Introduction
Key Teaching Points
|
Atrial fibrillation (AF) originating from a persistent left superior vena cava (PLSVC) has been rarely described.1, 2, 3 In this report, we present a challenging case of PLVSC electrical isolation during AF and atrial tachycardia (AT).
Case presentation
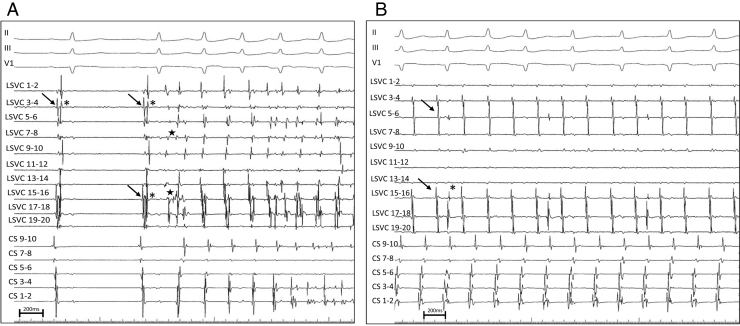
A 56-year-old woman was referred for recurrence of symptomatic paroxysmal AF and AT after pulmonary vein isolation (PVI). Preprocedure imaging demonstrated the presence of a PLSVC. Reconnection of right superior and right inferior pulmonary veins (PV) was observed, and both veins were successfully reisolated. Postisolation, AF initiated spontaneously multiple times. During mapping of non-PV triggers, the circular mapping catheter (Lasso; Biosense-Webster) was positioned via the coronary sinus (CS) into the PLSVC (Figure 1A). During the first 2 beats in sinus rhythm, left atrial (LA) far-field signals (arrow) followed by PLSVC potentials (asterisk) were recorded on the circular mapping catheter. Then, during AF initiation (star), reversal of the activation sequence was observed on the circular mapping catheter with PLSVC potentials recorded first, followed by LA far-field signals. Electrical isolation of the PLSVC was performed using an irrigated-tip radiofrequency ablation catheter, from inside the PLSVC and from the adjacent endocardial surface of the LA. Atrial burst pacing was then performed and a focal AT was induced. The AT (cycle length 250–260 ms) was mapped and localized to the floor of the markedly enlarged CS at its mid-portion, prior to its junction with the PLSVC. During AT, additional potentials (asterisk) were observed on the circular mapping catheter (Figure 1B). Successful ablation of the focal AT was performed at the site of earliest activation. Based on these tracings, was electrical isolation of the PLSVC achieved?
Figure 1.
A: Simultaneous recordings of surface ECG leads II, III, and V1 and intracardiac coronary sinus (CS) and persistent left superior vena cava (PLSVC) electrograms during atrial fibrillation (AF) initiation. The circular mapping catheter is positioned within the PLSVC. During the first 2 sinus beats, left atrial far-field signals (arrow) are recorded first, followed by PLSVC signals (asterisk). During AF initiation, a reversal of the activation sequence is observed, with PLSVC potentials recorded very early (star) compared to the P wave on the surface ECG and to the atrial signals recorded on the CS catheter. Of note, the atrial activation sequence is from distal to proximal on the CS during AF initiation, indicating a probable left atrial origin. The AF cycle length is shorter in the PLSVC than in the CS. B: The circular mapping catheter is still positioned within the PLSVC during the atrial tachycardia originating from the CS. Spontaneous PLSVC potentials (*) distinct from the atrial far-field signals (A) are observed on the circular mapping catheter.
Commentary
Isolated PLSVC is the most common congenital thoracic venous anomaly, with a prevalence of 0.3%–0.5% in the general population. It is usually asymptomatic and hemodynamically insignificant and may or may not connect to the right superior vena cava via a bridging innominate vein. Rarely, the caudal right superior cardinal vein regresses, leading to an absent right superior vena cava, an anomaly that often coexists with other congenital heart defects such as an atrial septal defect, bicuspid aortic valve, aortic coarctation, CS ostial atresia, or cor triatriatum. In the vast majority of individuals, the PLSVC courses between the LA appendage (LAA) and left superior pulmonary vein before draining into the right atrium via the CS. Rarely it drains into the LA, resulting in a right-to-left shunt.
