of Mrf-2 by small interference RNA (siRNA) there was a potent inhibition of hormone-induced lipid accumulation, and significant decreases in the expression of the adipogenic transcription factors CCAAT/enhancer-binding protein (C/EBP) α and peroxisome proliferator-activated receptor-γ and the mature adipocyte genes they control. Transduction of Mrf-2−/− MEFs with a retroviral vector expressing the longer Mrf-2 splice variant (Mrf-2B) stimulated both gene expression and lipid accumulation. Because 3T3-L1 cells are committed to the adipocyte lineage, we used this simpler model system to examine the effects of Mrf-2 deficiency on adipocyte maturation. Analyses of both mRNA and protein revealed that knockdown of Mrf-2 in 3T3-L1 cells prolonged the expression of C/EBP homologous protein-10, a dominant-negative form of C/EBP. Consistent with these findings, suppression of Mrf-2 also inhibited the DNA-binding activity of C/EBPβ. These data suggest that Mrf-2 facilitates the induction of the two key adipogenic transcription factors C/EBPα and peroxisome proliferator-activated receptor-γ indirectly by permitting hormone-mediated repression of the adipogenic repressor C/EBP homologous protein-10.
WHITE ADIPOSE TISSUE plays an important role in energy balance by accumulating triglycerides when energy substrates are abundant, and liberating glycerol and fatty acids during starvation. Recent data suggest that both lipogenesis and adipocyte differentiation are dynamically regulated to accommodate changes in energy balance (1). Therefore, understanding the mechanisms that control these processes may be the key to understanding how excess energy input leads to obesity and its complications.
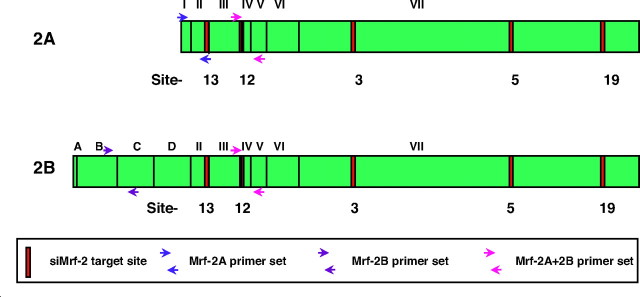
Modulator recognition factors-1 and -2 (Mrf-1, 2) were cloned in our laboratory by screening an expression library for proteins that recognize specific viral DNA sequences (2, 3). Mrf-2 has two splicing variants, Mrf-2A and -2B, as shown in Fig. 1. Exons III–VI, which encode the ARID (AT-rich interaction domain) DNA-binding motif are found in both proteins, as are exons II and VII. Mrf-2A is truncated at the N-terminal and lacks exons A-D of Mrf-2B (4, 5). Little is known about the differences in the functions between Mrf-2A and 2B, but it has been reported that Mrf-2B is a more potent inhibitor of cell growth in NIH-3T3 fibroblasts (4).
Fig. 1.
Structures of Two Splicing Variants of Mrf-2 and Locations of Small Interference RNAs Directed to Mrf-2
The differences in the two splicing variants, Mrf-2A and Mrf-2B are shown. Exons III-VI encode the ARID DNA-binding motif that is found in both Mrf-2A and Mrf-2B, as are exons II and VII. Mrf-2A lacks exons A-D of Mrf-2B; exon I encodes only the first codon of Mrf-2A. This figure also depicts the locations of small interference RNAs directed to Mrf-2, and of PCR primer sets that specifically detect Mrf-2A (blue), Mrf-2B (purple), or both (red). All five of the siMrf-2 target sites are common to both Mrf-2A and Mrf-2B.
To study the functions of Mrf-2 further, we generated mice lacking both Mrf-2A and 2B (6). Mrf-2 knockout mice are lean, with reduction in both brown and white adipose tissues, and marked lipodystrophy in both inguinal and gonadal white adipose depots. Mrf-2 is widely expressed in adult mouse tissues and therefore, the lean phenotype of Mrf-2−/− mice could arise from a variety of mechanisms. One hypothesis that could explain both the leanness and the lipodystrophy in these mice is that Mrf-2 expression is required for adipogenesis. To test this hypothesis, we examined the effects of Mrf-2 deficiency on adipogenesis using two different in vitro model systems. In the first model, we compared in vitro adipogenesis in primary fibroblast lines (mouse fibroblasts; MEFs) derived from Mrf-2−/− and Mrf-2+/+ embryos. Here we show that the Mrf-2−/− MEF lines have a significant defect in in vitro adipogenesis, compared with MEFs from their wild-type littermates.
Embryonic fibroblast cultures contain heterogeneous cells populations and may include mesenchymal stem cells, preadipocytes, and other cell types. Therefore, the observed effects of Mrf-2 deficiency in this model could result from a reduction in the commitment of precursors to the adipocyte lineage, or a reduction in adipocyte maturation, or both. Because murine 3T3-L1 preadipocytes are committed to the adipocyte lineage, we elected to use this simpler system to determine whether Mrf-2 is required for adipocyte maturation.
The process of in vitro adipogenesis has been examined in considerable detail in 3T3-L1 cells. When treated with hormone mixtures that contain 3-isobutyl-1-methylxanthine (IBMX), dexamethasone (Dex), and insulin (Ins), these cells divide one or two times in a process called mitotic clonal expansion (7), and CCAAT/enhancer-binding proteins β and δ (C/EBPβ and -δ) are induced (8, 9). C/EBPβ and -δ cooperate to induce C/EBPα and peroxisome proliferator-activated receptor-γ (PPARγ) (8, 10). C/EBPα and PPARγ maintain the expression of one another, and activate transcription of many genes that are characteristic of mature adipocytes, such as adipose fatty acid-binding protein (aP2) (11, 12), phosphoenolpyruvate carboxykinase (PEPCK) (13, 14), lipoprotein lipase (15), and perilipin (16, 17).
Another member of the C/EBP family, C/EBP homologous protein-10 (CHOP-10; also called growth arrest and DNA damage 153–GADD 153), has inhibitory effects on C/EBPα, -β, and -δ. In CHOP-10, proline replaces the basic region alanine and lysine residues that are essential for DNA-binding activity in other C/EBP family members (18). In the absence of CHOP-10, the other C/EBPs form homodimers, and activate transcription by binding to DNA; in the presence of CHOP-10, C/EBPs form heterodimers with CHOP-10 that cannot bind to DNA, and consequently the expression of C/EBP-dependent genes is suppressed (18, 19). Expression of CHOP-10 falls during clonal expansion, when C/EBPβ activity is increasing (19). The failure to decrease CHOP-10 under some experimental conditions causes inhibition of adipogenesis, primarily due to a decrease in C/EBPβ activity (19, 20, 21, 22). Here we show that knockdown of Mrf-2 inhibits adipocyte maturation in 3T3-L1 preadipocytes, and provide preliminary evidence that this is due to persistent expression of CHOP-10 in the early phases of this process.
RESULTS
Mrf-2−/− MEFs Have a Significant Deficit in in Vitro Adipogenesis
The propagation of mouse embryo fibroblasts is subject to considerable variation. This may be due to variations in timing of the pregnancies, the fairly crude dissections of mouse embryos or variations in culturing and freezing of the lines. As a result, even wild-type MEF lines show considerable variation in their potential for in vitro adipogenesis. Therefore, in examining the effects of the Mrf-2 knockout on adipogenesis, we sought to minimize these variables by comparing knockout and wild-type MEFs from the same litters. Figure 2A shows a typical experiment. It can be seen that the number of adipocytes in either of the two Mrf-2−/− MEF cultures was significantly lower than in the Mrf-2+/+ MEF culture from d 6–12 of hormone treatment. These results are typical of similar experiments in which one or more wild-type MEF lines were compared with one or more knockout MEF lines from four separate litters. In each case, the number of fat cells produced was significantly lower for the knockout line than for the paired wild-type line.
Fig. 2.
