Introduction
KEY TEACHING POINTS
|
Early repolarization (ER) is a common electrocardiographic finding and is diagnosed by the presence of a J-wave amplitude of ≥0.1 mV in ≥2 contiguous inferior or lateral leads of a standard 12-lead electrocardiogram (ECG), which is manifested as QRS slurring or notching.1 The ER pattern is observed in 1%–13% of cases in the general population and is mainly thought to be benign.2 However, Haïssaguerre et al3 have underlined the potential malignant role of an ER pattern in idiopathic ventricular fibrillation (VF) patients. Of 206 idiopathic VF cases and 412 controls, ER pattern was more frequently present in patients with idiopathic VF than in those in the control group (31% vs 5%). In addition, a population-based study from Finland including 10.864 middle-aged people found an ER pattern in 630 individuals leading to an increased risk of cardiac death.4 An ER pattern on the ECG in patients resuscitated from VF is called early repolarization syndrome (ErS).1
So far, variants in several ion-channel genes have been associated with ErS. Variants in KCNJ8 are associated with a gain of function with an increased outward potassium current,5, 6 and variants in the subunits of the cardiac L-type calcium channel (CACNA1C, CACNB2, CACNA2D1) lead to a reduced inward calcium current.7 Finally, a variant in SCN5A causes a loss of function of the sodium channel, which leads to decreased inward sodium current.8
We present 2 cases with idiopathic VF storm and intermittent ER pattern and a potential recognized association with variants in the ankyrin-2 gene (ANK2).
Case report 1
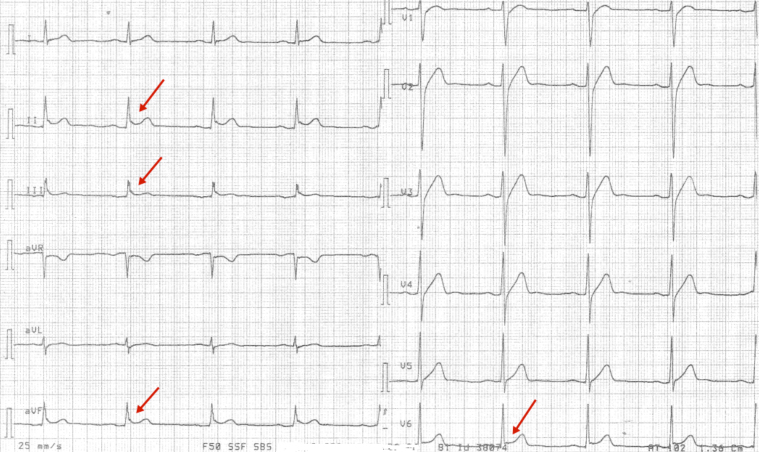
A 29-year-old man with a previous medical history of meningitis collapsed in his home. He had family history of sudden cardiac death. The paramedics arrived 3 minutes later and used direct current cardioversion to convert a VF to sinus rhythm. On his arrival at the hospital, echocardiography results were normal with left ventricular ejection fraction (LVEF) >60%. Coronary angiogram was normal without atherosclerosis. The patient was subjected to 24 hours of hypothermia treatment, with a target temperature of 33°C. A 12-lead ECG recorded after hypothermia treatment, during normal body temperature, showed inferior-lateral QRS slurring (Figure 1).
Figure 1.
Case 1: A 12-lead electrocardiogram with inferolateral early repolarization pattern with J-point elevation and QRS slurring after hypothermia treatment (red arrow).
Repeated VF episodes during intensive care were observed, and amiodarone was started with apparent effect. Before discharge, an implantable cardioverter-defibrillator (ICD) was implanted.
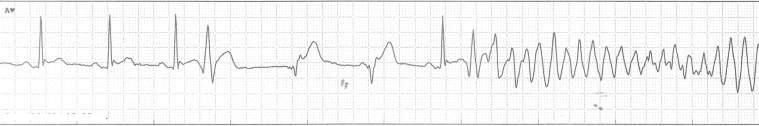
Twelve days later, the patient was readmitted to hospital because of appropriate shock therapies from the ICD that resulted from repeated VF episodes. During the next 18 days in the hospital, he had 6 more episodes of VF. All episodes were preceded by a premature ventricular contraction (PVC) (Figure 2). Medical antiarrhythmic therapy was sequentially tested with amiodarone (initial 600 mg twice a day, reduced to 200 mg once a day), sotalol (160 mg twice a day), metoprolol (75 mg/d), and flecainide (100 mg twice a day), but recurrent arrhythmia was observed with each of these drugs. Combinations of antiarrhythmic drugs were not tested.
Figure 2.
Case 1: Telemetry tracing. Ventricular fibrillation preceded by a ventricular extrasystole.
Because of treatment-resistant VF, the patient was offered radiofrequency ablation to eliminate the focus for the monomorphic PVCs. The procedure seemed successful in suppressing the PVCs, but on reentering the ward, the patient had recurrent VF storm. He was readmitted to the EP laboratory for supplementary radiofrequency ablation. Because of VF storm with need for 80 external defibrillations despite the ablation attempt, intravenous isoproterenol infusion was started, which completely suppressed the malignant arrhythmias. The isoproterenol infusion was gradually reduced and replaced with quinidine sulfate 300 mg twice a day. The rhythm stabilized on quinidine sulfate treatment, and the ER pattern in the ECG was markedly reduced. During a 36-month follow-up period on quinidine sulfate, no arrhythmias have been observed.