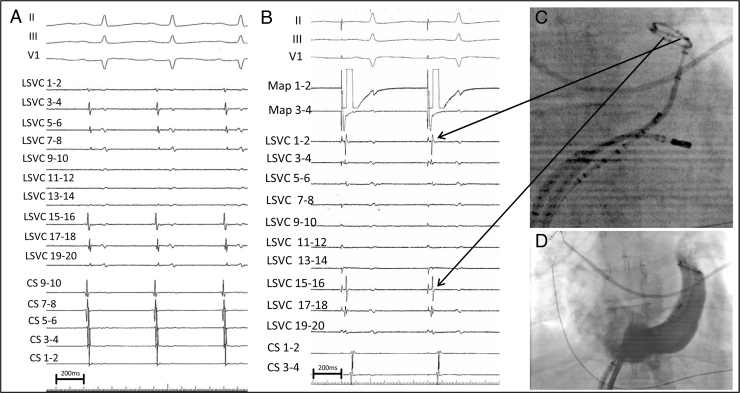
Similar to all major thoracic veins (the PV, superior vena cava, and vein of Marshall), the PLSVC may be associated with sleeves of arrhythmogenic myocardial tissue. These electrically active fibers are capable of generating rapid repetitive discharges through a combination of localized re-entry, or are enhanced automaticity owing to persistence of the ectopic pacemaker in the left common cardinal vein.4. Connections between the lateral LA region near the anterior aspect of the left PV ostia and the anteromedial aspect of the PLSVC between proximal and mid-PLSVC levels have been described.1 Ablation of these connections can result in electrical isolation. In this particular case, electrical isolation of the PLSVC from both the endocardial LA and CS was performed owing to the presence of separate connections from the PLSVC to the LA, and to the distal CS. Postisolation, entrance block of conduction from the LA to the PLSVC could be confirmed by disappearance of PLSVC potentials during sinus rhythm (Figure 2A). Owing to the anatomic proximity between the LAA and PLSVC, far-field signals from the LAA were observed on the circular mapping catheter after electrical PLSVC isolation. Pacing maneuvers from the LAA were performed to confirm the LAA origin of these signals (Figure 2B). LSVC pacing maneuvers during sinus rhythm were also performed, though it was not possible to unequivocally confirm exit block, since PLSVC capture could not be demonstrated. Of note, PLSVC pacing maneuvers should be performed with great care to avoid inadvertent direct capture of the adjacent LA, which may result in misinterpretation of apparent exit conduction. In contrast, when the pacing site does not correspond to the site of a connection, capture is limited to the local structure, with delayed and passive activation of the second structure.
Figure 2.
A: Simultaneous recordings of surface ECG leads II, III, and V1 and intracardiac coronary sinus (CS) and persistent left superior vena cava (PLSVC) electrograms during sinus rhythm after PLSVC isolation. The circular mapping catheter is positioned within the PLSVC. Note the complete absence of PLSVC potentials compared with the baseline recording during the first 2 beats in Figure 1A. B: Pacing is performed from the ablation catheter (Map 1–2) positioned in the left atrial appendage (LAA). The potentials recorded on the circular mapping catheter immediately follow the stimulus artifact on the anterior bipoles (arrows), confirming that these potentials represent LAA far-field signals. C: Shown is a fluoroscopic image of the circular mapping catheter positioned in the PLSVC in front of the LAA, the CS catheter, and the ablation catheter within the CS during AT ablation. Bipoles 3–4, 15–16, and 17–18 are anterior and close to the LAA (arrows). D: An angiographic image (anteroposterior view) of the CS and PLSVC is depicted.