In Vitro Adipogenesis in Mrf-2−/− and Mrf-2+/+ Mouse Embryo Fibroblasts
A, Time-course of adipogenesis. Two Mrf-2−/− MEF lines (red symbols) and one Mrf-2+/+ MEF line (blue symbols) from the same litter were plated at the same densities and treated with adipogenic hormones for 12 d. At the indicated time points, fat cells were counted as described in Materials and Methods. Values are means, ± se for quadruplicate wells. * and **, Significant differences between both Mrf-2−/− MEF lines and the Mrf-2+/+ MEF line, in pairwise comparisons using a two-tailed Student’s t-test, P < 0.03 and P < 0.001, respectively. The right-hand panels show representative low-power fields of Oil Red O-stained cultures at d 12. The results are representative of independent experiments using paired Mrf-2−/− and Mrf-2+/+ MEF lines from four different litters. B, Gene expression in adipogenesis. The three MEF lines shown in Part A, plus one Mrf-2−/− and two Mrf-2+/+ MEF lines from a different litter were plated in six-well plates and treated with adipogenic hormones. At the indicated time points, RNA was extracted from each MEF line. cDNA was prepared from all of the samples using the same reaction mix, and expression of the indicated genes, normalized to 18S RNA was measured using real-time PCR. *, Significant differences between the Mrf-2−/− and Mrf-2+/+ MEF lines, using an unpaired t test, P < 0.05.
As expected, the expression of multiple markers of mature adipocytes was also reduced in Mrf-2−/− MEFs compared with MEFs from wild-type littermates. Figure 2B shows real-time RT-PCR data for RNA samples derived from the same cells depicted in Fig. 2A, plus RNA samples that were obtained in a duplicate experiment using MEF lines from another litter. The second litter included one Mrf-2−/− embryo and two Mrf-2+/+ embryo, and therefore, we were able to compare three RNA samples of each genotype at each time point. As can be seen, the expression of the adipocyte markers fatty acid synthase (FAS), perilipin, PEPCK, and aP2 was significantly reduced in the Mrf-2−/− MEFs after 12 d of hormone treatment. Interestingly, the adipogenic transcription factors C/EBPα and PPARγ were also reduced in the Mrf-2−/− MEFs at d 12.
Overexpression of Mrf-2B Stimulates Lipogenesis and Adipocyte Gene Expression in Mrf-2−/− MEFs
Mrf-2−/− MEFs lack expression of both Mrf-2A and Mrf-2B. Therefore, it was not clear whether the deficit in adipogenesis in these cells is due to the lack of Mrf-2A, Mrf-2B or both. To address this, we prepared retroviral vectors that express these proteins and tested their effects on adipogenesis in Mrf-2−/− MEFs. Adipogenesis was not stimulated in Mrf-2−/− MEFs that were transduced with the Mrf-2A expressing retrovirus (data not shown) but was stimulated modestly in Mrf-2−/− MEFs that were transduced with the Mrf-2B-expressing retrovirus, compared with both untreated MEFs and MEFs that were transduced with the LacZ control retrovirus (Fig. 3A). The Mrf-2B retrovirus also stimulated the expression of C/EBPα, PPARγ, and mature adipocyte markers, including aP2, PEPCK, FAS, and perilipin (Fig. 3B). Interestingly, the expression of the Mrf-2B transgene was transient, reaching a peak at 48 h after transduction (d 0) and declining rapidly by d 2 of hormone treatment. Although Mrf-2B transgene expression remained above background levels at d 12 (Fig. 3B, upper right panel; note that this is a log scale), the peak of expression occurred in the early phases of adipogenesis. By contrast, most of the stimulation of gene expression occurs at d 12. This would suggest that either the modest overexpression of Mrf-2B that remains at d 12 (2-fold over background) is sufficient to stimulate the expression of the late genes, or that these are downstream effects of the high level of Mrf-2B expression at early time points. In this regard, it is interesting to note that transduction with the Mrf-2B retrovirus inhibits CHOP-10 expression before the addition of adipogenic hormones at d 0, compared with either LacZ retrovirus or no treatment (Fig. 3B, lower right panel). An increase in CHOP-10 expression coincides with the decrease in Mrf-2B expression in Mrf-2B retrovirus-transduced cells. CHOP-10 expression decreases after d 2 of hormone treatment, but it is not lower in the Mrf-2B-transduced cells than in the untreated cells. Taken together, these results suggest that overexpression of Mrf-2B in the early stages of hormone treatment is sufficient to stimulate both adipogenesis and adipocyte-specific gene expression several days later.
Fig. 3.
Transduction of Mrf-2−/− MEFs with Retroviral Vectors that Express Mrf-2B Stimulates Adipogenesis and Adipocyte-Specific Gene Expression
A, Adipogenesis. Mrf-2−/− MEFs were plated in tissue culture flasks and transduced the following day (d −2) with retrovirus expressing Mrf-2B (blue symbols and lines), a control retrovirus expressing LacZ (green symbols and lines) or no retrovirus (red symbols and lines). *, Significant differences between the Mrf-2B-transduced cells and both untreated and LacZ-transduced cells. After 2 d (d 0) the cells were treated with adipogenic hormones and fat cells were counted every other day, beginning at d 6. The right-hand panels are bright-field photomicrographs of Oil Red O-stained cells at d 12. B, Gene expression. RNA was isolated from replicate flasks at the indicated time points and analyzed by real-time RT-PCR using primers for the indicated genes. The d 0 sample was from a single untransfected flask. Transgene refers to either human Mrf-2B or LacZ, as indicated. Transgene expression was quantitated using serial dilutions of the pFB-Neo-Mrf-2B or PFB-Neo-LacZ plasmids; data for the untransfected cells were obtained using the pFB-Neo-Mrf-2B plasmid as standard. The data are representative of separate experiments using three different Mrf-2−/− MEF lines.
Because all MEF lines contain mixtures of uncommitted mesenchymal stem cells and committed adipocyte precursors, the endpoint of the in vitro adipogenesis experiments reflects the contributions of both cell populations. Therefore, the defect in adipogenesis in Mrf-2−/− MEFs could result from impaired commitment to the adipocyte lineage, impaired maturation of committed precursors, or both, and the overexpression of Mrf-2B could affect either or both of these processes. To evaluate the role of Mrf-2 in adipocyte maturation, we used the 3T3-L1 preadipocyte line.
Knockdown of Mrf-2 by Small Interference RNA Inhibits Adipogenesis in 3T3-L1 Preadipocytes
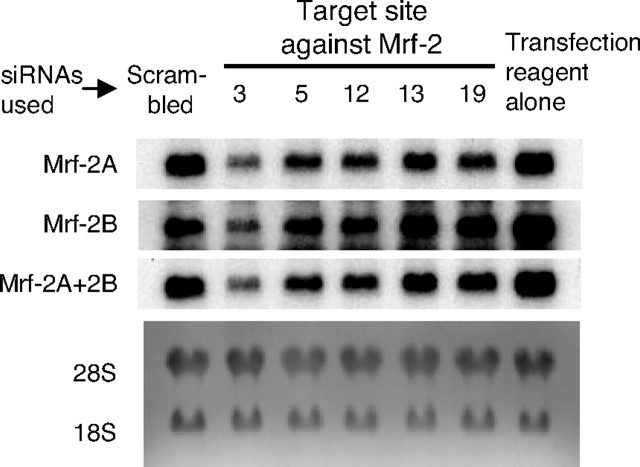
We prepared five siRNAs that were targeted to sequences common to both Mrf-2A and Mrf-2B (Fig. 1 and Table 1), and tested their ability to knock down Mrf-2A and Mrf-2B expression in 3T3-L1 preadipocytes. 3T3-L1 preadipocytes were treated with transfection reagent alone, with scrambled siRNA, or with siRNAs targeted to Mrf-2 (siMrf-2). Total RNA was extracted the following day, and Northern blot analyses were carried out using probes specific to Mrf-2A, Mrf-2B, or both. As shown in Fig. 4, the expression levels of both Mrf-2A and Mrf-2B were unchanged in scrambled siRNA-treated cells compared with cells treated with transfection reagent alone. Treatment of 3T3-L1 preadipocytes with siRNAs targeted to all five sites led to down-regulation of both Mrf-2A and 2B, compared with the controls. Because all five of the siMrf-2 targets are common to both Mrf-2A and Mrf-2B, it is reasonable that both splicing variants would be affected similarly by any given siRNA. The site 13 and 19 siMrf-2s had the smallest effects, the site 5 and 12 siMrf-2s had intermediate effects, and site 3 siMrf-2 had the strongest effect.
Table 1.
