Abstract
Leaves and storage roots of sweet potato plants (Ipomea batatas) showed the same qualitative isoperoxidase patterns and a similar distribution of distinctive isoperoxidases between the cell protoplast and cell wall free, ionically bound, and covalently bound fractions. No changes in the qualitative isoenzyme spectrum were found in relation to age, mechanical injury, or ethylene action. Thus, as in tobacco plants, the cell isoperoxidases in sweet potato did not reflect the possible differential mRNA synthesis in relation to organ, age, or injury. Transcription does not seem to be a limiting factor in injury- and ethylene-dependent peroxidase enhancement during the first 24 hr.
The contribution of the wall ionically bound and protoplast fractions was highest in young and old leaves, respectively. In the protoplast and wall ionically and covalently bound fractions, 14, 6, and 5 isoenzyme bands were found; in addition, 4 bands, not detected in the protoplast, were also revealed in the covalently bound fraction. The distinctive “juvenile” and, developing with age, “mature” isoforms were mainly found in the ionically bound and protoplast fractions, respectively.
The injury-enhanced and/or ethylene-enhanced peroxidase development was most pronounced in young leaves. Ethylene suppressed some injury-enhanced, had no effect on some other injury-enhanced, and greatly promoted some of the injury-unaffected or enhanced isoperoxidases. After ethylene removal, an increase in the “mature” isoforms was found in the protoplast of intact leaves.
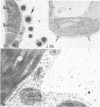
Electron microscopy of leaves revealed peroxidase in membrane-bound vesicles located mainly in the vacuole; a thin layer of reaction products was also found on the wall's outer surface. No Golgi apparatus were seen in the cells of control or ethylene-treated intact leaves. In ethylene-treated intact or injured leaves accumulations of reaction products between the plasmalemma and wall were also found. Numerous Golgi apparatus with dark stained vesicles were seen in injured, and especially in injured and ethylene-treated leaves; the vacuolar bodies seemed to occur in very great number.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birecka H., Briber K. A., Catalfamo J. L. Comparative studies on tobacco pith and sweet potato root isoperoxidases in relation to injury, indoleacetic Acid, and ethylene effects. Plant Physiol. 1973 Jul;52(1):43–49. doi: 10.1104/pp.52.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Catalfamo J. L. Cell wall and protoplast isoperoxidases in tobacco plants in relation to mechanical injury and infection with tobacco mosaic virus. Plant Physiol. 1975 Apr;55(4):611–619. doi: 10.1104/pp.55.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birecka H., Miller A. Cell wall and protoplast isoperoxidases in relation to injury, indoleacetic Acid, and ethylene effects. Plant Physiol. 1974 Apr;53(4):569–574. doi: 10.1104/pp.53.4.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrispeels M. J. Synthesis and secretion of hydroxyproline containing macromolecules in carrots. I. Kinetic analysis. Plant Physiol. 1969 Aug;44(8):1187–1193. doi: 10.1104/pp.44.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashek W. V. Synthesis and Transport of Hydroxyproline-rich Components in Suspension Cultures of Sycamore-Maple Cells. Plant Physiol. 1970 Dec;46(6):831–838. doi: 10.1104/pp.46.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner M., Chrispeels M. J. Involvement of the Golgi Apparatus in the Synthesis and Secretion of Hydroxyproline-rich Cell Wall Glycoproteins. Plant Physiol. 1975 Mar;55(3):536–541. doi: 10.1104/pp.55.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- Hanson A. D., Kende H. Ethylene-enhanced Ion and Sucrose Efflux in Morning Glory Flower Tissue. Plant Physiol. 1975 Apr;55(4):663–669. doi: 10.1104/pp.55.4.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew J. Y., Shannon L. M. Incorporation of carbohydrate residues into peroxidase isoenzymes in horseradish roots. Plant Physiol. 1973 Nov;52(5):462–465. doi: 10.1104/pp.52.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu E. H., Lamport D. T. An Accounting of Horseradish Peroxidase Isozymes Associated with the Cell Wall and Evidence that Peroxidase Does Not Contain Hydroxyproline. Plant Physiol. 1974 Dec;54(6):870–876. doi: 10.1104/pp.54.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan C. A. Assay and Biochemical Properties of the Proteinase Inhibitor-inducing Factor, a Wound Hormone. Plant Physiol. 1974 Sep;54(3):328–332. doi: 10.1104/pp.54.3.328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon L. M., Uritani I., Imaseki H. De novo synthesis of peroxidase isozymes in sweet potato slices. Plant Physiol. 1971 Apr;47(4):493–498. doi: 10.1104/pp.47.4.493. [DOI] [PMC free article] [PubMed] [Google Scholar]