During AT, PLSVC potentials at a cycle length of 1000 ms were observed (Figure 1B). In this case, they most likely represent dissociated PLSVC activity, confirming entrance block from both the CS to the LSVC and from the LA to the PLSVC. The differential diagnosis includes 4:1 conduction block from the LA to the PLSVC, which could not be entirely excluded. The transient nature of the potentials observed during AT precluded additional diagnostic maneuvers. Nevertheless, entrance block was confirmed during LAA pacing maneuvers after restoration of sinus rhythm. In contrast to recordings during sinus rhythm, these potentials do not represent exit block but instead act as confirmation of entrance block, since spontaneous PLSVC firing can only occur once the vein is no longer inhibited (ie, overdriven) by conduction of electrical activity from the LA. Of note, the exact anatomic distribution of CS musculature in this patient is unknown. Precise identification of the dissociated potentials during AT requires accurate knowledge of anatomic characteristics and of the relationship between the various atrial potentials recorded in the PLSVC during sinus rhythm. Activation mapping localized the AT to a focal source in the mid CS, below the junction with the PLSVC, where focal ablation interrupted the tachycardia and resulted in a persistent sinus rhythm. After electrical isolation of the PLSVC and ablation of the focal AT, no arrhythmia could be induced during atrial burst pacing with or without isoproterenol infusion.
In conclusion, the main teaching points of this case are as follows: (1) Electrical isolation of a PLSVC, when present, should be considered during AF ablation procedures given the potential arrhythmogenicity of this structure. (2) Owing to the anatomic proximity between the LAA and PLSVC, far-field signals from the LAA may be observed on a circular mapping catheter after electrical isolation of the PLSVC. (3) In contrast to recordings during sinus rhythm, dissociated potentials during AT (or AF) do not represent exit block but instead act as confirmation of entrance block, since spontaneous firing can only occur once the vein is no longer inhibited by conduction of electrical activity from the LA.
Footnotes
Dr Macle reports receiving lecture fees from St. Jude Medical, Biosense-Webster, Bristol Myers Squibb, and Pfizer and grant support from St. Jude Medical and Biosense-Webster. Dr Andrade reports receiving lecture fees from Medtronic, Bayer, and Biosense-Webster; consulting fees from Medtronic, Bayer, and Biotronik; and grant support from Medtronic. Dr Khairy reports receiving consulting fees from Boehringer-Ingelheim and grant support from Boehringer-Ingelheim, Actelion, Bayer, Medtronic, and St Jude Medical.
References
- 1.Hsu L.F., Jaïs P., Keane D., Wharton J.M., Deisenhofer I., Hocini M., Shah D.C., Sanders P., Scavée C., Weerasooriya R., Clémenty J., Haïssaguerre M. Atrial fibrillation originating from persistent left superior vena cava. Circulation. 2004;109:828–832. doi: 10.1161/01.CIR.0000116753.56467.BC. [DOI] [PubMed] [Google Scholar]
- 2.Liu H., Lim K.T., Murray C., Weerasooriya R. Electrogram-guided isolation of the left superior vena cava for treatment of atrial fibrillation. Europace. 2007;9:775–780. doi: 10.1093/europace/eum118. [DOI] [PubMed] [Google Scholar]
- 3.Wissner E., Tilz R., Konstantinidou M., Metzner A., Schmidt B., Chun K.R., Kuck K.H., Ouyang F. Catheter ablation of atrial fibrillation in patients with persistent left superior vena cava is associated with major intraprocedural complications. Heart Rhythm. 2010;7:1755–1760. doi: 10.1016/j.hrthm.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 4.Peltier J., Destrieux C., Desme J., Renard C., Remond A., Velut S. The persistent left superior vena cava: anatomical study, pathogenesis and clinical considerations. Surg Radiol Anat. 2006;28:206–210. doi: 10.1007/s00276-005-0067-7. [DOI] [PubMed] [Google Scholar]