Sequences of Small Interference RNAs Targeted to Mrf-2
| Targets | 5′–3′ | |
|---|---|---|
| Scrambled | Sense | CCUUCAGCCUAAACCCAGGUAAATA1 |
| Antisense | UAUUUACCUGGGUUUAGGCUGAAGGCC | |
| Site 3 | Sense | GCCAAACCCUGCUGCUUUACAGAGA |
| Antisense | UCUCUGUAAAGCAGCAGGGUUUGGCUU | |
| Site 5 | Sense | CAAGAACCCACAUAAACUAACCAGC |
| Antisense | GCUGGUUAGUUUAUGUGGGUUCUUGGG | |
| Site 12 | Sense | ACAGAUUAACCUUUGGACUAUGUTT |
| Antisense | AAACAUAGUCCAAAGGUUAAUCUGUUU | |
| Site 13 | Sense | CAAUAACUGUGACGGUAAAGUUATC |
| Antisense | GAUAACUUUACCGUCACAGUUAUUGUU | |
| Site 19 | Sense | GAACCAGACGGUGCUUUCUCCUCTC |
| Antisense | GAGAGGAGAAAGCACCGUCUGGUUCUU |
The two nucleotides at the 3′-end of the sense strand (underlined) are deoxynucleotides.
Fig. 4.
Knockdown of Mrf-2 mRNA Expression in 3T3-L1 Cells by siRNAs Targeted to Various Sites in Mrf-2
Confluent 3T3-L1 cells were treated with five siRNAs targeted to Mrf-2, as listed in Table 1. After 6 h, the medium was replaced, and cells were incubated for 18 h. Total RNA was extracted and Northern blotting was carried out using probes to Mrf-2A, Mrf-2B and Mrf-2A +2B successively.
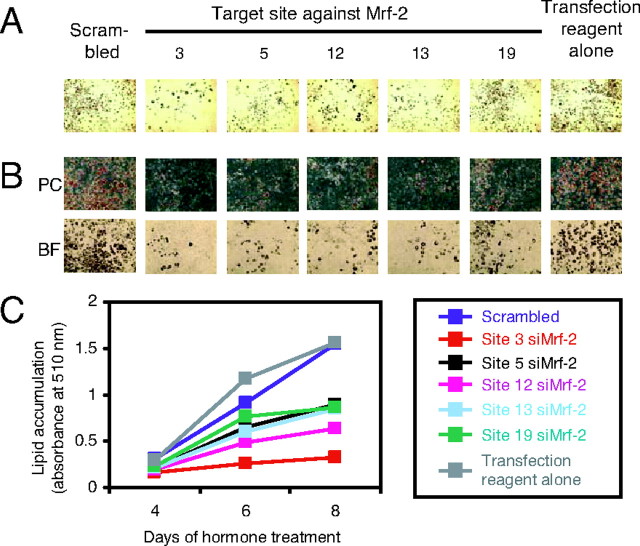
Having established that our siMrf-2s knock down Mrf-2 expression, we next tested whether they would inhibit adipogenesis. (In all of these experiments, the siRNAs were added 1 d before the addition of the adipogenic mixture; see Materials and Methods.) As shown in Fig. 5, all of the siMrf-2s inhibited the formation of adipocytes. Site 3 siMrf-2 inhibited adipogenesis the most, which is consistent with our observation that it had the strongest effects on Mrf-2 expression (Fig. 4). To quantitate lipid accumulation, Oil Red O was extracted after staining, and the absorbance of the extracts was measured (Fig. 5C). As expected, lipid accumulation was decreased in cells treated with all five siMrf-2s compared with the controls. Site 3 siMrf-2 was most effective, with a decrease of about 80% at d 8.
Fig. 5.
Inhibition of Adipogenesis in 3T3-L1 Cells by Knockdown of Mrf-2 mRNA
3T3-L1 cells were seeded and allowed to reach confluency. One day after the cells were confluent, they were transfected with the indicated siRNAs. Twenty-four hours after siRNA treatment (designated as d 0), adipogenesis was initiated by adding IBMX, Dex, and Ins. At d 4, 6, and 8, cells were fixed and stained with Oil Red O. A, Photomicrographs at low magnification under bright-field at d 8. B, Photomicrographs at high magnification under phase contrast (PC) and bright-field (BF) at d 8. C, Oil Red O was extracted with 2-propanol, and the absorbances of the extracts at 510 nm were measured to quantitate lipid accumulation at d 4, 6, and 8. The results shown are representative of two independent experiments.
To reduce the chances of artifactual effects in siRNA experiments, it is important to show that down-regulation of the target mRNA produces consistent phenotypic effects that are not dependent on a specific target site within the gene of interest (23). In this study, we used five different siRNAs targeted to both Mrf-2A and Mrf-2B (Fig. 1), and observed similar knockdown effects on both transcripts (Figs. 4 and 5). All five siRNAs also inhibited adipogenesis (Fig. 5), and this effect correlated well with the extent of the reductions in Mrf-2A and Mrf-2B. Therefore, we conclude that the inhibition of adipogenesis by knockdown of Mrf-2 is not an artifact, and that Mrf-2 is required for adipocyte maturation. In our subsequent experiments, we used both sites 3 and 12 siMrf-2s for knockdowns and scrambled siRNA for controls. (Results for site 3 are presented here; results for site 12, which were essentially identical, are presented in the supplemental data published on The Endocrine Society’s Journals Online web site at http://mend.endojournals.org).
Expression Profile of Mrf-2 during Adipogenesis
In the experiments shown in Fig. 5, we introduced siMrf-2 by transient transfection 1 d before induction of adipogenesis, and yet, we observed decreases in fat accumulation 5–9 d later. To determine whether these effects were due to a transient knockdown of Mrf-2 in the early stages of adipogenesis or a persistent knockdown that lasted throughout adipogenesis, we measured Mrf-2 expression at multiple time points after transfection. As shown in Fig. 6A (Northern blot), and Fig. 6B (real-time RT-PCR), siMrf-2 decreased the expression of Mrf-2A by 60∼80% from d 0–2, and this effect disappeared by d 6. Mrf-2B expression was decreased most at d 0, but the effects of siMrf-2 were difficult to detect on d 1 and 2, owing to the severe decrease of Mrf-2B in the control on those days. Nonetheless, Mrf-2B expression had clearly begun to recover by d 3, and was at normal levels from d 4–6. Although we have generated antibodies to human Mrf-2 peptides, they do not cross-react well with the mouse proteins, and they are unsuitable for Western blot analysis. Therefore, we were unable to track Mrf-2A and Mrf-2B protein expression after treatment with siMrf-2.
Fig. 6.
Mrf-2 mRNA Expression during Adipogenesis in Scrambled siRNA- or siMrf-2-Treated 3T3-L1 Cells
Adipogenesis was initiated by IBMX, Dex, and Ins after treatment of confluent 3T3-L1 cells with scrambled siRNA or site 3 siMrf-2 as described in Materials and Methods. Total RNA was extracted at the indicated time points. Panel A, Northern blotting. Total RNA was separated on denaturing agarose gels, and transferred to a Zeta-Probe membrane. The membrane was hybridized successively with probes specific to Mrf-2A, Mrf-2B, or both, and mRNA transcripts were visualized by autoradiography. C, Scrambled siRNA treatment; KD indicates siMrf-2 treatment. Panel B, Real-time RT-PCR. Open columns, Scrambled siRNA treatment; closed columns, siMrf-2 treatment. The results shown are representative of two independent experiments.
SiMrf-2 Inhibits the Expression of Adipogenic Genes
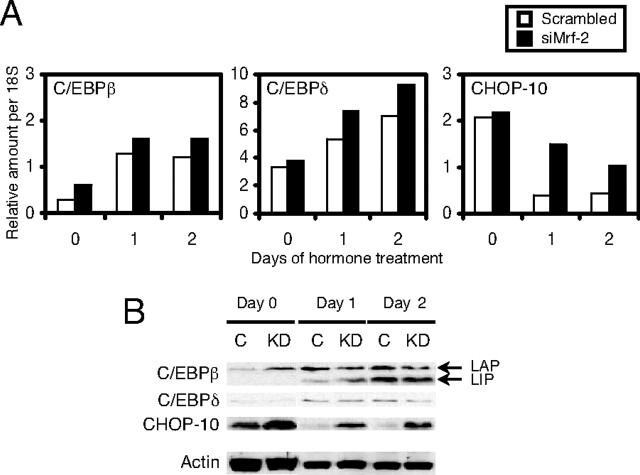
Because the effects of siMrf-2 were strongest at the early stages of adipogenesis, we measured its effects on the expression of C/EBPβ and -δ, which are known to be induced at this time. Surprisingly, expression of mRNA encoding these C/EBPs was slightly higher in siMrf-2-treated cells than in controls (Fig. 7A). Western blot analyses revealed that there was little or no change in the expression of either the active form of C/EBPβ (designated as LAP in Fig. 7B), the dominant-negative form of C/EBPβ [designated as LIP in Fig. 7B (24)] or C/EBPδ. Expression of CHOP-10, which acts as a dominant-negative inhibitor of C/EBPs, decreases after induction of adipogenesis (19), and we observed the same phenomenon in scrambled siRNA-treated 3T3-L1 cells (open column in Fig. 7A and lanes C in Fig. 7B). Interestingly, CHOP-10 mRNA and protein remained at much higher levels after induction of adipogenesis when 3T3-L1 preadipocytes were treated with siMrf-2 (closed columns in Fig. 7A, and lanes KD in Fig. 7B). The increased expression of CHOP-10 after treatment with siMrf-2 would be expected to decrease the expression of C/EBPβ-dependent genes. Therefore, we examined the effects of siMrf-2 on the expression of C/EBPα and PPARγ. In control cells, the expression of C/EBPα mRNA became detectable at d 2, and reached a maximum from d 6–8 (Fig. 8A, open columns). This induction was weaker at every time point in 3T3-L1 cells treated with siMrf-2 (Fig. 8A, closed columns). C/EBPα has two alternative translation products: a full-length 42-kDa isoform (p42) and a truncated 30-kDa isoform (p30). Although both isoforms are active transcription factors, their expression cannot be evaluated by real-time RT-PCR because these isoforms arise from the same transcript (25). Therefore, we also measured the levels of these isoforms using Western blots (Fig. 8B). In control cells, both C/EBPα p42 and p30 gradually increased from d 2–4 and reached a maximum at d 6–8 (Fig. 8B, lanes C). SiMrf-2 decreased the levels of both p42 and p30 from d 2–8 after adipogenesis. The profile of PPARγ mRNA expression was similar to that of C/EBPα in scrambled siRNA-treated cells (Fig. 8A, open columns). As it was for C/EBPα, expression of PPARγ mRNA was decreased in siMrf-2-treated 3T3-L1 cells (Fig. 8A, closed columns). PPARγ has two transcriptional variants (PPARγ1 and PPARγ2), with PPARγ2 being the adipocyte-specific form (12). Because we used primer sets that detect both isoforms of PPARγ, the two variants were measured separately using Western blot analysis. Immunoblotting revealed that PPARγ1 did not change, but PPARγ2 expression was decreased from d 4–8 in 3T3-L1 cells treated with siMrf-2 (Fig. 8B). As expected, reduced expression of C/EBPα and PPARγ in 3T3-L1 cells treated with siMrf-2 suppressed expression of their target genes, such as aP2, PEPCK, perilipin, and fatty acid synthase at the late stages of adipogenesis (Fig. 8A). Immunoblotting showed that knockdown of Mrf-2 also suppressed expression of the proteins encoded by these genes (Fig. 8B).
Fig. 7.
Expression of C/EBP Family Members during Adipogenesis in Scrambled siRNA- or siMrf-2-Treated 3T3-L1 Cells
Adipogenesis was initiated by IBMX, Dex, and Ins 1 d after treatment of confluent 3T3-L1 cells with scrambled siRNA or site 3 siMrf-2. Panel A, Real-time RT-PCR. Open columns, Scrambled siRNA treatment; closed columns, siMrf-2 treatment. Panel B, Immunoblotting. Whole-cell extracts (25 μg) were subjected to SDS-PAGE and transferred to nitrocellulose membranes. The membranes were blotted successively with antibodies to C/EBPβ, C/EBPδ, and CHOP-10, as indicated. C, Scrambled siRNA treatment; KD, siMrf-2 treatment; LAP, liver-enriched activator protein; LIP, liver-enriched inhibitory protein. The results shown are representative of two independent experiments.
Fig. 8.
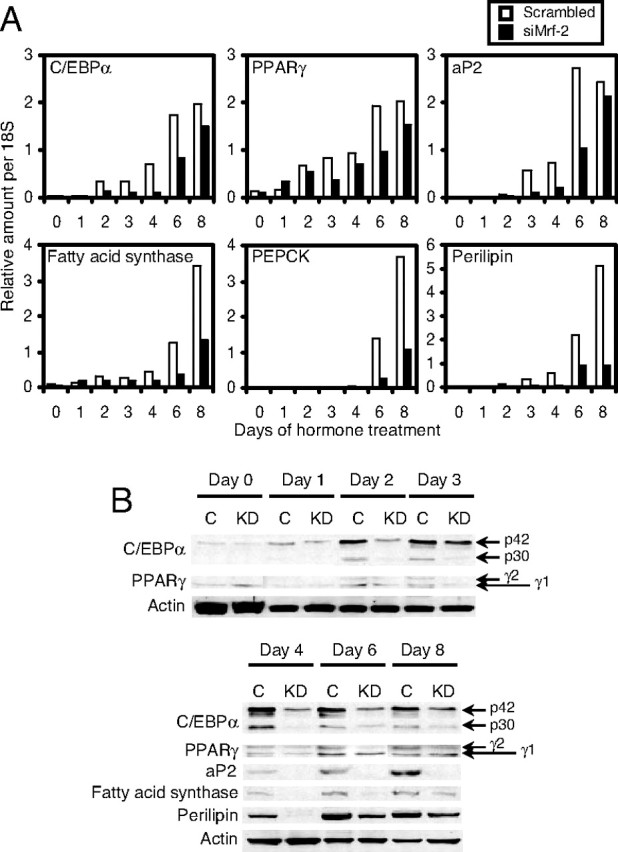
Expression of Adipogenic Transcription Factors and Their Target Genes during Adipogenesis in 3T3-L1 Cells Treated with Scrambled siRNA or siMrf-2
Adipogenesis was initiated by IBMX, Dex, and Ins after treatment of confluent 3T3-L1 cells with scrambled siRNA or site 3 siMrf-2. Total RNA or whole cell extracts were obtained at the indicated time points and gene and protein expression were measured as shown in Fig. 5. Panel A, Real-time RT-PCR. Open columns, Scrambled siRNA treatment; closed columns, siMrf-2 treatment. Panel B, Immunoblotting. C, Scrambled siRNA treatment; KD, siMrf-2 treatment; p42, full-length C/EBPα; p30, its alternative translation product. The results shown are representative of two independent experiments.
SiMrf-2 Decreases C/EBPβ DNA-Binding Activity at the Early Stages of Adipogenesis
Sustained expression of CHOP-10 protein in siMrf-2 treated 3T3-L1 cells (Fig. 7) might inhibit adipogenesis by decreasing C/EBPβ DNA-binding activity (19, 20, 21, 22, 26). We tested this directly by preparing nuclear extracts from control and siMrf-2-treated cells on d 0 and 1 of adipogenesis and comparing C/EBPβ protein levels and DNA-binding activity. A portion of each nuclear extract was subjected to immunoblot analysis for measurement of C/EBPβ and CHOP-10 as shown in Fig. 9A. Knockdown of Mrf-2 did not change the expression of either C/EBPβ-LAP or C/EBPβ-LIP. As we saw in previous experiments, the expression of CHOP-10 protein was higher in the siMrf-2-treated 3T3-L1 cells at d 0, and although it decreased by d 1, it remained substantially higher than the control level (Fig. 9A). An EMSA was carried out using the same nuclear extracts and the results are shown in Fig. 9B. When nuclear extracts from d 0 were used, C/EBPβ-DNA complexes did not appear (lanes 2–5), and this was consistent with the lack of C/EBPβ expression seen in Fig. 8A. A low molecular weight DNA-protein complex did appear in these lanes, but it did not appear to contain C/EBPβ because it was not supershifted by anti-C/EBPβ antibodies. In nuclear extracts from d 1, higher molecular weight DNA-protein complexes were observed (lanes 6 and 10), and these complexes were super-shifted by anti-C/EBPβ antibodies (lanes 8 and 12). No shifts were observed, however, when anti-C/EBPδ antibody was used (lanes 9 and 13). The C/EBPβ-protein complexes disappeared in the presence of excess unlabeled probe (lanes 7 and 11), but not in the presence of excess DNA that lacked a C/EBPβ-binding site (data not shown). C/EBPβ DNA-binding activity was reduced in nuclear extracts from siMrf-2-treated cells, compared with that in scrambled siRNA-treated controls (compare lanes 6 and 10, 8 and 12). As a control, we also measured DNA binding by NF-1 in the same nuclear extracts, and found that it was unaffected by siMrf2 (data not shown). The reduction in C/EBPβ DNA-binding activity in the absence of a change in C/EBPβ proteins, coupled with the increase in CHOP-10 protein, strongly implicate CHOP-10 as the mediator of the siMrf-2-dependent inhibition of adipogenesis.
Fig. 9.

Effect of Knockdown of Mrf-2 on C/EBPβ DNA-Binding Activity at the Early Stages of Adipogenesis
Adipogenesis was initiated by IBMX, Dex, and Ins after treatment of confluent 3T3-L1 cells with scrambled siRNA or site 3 siMrf-2. Nuclear extracts were prepared at the indicated time points. Panel A, Immunoblot analysis of nuclear extracts (10 μg). Panel B, EMSAs. Nuclear extracts were incubated with a radiolabeled oligonucleotide probe corresponding to the C/EBPβ binding site in the C/EBPα promoter, in the presence or absence of a 100-fold excess of unlabeled probe, anti-C/EBPβ or anti-C/EBPδ antibody (0.4 μg), as indicated. C, Scrambled siRNA treatment; KD, siMrf-2 treatment. The results shown are representative of two independent experiments.
DISCUSSION
Our results show that expression of the ARID transcription factor gene Mrf-2 is required for adipogenesis in two different model systems. In vitro adipogenesis was strongly inhibited in Mrf-2−/− MEFs compared with Mrf-2+/+ MEFs, and in 3T3-L1 cells after knockdown of Mrf-2 by siRNA. In vitro adipogenesis was also stimulated in Mrf-2−/− MEFs after overexpression of Mrf-2B (the longer splice variant of the Mrf-2 gene). The loss or restoration of Mrf-2 expression was associated with reduced or enhanced expression, respectively, of the adipogenic transcription factors C/EBPα and PPARγ, and the downstream genes they regulate.
The defect in adipogenesis was consistently observed in Mrf-2−/− MEFs when they were compared with Mrf-2+/+ MEFs from the same litter. This was true for independent preparations from four different litters, rendering it extremely unlikely that this is an artifact relating to the propagation of the Mrf-2−/− MEFs. Primary MEF cultures contain mixed cell populations that may include mesenchymal stem cells as well as preadipocytes (27). Therefore, the defect in adipogenesis in Mrf-2−/− MEFs could indicate a requirement for Mrf-2 in commitment to the adipocyte lineage, and/or a requirement for Mrf-2 in adipocyte maturation. Similarly, the overexpression of Mrf-2B could affect either of these processes. To assess the effects of Mrf-2 deficiency on adipocyte maturation, we used siRNA to knock down Mrf-2A and Mrf-2B expression in 3T3-L1 cells, which are committed to the adipocyte lineage.
Adipogenesis in 3T3-L1 cells requires the expression of a well-characterized succession of transcription factors: early induction of C/EBPβ and C/EBPδ leads to the induction of C/EBPα and PPARγ, and the activities of these latter transcription factors activate the expression of adipocyte-specific genes. In this report, we demonstrated that expression of CHOP-10, the dominant-negative form of C/EBP, was prolonged at the early stages of adipogenesis by knockdown of Mrf-2A-plus -Mrf-2B, using either siMrf-2 site-3 or site-12 (Fig. 7; and Supplemental Fig. 3). This led to a decrease in C/EBPβ DNA-binding activity (Fig. 9; and Supplemental Fig. 4), followed by decreases in C/EBPα and PPARγ expression (Fig. 8; and Supplemental Fig. 2). Taken together, these data lead us to the attractive hypothesis that knockdown of Mrf-2 suppresses adipogenesis in 3T3-L1 cells primarily by inhibiting the reduction in CHOP-10 expression that normally occurs as an early response to adipogenic factors.
Although our hypothesis is that siMrf-2 suppresses the expression of C/EBPα and PPARγ and their target genes indirectly through the inhibition of C/EBPβ by CHOP-10, many other explanations are possible. One possibility is that transcriptional cofactors mediate the effects of siMrf-2 on these genes. To test this possibility, we measured the effects of siMrf-2 on the expression of thyroid hormone receptor-associated proteins 220 (28), CREB-binding protein (29), p300 (29, 30), and PPARγ coactivator-2 (31), which are known to be coactivators of C/EBPα and/or PPARγ during adipogenesis. Because we did not find any changes in the expression of these coactivators in siMrf-2-treated 3T3-L1 cells (data not shown), they do not appear to mediate the effects of siMRF-2.
Another possibility is that Mrf-2A or Mrf-2B interacts directly with C/EBPα, PPARγ, their promoters, or the promoters of their downstream targets. Although it is important to test this directly, this has proven difficult in practice. Both Mrf-2A and Mrf-2B produce inconsistent results in transient cotransfection assays with a variety of reporter systems. These proteins are tightly bound to the nuclear matrix (Whitson, R. H., unpublished results), and like other nuclear matrix proteins, they may only modulate the activities of their target genes in the context of higher-order chromatin structures. This is true for the nuclear matrix protein SATB1 (32).
The expression pattern for Mrf-2B, after retroviral transduction also suggests that Mrf-2B exerts indirect effects on C/EBPα and PPARγ (Fig. 3B). Whereas Mrf-2B expression peaks at d 0, and remains elevated through d 2, it is barely above background levels by d 12 when the effects on C/EBPα and PPARγ and the downstream genes are strongest.
Because suppression of Mrf-2 expression has similar effects on in vitro adipogenesis in both MEFs and 3T3-L1 cells, it is reasonable to propose that this occurs by similar mechanisms in the two cell types. We do not have evidence, however, to support a role for Mrf-2 in suppressing CHOP-10 expression in MEFs. Transduction of Mrf-2−/− MEFs with Mrf-2B-expressing retrovirus did suppress CHOP-10 expression (Fig. 3B), but CHOP-10 expression was not consistently elevated in Mrf-2−/− MEFs. In MEF preparations from some litters, CHOP-10 mRNA expression was significantly higher in Mrf-2−/− MEFs compared with Mrf-2+/+ MEFs. In MEFs from other litters, however, CHOP-10 expression was equal in Mrf-2−/− and Mrf-2+/+ lines, both before and during adipogenesis. This variability in expression was also observed for other transcription factors that are known to regulate early events in adipogenesis, including C/EBPβ, KLF2, KLF5, and KLF15 (Whitson, R. H., unpublished results). We do not know the reasons for the greater variability of CHOP-10 expression in MEFs vs. 3T3-L1 cells, but a likely explanation is that adipogenesis in the MEF model is far more complex than it is in 3T3-L1 model. MEF cultures include cells that are committed to other fates, such as bone or muscle, as well as uncommitted stem cells, and preadipocytes. Therefore, it is possible that the presence of these other cell types masks the effects of Mrf-2 deficiency on CHOP-10 expression at the early stages of adipogenesis. This is supported by the fact that CHOP-10 is known to have both positive and negative effects on other differentiation processes, such as osteogenesis (33). The presence of these other cell types contributes less noise to the measurement of adipocyte-specific genes, however, and therefore, we were able to discern statistically significant differences in the expression of these genes at the endpoint of our experiments (Fig. 2B).
Although our data strongly support a role for Mrf-2 in adipocyte maturation, many questions remain to be answered. Because the expression of both Mrf-2A and Mrf-2B was diminished by all of our siRNAs, it is not clear whether the inhibition of adipocyte maturation in 3T3-L1 cells is due to suppression of Mrf-2A or Mrf-2B or both. Selective knockdown of Mrf-2A by siRNA is impractical because Mrf-2A differs from Mrf-2B by only a single codon. Selective knockdown of Mrf-2B has also proven difficult, and the effects of siRNAs on both Mrf-2B expression and adipogenesis are both quite weak in 3T3-L1 cells. Retroviral vectors that express Mrf-2A or Mrf-2B do not stimulate adipogenesis in 3T3-L1 cells, either in the presence or absence of adipogenic factors (data not shown). Thus, it remains possible that the combined expression of Mrf-2A and Mrf-2B is required for adipocyte maturation in 3T3-L1 cells. By contrast, overexpression of Mrf-2B stimulates lipogenesis and the expression of C/EBPα and PPARγ and their downstream genes in Mrf-2−/− MEFs in the presence of Ins, Dex, and IBMX (Fig. 3B). Overexpression of Mrf-2A does not stimulate adipogenesis in Mrf-2−/− MEFs, however. Thus, it would appear that Mrf-2B is the more critical factor for facilitating adipogenesis in MEFs. Although it is tempting to conclude that Mrf-2B also plays the critical role in facilitating adipocyte maturation in 3T3-L1 cells, it is also possible that overexpression of Mrf-2B stimulates the commitment of stem cells to the adipocyte lineage in MEF cultures, and adipocyte maturation requires Mrf-2A or a combination of Mrf-2A and Mrf-2B. Knockdown and overexpression of Mrf-2B in mesenchymal stem cell lines may serve to clarify this. Finally, although our in vitro data suggest that the lipodystrophy in Mrf-2−/− mice could result from cell-autonomous effects, we must caution that lack of Mrf-2 expression in other tissues could also contribute to this phenotype. The best way to address this question is to propagate mice with an adipose-specific Mrf-2 knockout.
In summary, we found that the loss of Mrf-2A and Mrf-2B expression in Mrf-2−/− MEFs and knockdown of Mrf-2A-plus-Mrf-2B by siRNA in 3T3-L1 cells resulted in the inhibition of adipogenesis along with decreases in the expression of C/EBPα, PPARγ, and adipogenic genes. These data support a role for Mrf-2 in adipocyte maturation, and provide at least a partial explanation for the leanness and lipodystrophy we have observed in Mrf-2−/− mice (6). In 3T3-L1 preadipocytes, siRNA directed against Mrf-2A plus Mrf-2B also prolonged the expression of CHOP-10, and this led to the inhibition of C/EBPβ DNA-binding activity. It is not yet clear, however, which of the Mrf gene products facilitates the suppression of CHOP-10 in 3T3-L1 cells, or whether the same mechanism is at work in MEFs. These data and further investigations may lead to the development of novel treatments for obesity that target the activity of the Mrf-2 gene products.
MATERIALS AND METHODS
The 3T3-L1 preadipocyte cell line was purchased from American Type Culture Collection (Manassas, VA). DMEM high-glucose medium was purchased from Irvine Scientific (Santa Ana, CA). Fetal bovine serum was purchased from Omega Scientific (Tarzana, CA). Lipofectamine 2000 and TRIzol reagent were purchased from Invitrogen (Carlsbad, CA). RNasin ribonuclease inhibitor was purchased from Promega (Madison, WI). Avian myeloblastosis virus reverse transcriptase was purchased from Life Sciences, Inc. (St. Petersburg, FL). Real-time PCR Detection System, iQ SYBR Green supermix reagent, Zeta probe membranes, and Trans-blot nitrocellulose membranes were purchased from Bio-Rad (Hercules, CA). Protease inhibitor cocktail was purchased from Roche (Indianapolis, IN). NE-PER Nuclear and Cytoplasmic Extraction Reagents and MicroBCA assay kit were purchased from Pierce Biotechnology (Rockford, IL). Enhanced chemiluminescence (ECL) system was purchased from Amersham Bioscience (Piscataway, NJ). ULTRAhyb solution was purchased from Ambion (Austin, TX). [α-32P]deoxy-CTP and [γ-32P]ATP were purchased from PerkinElmer (Boston, MA). Other reagents were purchased from Sigma (St. Louis, MO).
Anti-C/EBPα (14AA), C/EBPβ (C-19)X, C/EBPδ (C-22)X, GADD153 (B-3), PPARγ (H-100), FAS (H-300), Actin (C-11)-R antibodies and horseradish peroxidase (HRP)-conjugated antirabbit IgG antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-aP2 antibody was purchased from Cayman Chemical (Ann Arbor, MI). Anti-perilipin A antibody was purchased from Affinity Bioreagents (Golden, CO). HRP-conjugated antimouse IgG antibody was purchased from Sigma.
MEF Culture
Male and female Mrf-2+/− mice were mated as harems (one male, two females) and embryonic d 0.5 was determined as the morning on which a mating plug appeared. Females were weighed daily to follow the course of the pregnancies, and euthanized at embryonic d 14.5. The embryos were separated from their yolk sacs, which were digested with proteinase K. The digests were then analyzed by PCR to determine genotypes. Genotypes were confirmed by also analyzing DNA extracted from the MEF cultures. The embryos were rinsed in ice-cold sterile saline containing streptomycin and penicillin, the heads and livers were removed, and the remainder of the embryo was minced. The minced tissue was digested with trypsin, and the digests were plated in 75 cm2 tissue culture flasks. The rapidly growing cells were passed twice at 1:3, then trypsinized and frozen in liquid nitrogen. In all of the MEF experiments, the cells frozen at passage two were thawed and plated in high-glucose DMEM, supplemented with 10% fetal bovine serum, penicillin and streptomycin. Cell viability, as determined by Trypan Blue exclusion, ranged from 95–100% and there was no difference in the viability of Mrf-2−/− MEF vs. Mrf-2+/+ MEFs. Cells were plated at a density of 1.5 × 105 viable cells/cm2. For in vitro adipogenesis studies, the normal culture medium was replaced the day after seeding with the same medium containing 0.5 μg/ml Ins, 1 μm Dex, and 0.5 mm IBMX. This medium was replaced every other day for a maximum of 12 d.
Adipocytes were counted in growing MEF cultures as follows: Mrf-2−/− and Mrf-2+/+ MEF lines were plated in four replicate wells of 24-well tissue culture plates, and treated with adipogenic medium as described above. At the time points indicated in the figures, low power bright-field images were taken of three randomly selected fields. The adipocytes in each image were counted using ImagePro software (Media Cybernetics, Inc., Bethesda, MD), and means and standard error of the counts (12 images per line per time point) were calculated.
Retroviral Transduction
A retroviral vector expressing human Mrf-2B was constructed by cloning the cDNA sequence for this protein into the pFB-Neo vector from Stratagene (La Jolla, CA). This recombinant vector or a control pFB-Neo-LacZ vector was cotransfected into 293T cells along with pVPack-VSV-G (also from Stratagene) and a gag-pol expressing vector. Retroviral supernatants were harvested 24–48 h later and concentrated by polyethylene glycol precipitation. The LacZ retrovirus preparations were titered by staining transduced cells for β-galactosidase, and by real-time RT-PCR measurements of LacZ gene expression (see below). The Mrf-2B retrovirus preparations were titered by RT-PCR, and the multiplicity of infection was estimated by comparing multiplicity of infection (MOI) to gene expression in the LacZ-transduced cells. For the experiments depicted in Fig. 3, Mrf-2−/− MEFs were plated from frozen stocks, and transduced the next day (d −2) with either Mrf-2B or LacZ at an MOI of approximately 1. (Transductions at higher MOIs were generally toxic.) Forty-eight hours later (d 0) the cells were treated with adipogenic factors. RNA was isolated from replicate cultures at the indicated times, and gene expression was measured using real-time RT-PCR as described below.
3T3-L1 Cell Culture and Treatment
3T3-L1 preadipocytes were maintained in high-glucose DMEM supplemented with 10% fetal bovine serum, penicillin, and streptomycin (growth medium; GM). For adipogenesis experiments, cells were seeded at 1.56 × 104/cm2, and grown to confluence for 40∼42 h. Two days after confluence, designated as d 0, adipogenesis was initiated by adding 0.5 mm IBMX, 0.25 μm Dex, and 10 μg/ml Ins. Two days later (d 2), the medium was replaced with GM supplemented with 10 μg/ml Ins. This medium was replaced every 2 d. Antibiotics were not added during adipogenesis.
Small Interference RNA Treatment
Twenty-seven-nucleotide siRNAs targeted to Mrf-2 were synthesized by Integrated DNA Technology (Coralville, IA). The locations and sequences of these siRNAs are shown in Fig. 1 and Table 1, respectively. One day after confluence (d −1), cells were washed and replaced with GM without serum, and transfected with 40 nm siRNAs using lipofectamine 2000 according to the manufacturer’s instructions. Six hours later, the medium was replaced, and the cells were cultured for 18 h before the addition of the adipogenic mixture on d 0.
RNA Analysis
Total RNA was extracted using TRIzol reagent. cDNA was synthesized in 40 μl of 25 mm Tris·HCl (pH 8.3), 50 mm KCl, 2 mm dithiothreitol, and 5 mm MgCl2, 312.5 μm deoxynucleotide triphosphates, 1 μm random hexamer, 20 U RNasin ribonuclease inhibitor, 10 U Avian myeloblastosis virus reverse transcriptase, and 1 μg of template RNA. The reaction was carried out at 20 C for 10 min, 37 C for 60 min, 55 C for 10 min, and then 93 C for 5 min. After this, the reaction mixtures were diluted 20-fold with water, and mRNA expression levels were determined using real-time PCR. Real-time PCR was performed with a MyiQ Single-Color Real-Time PCR Detection System, using the iQ SYBR Green supermix reagent. Primer sets (final concentration 0.4 μm) are listed in Table 2. mRNA expression was normalized to 18S ribosomal RNA. To quantitate transgene expression in the retrovirus-transduced MEFs, real-time PCR was run using pFB-Neo Mrf-2B or pFB-Neo-LacZ plasmids as standards.
Table 2.
Sequences of RT-PCR Primer Sets
| Molecule | Forward 5′–3′ | Reverse 5′–3′ |
|---|---|---|
| 18S rRNA | ATGCGGCGGCGTTATTCC | ATCTGTCAATCCTGTCCGTGTC |
| Mouse Mrf-2A | AAGCCGATATCCACACCAAGAC | GGGTCCTTTGAGCCACTGAAG |
| Mouse Mrf-2B | CAACTTTTATCCAGCTCGAAAC | GAGAAATCAGAATGCGCCC |
| Mouse Mrf-2A +2B | AGAAAAACGCCCATCGAGC | CTCCCAGGATTACCACCTAAC |
| Human Mrf-2A + 2B | GATCAGAAGGCCACAAGCTTC | GTACTGGGGTGCACGGATGAC |
| C/EBPβ | GCAAGAGCCGCGACAAG | GGCTCGGGCAGCTGCTT |
| C/EBPδ | ACGACGAGAGCGCCATC | TCGCCGTCGCCCCAGTC |
| CHOP-10 | AACAGAGGTCACACGCACATC | TCGTTCTCCTGCTCCTTCTCC |
| C/EBPα | GCTGAGCCGTGAACTGGAC | CGACCCGAAACCATCCTCTG |
| PPARγ | CGCCAAGGTGCTCCAGAAG | GTCAGCGGGTGGGACTTTC |
| aP2 | AAGAAGTGGGAGTGGGCTTTG | CTCTTCACCTTCCTGTCGTCTG |
| PEPCK | CAGTGAGGAAGTTCGTGGAAGG | CAGTGAGAGCCAGCCAACAG |
| FAS | CCGTGTGACCGCCATCTATATC | GTGAGGTTGCTGTCGTCTGTAG |
| TRAP220 | CCCTGTGAATGACTCCCTTGTG | TTTCAGCCTTCCTCCGAATAGC |
| CREB-binding protein | GGCAATGAGTGTGAACAGTGTG | TGGAAGCAGGAGGTGGAGTC |
| p300 | TCAACCACCACCAGCAACAG | GCAGAAGGAGCAGCAGGAAG |
| PGC-2 | CAAGGAGCAGATCGTGGAGATG | TTCAGGGTAGGGTGTCAGTCC |
| LacZ | TCTCGTTGCTGCATAAACC | CGTTTCACCCTGCCATAAAG |
For Northern blotting, 10 μg of total RNA were treated with glyoxal and dimethyl sulfoxide, and resolved on 1% agarose gels under denaturing conditions (2.1 m formaldehyde). After transfer to Zeta-Probe membranes, hybridization was carried out using oligonucleotide probes in ULTRAhyb solution. Oligonucleotide probes for Mrf-2A, −2B, or −2A+2B were synthesized using RT-PCR and the same primer sets listed in Table 2, then the antisense strand was labeled using [α-32P] deoxy-CTP and the reverse primer.
Preparation of Protein Samples
Cell monolayers were washed with ice-cold PBS, and cells were harvested in ice-cold buffer containing 50 mm Tris·HCl (pH 7.4), 100 mm NaCl, 4% Nonidet P-40, 0.4% sodium dodecyl sulfate, 1 mm phenylmethylsulfonyl fluoride, and protease inhibitor cocktail. Lysates were incubated on ice for 15 min, and stored at −80 C. For preparation of cytoplasmic and nuclear extracts, NE-PER Nuclear and Cytoplasmic Extraction Reagents were used. Protein concentration was determined using a MicroBCA assay kit.
Immunoblotting
Whole cell extracts were resolved by SDS-PAGE and transferred to Trans-blot nitrocellulose membranes. After transfer, membranes were blocked with 5% nonfat dry milk in Tris-buffered saline, 0.1% Tween 20, and incubated with primary antibodies. HRP-conjugated secondary antibodies and ECL reagents were used for detection of proteins.
Oil Red O Staining
Oil Red O staining was performed using a slight modification of the procedure described by Kinkel et al. (34). Briefly, cell monolayers were washed with PBS, and fixed with 3.7% formaldehyde in PBS for 10 min at room temperature. After washing, cells were stained with 0.18% Oil Red O solution in 60% 2-propanol for 15 min. Cells were washed with water and photographed. To quantitate lipid staining, Oil Red O was extracted by adding 100% 2-propanol, and absorbances of the extracts at 510 nm were measured.
EMSAs
A double-stranded oligonucleotide probe corresponding to the C/EBPβ binding site in the C/EBPα promoter (19) (5′-GCG TTG CGC CAC GAT CTC TC-3′) was used for EMSAs. The binding reaction contained 0.01 pmol of the [γ-32P]ATP-labeled probe, 1 μg of poly(deoxyinosinic-deoxycytidylic) acid, and 1 μg of nuclear extract in 30 μl of buffer containing 10 mm HEPES (pH 7.5), 0.1 mm EDTA, 5% glycerol, 100 mm NaCl, 0.3 m urea, and 0.3% Nonidet P-40. Where indicated, a 100-fold excess of unlabeled probe or 0.4 μg of anti-C/EBPβ or -δ antibody were added 30 min before the addition of labeled probe. After the reaction bound and free probe were separated on 4% nondenaturing polyacrylamide gels in 0.25× TBE buffer and visualized by autoradiography.
NURSA Molecule Pages:
Nuclear Receptors: PPARγ.
Footnotes
This research is partially supported by the IDEN foundation. This work is not supported in whole or part by National Institutes of Health.
Current address for T.Y.: Phytomedicines & Nutraceuticals Research Laboratory, Healthcare Research Institute, Wakunaga Pharmaceutical Co. Ltd., Akitakata-shi, Hiroshima 739-1195, Japan.
Disclosure Summary: Authors T.Y., R.H.W. and S.L. have nothing to declare. K.I. is presently consulting for Wakunaga Pharmaceutical Co. Ltd. in Japan.
First Published Online October 25, 2007
Abbreviations: aP2, Adipose fatty acid-binding protein; ARID, AT-rich interaction domain; C/EBP, CCAAT/enhancer-binding proteins; CHOP-10, C/EBP homologous protein-10; Dex, dexamethasone; FAS, fatty acid synthase; HRP, horseradish peroxidase; IBMX, 3-isobutyl-1-methylxanthine; Ins, insulin; MEF, mouse embryo fibroblasts; MOI, multiplicity of infection; Mrf-2, modulator recognition factor-2; PEPCK, phosphoenolpyruvate carboxykinase; siRNA, small interference RNA; PPARγ, peroxisome proliferator-activated receptor-γ.
References
- 1.Spiegelman BM, Flier JS 1996. Adipogenesis and obesity: rounding out the big picture. Cell 87:377–389 [DOI] [PubMed] [Google Scholar]
- 2.Huang TH, Oka T, Asai T, Okada T, Merrills BW, Gertson PN, Whitson RH, Itakura K 1996. Repression by a differentiation-specific factor of the human cytomegalovirus enhancer. Nucleic Acids Res 24:1695–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitson RH, Huang T, Itakura K 1999. The novel Mrf-2 DNA-binding domain recognizes a five-base core sequence through major and minor-groove contacts. Biochem Biophys Res Commun 258:326–331 [DOI] [PubMed] [Google Scholar]
- 4.Watanabe M, Layne MD, Hsieh CM, Maemura K, Gray S, Lee ME, Jain MK 2002. Regulation of smooth muscle cell differentiation by AT-rich interaction domain transcription factors Mrf2α and Mrf2β. Circ Res 91:382–389 [DOI] [PubMed] [Google Scholar]
- 5.Yuan YC, Whitson RH, Liu Q, Itakura K, Chen Y 1998. A novel DNA-binding motif shares structural homology to DNA replication and repair nucleases and polymerases. Nat Struct Biol 5:959–964 [DOI] [PubMed] [Google Scholar]
- 6.Whitson RH, Tsark W, Huang TH, Itakura K 2003. Neonatal mortality and leanness in mice lacking the ARID transcription factor Mrf-2. Biochem Biophys Res Commun 312:997–1004 [DOI] [PubMed] [Google Scholar]
- 7.Bernlohr DA, Bolanowski MA, Kelly Jr TJ, Lane MD 1985. Evidence for an increase in transcription of specific mRNAs during differentiation of 3T3-L1 preadipocytes. J Biol Chem 260:5563–5567 [PubMed] [Google Scholar]
- 8.Rosen ED, Spiegelman BM 2000. Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16:145–171 [DOI] [PubMed] [Google Scholar]
- 9.Yeh WC, Cao Z, Classon M, McKnight SL 1995. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev 9:168–181 [DOI] [PubMed] [Google Scholar]
- 10.Hamm JK, Park BH, Farmer SR 2001. A role for C/EBPβ in regulating peroxisome proliferator-activated receptor gamma activity during adipogenesis in 3T3-L1 preadipocytes. J Biol Chem 276:18464–18471 [DOI] [PubMed] [Google Scholar]
- 11.Christy RJ, Yang VW, Ntambi JM, Geiman DE, Landschulz WH, Friedman AD, Nakabeppu Y, Kelly TJ, Lane MD 1989. Differentiation-induced gene expression in 3T3-L1 preadipocytes: CCAAT/enhancer binding protein interacts with and activates the promoters of two adipocyte-specific genes. Genes Dev 3:1323–1335 [DOI] [PubMed] [Google Scholar]
- 12.Tontonoz P, Hu E, Graves RA, Budavari AI, Spiegelman BM 1994. mPPARγ2: tissue-specific regulator of an adipocyte enhancer. Genes Dev 8:1224–1234 [DOI] [PubMed] [Google Scholar]
- 13.Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM 1995. PPARγ2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol 15:351–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olswang Y, Blum B, Cassuto H, Cohen H, Biberman Y, Hanson RW, Reshef L 2003. Glucocorticoids repress transcription of phosphoenolpyruvate carboxykinase (GTP) gene in adipocytes by inhibiting its C/EBP-mediated activation. J Biol Chem 278:12929–12936 [DOI] [PubMed] [Google Scholar]
- 15.Schoonjans K, Peinado-Onsurbe J, Lefebvre AM, Heyman RA, Briggs M, Deeb S, Staels B, Auwerx J 1996. PPARα and PPARγ activators direct a distinct tissue-specific transcriptional response via a PPRE in the lipoprotein lipase gene. EMBO J 15:5336–5348 [PMC free article] [PubMed] [Google Scholar]
- 16.Dalen KT, Schoonjans K, Ulven SM, Weedon-Fekjaer MS, Bentzen TG, Koutnikova H, Auwerx J, Nebb HI 2004. Adipose tissue expression of the lipid droplet-associating proteins S3–12 and perilipin is controlled by peroxisome proliferator-activated receptor-γ. Diabetes 53:1243–1252 [DOI] [PubMed] [Google Scholar]
- 17.Nagai S, Shimizu C, Umetsu M, Taniguchi S, Endo M, Miyoshi H, Yoshioka N, Kubo M, Koike T 2004. Identification of a functional peroxisome proliferator-activated receptor responsive element within the murine perilipin gene. Endocrinology 145:2346–2356 [DOI] [PubMed] [Google Scholar]
- 18.Ron D, Habener JF 1992. CHOP, a novel developmentally regulated nuclear protein that dimerizes with transcription factors C/EBP and LAP and functions as a dominant-negative inhibitor of gene transcription. Genes Dev 6:439–453 [DOI] [PubMed] [Google Scholar]
- 19.Tang QQ, Lane MD 2000. Role of C/EBP homologous protein (CHOP-10) in the programmed activation of CCAAT/enhancer-binding protein-β during adipogenesis. Proc Natl Acad Sci USA 97:12446–12450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang H, Lane MD, Tang QQ 2005. Effect of serum on the down-regulation of CHOP-10 during differentiation of 3T3-L1 preadipocytes. Biochem Biophys Res Commun 338:1185–1188 [DOI] [PubMed] [Google Scholar]
- 21.Carriere A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, Penicaud L, Casteilla L 2004. Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem 279:40462–40469 [DOI] [PubMed] [Google Scholar]
- 22.Batchvarova N, Wang XZ, Ron D 1995. Inhibition of adipogenesis by the stress-induced protein CHOP (Gadd153). EMBO J 14:4654–4661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cullen BR 2006. Enhancing and confirming the specificity of RNAi experiments. Nat Methods 3:677–681 [DOI] [PubMed] [Google Scholar]
- 24.Descombes P, Schibler U 1991. A liver-enriched transcriptional activator protein, LAP, and a transcriptional inhibitory protein, LIP, are translated from the same mRNA. Cell 67:569–579 [DOI] [PubMed] [Google Scholar]
- 25.Lin FT, MacDougald OA, Diehl AM, Lane MD 1993. A 30-kDa alternative translation product of the CCAAT/enhancer binding protein alpha message: transcriptional activator lacking antimitotic activity. Proc Natl Acad Sci USA 90:9606–9610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harmon AW, Patel YM, Harp JB 2002. Genistein inhibits CCAAT/enhancer-binding protein β (C/EBPβ) activity and 3T3-L1 adipogenesis by increasing C/EBP homologous protein expression. Biochem J 367:203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen ED, MacDougald OA 2006. Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7:885–896 [DOI] [PubMed] [Google Scholar]
- 28.Ge K, Guermah M, Yuan CX, Ito M, Wallberg AE, Spiegelman BM, Roeder RG 2002. Transcription coactivator TRAP220 is required for PPARγ2-stimulated adipogenesis. Nature 417:563–567 [DOI] [PubMed] [Google Scholar]
- 29.Takahashi N, Kawada T, Yamamoto T, Goto T, Taimatsu A, Aoki N, Kawasaki H, Taira K, Yokoyama KK, Kamei Y, Fushiki T 2002. Overexpression and ribozyme-mediated targeting of transcriptional coactivators CREB-binding protein and p300 revealed their indispensable roles in adipocyte differentiation through the regulation of peroxisome proliferator-activated receptor γ. J Biol Chem 277:16906–16912 [DOI] [PubMed] [Google Scholar]
- 30.Erickson RL, Hemati N, Ross SE, MacDougald OA 2001. p300 coactivates the adipogenic transcription factor CCAAT/enhancer-binding protein α. J Biol Chem 276:16348–16355 [DOI] [PubMed] [Google Scholar]
- 31.Castillo G, Brun RP, Rosenfield JK, Hauser S, Park CW, Troy AE, Wright ME, Spiegelman BM 1999. An adipogenic cofactor bound by the differentiation domain of PPARγ. EMBO J 18:3676–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohwi-Shigematsu T, Maass K, Bode J 1997. A thymocyte factor SATB1 suppresses transcription of stably integrated matrix-attachment region-linked reporter genes. Biochemistry 36:12005–12010 [DOI] [PubMed] [Google Scholar]
- 33.Shirakawa K, Maeda S, Gotoh T, Hayashi G, Shinomya K, Ehata S, Nishimura R, Mori M, Onozaki K, Hayashi H, Uematsu S, Akira S, Ogata E, Miyazono K 2006. CCAAT/Enhancer-binding protein homologous protein (CHOP) regulates osteoblast differentiation. Mol Cell Biol 26:6105–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kinkel AD, Fernyhough ME, Helterline DL, Vierck JL, Oberg KS, Vance TJ, Hausman GJ, Hill RA, Dodson MV 2004. Oil Red-O stains non-adipogenic cells: a precautionary note. Cytotechnology 46:49–56 [DOI] [PMC free article] [PubMed] [Google Scholar]