Case report 2
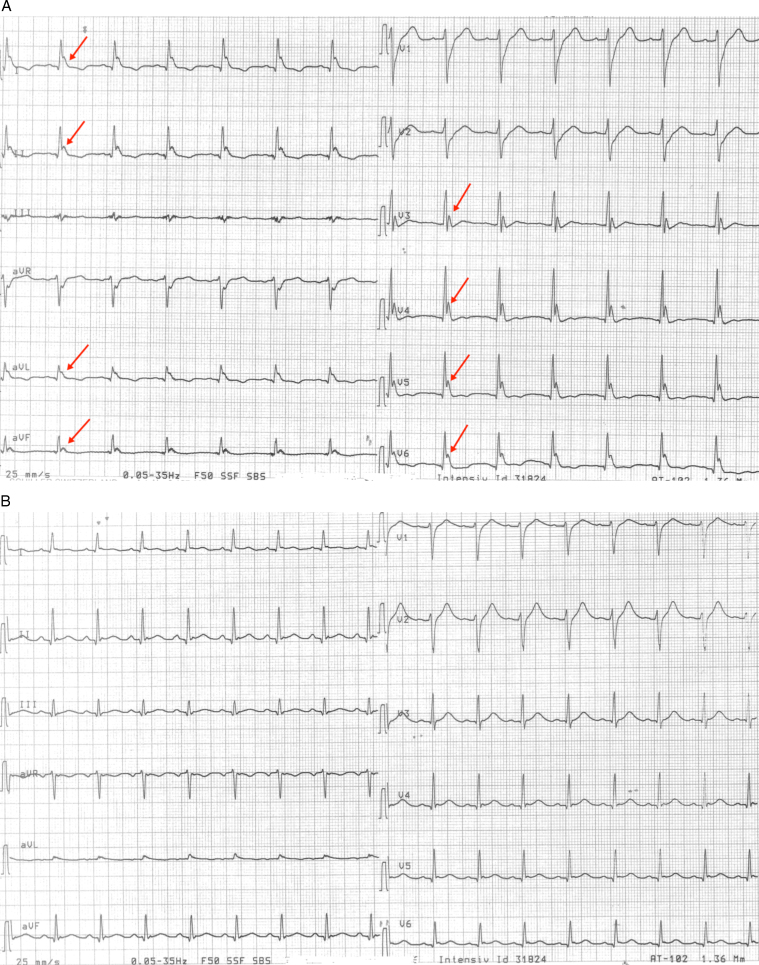
A 20-year-old man without known heart disease was hospitalized after cardiac arrest. He had no previous medical history and no family history of sudden cardiac death. He collapsed in his home with cardiac arrest, and basic resuscitation was started immediately. The paramedics arrived 10 minutes later and used direct current cardioversion to convert a VF. Return of spontaneous circulation was achieved after 1 hour of resuscitation. Results from coronary angiography and computed tomography of the cerebrum were normal. Laboratory findings revealed normal plasma potassium, sodium, calcium, and magnesium levels at admission and after 24 hours of hypothermia treatment with a target temperature of 33°C. An initial echocardiogram showed globally reduced contraction and LVEF of 20% with normal dimensions of left and right ventricles. The next morning, a 12-lead ECG showed an aggressive ER pattern in the inferior-lateral leads (Figure 3A). One hour later, the patient had recurrent VF, progressing into VF storm.
Figure 3.
Case 2: A: A 12-lead electrocardiogram with an aggressive inferior-lateral ER pattern during hypothermia treatment (red arrow). B: The electrocardiogram is completely normalized after administration of isoproterenol infusion.
Initially, he was treated with intravenous magnesium and amiodarone without any antiarrhythmic effect. After the start of isoproterenol infusion, the tendency to malignant arrhythmias ceased, and the ER pattern in the ECG was markedly reduced (Figure 3B). Echocardiography during isoproterenol infusion showed an LVEF of 55%, and on the second day after the infusion was discontinued, the LVEF was estimated at 35%. On the fourth day, cardiac magnetic resonance scanning was performed using late gadolinium enhancement, and the scans showed normal biventricular function, no myocardial edema, and normal cardiac tissue. An echocardiogram 1 month after discharge showed normal myocardial contraction with an LVEF of 60%. The reduced LVEF observed at admission likely was due to myocardial stunning.
An ICD was implanted. During a 16-month follow-up period without antiarrhythmic therapy, no arrhythmias have been observed.
Targeted next-generation DNA testing
Both patients were offered molecular genetic screening with a panel of 75 genes. For a full list of genes sequenced, see http://moma.dk/genetic-analysis, MOMA NGS Heart panel v1.
Genomic DNA was purified from blood, and concentration was measured with Invitrogen Quant-iT Picogreen (Thermo Fisher Scientific, Waltham, MA); 1 µg was used for TruSeq library preparation according to the manufacturer’s instructions (Illumina, San Diego, CA). Libraries were quantified by Kapa quantitative polymerase chain reaction (Kapa Biosystems, Wilmington, MA). Targeting of the 75 genes was performed using the NimbleGen EZ Choice in-solution capture system (Roche, Basel, Switzerland) following the manufacturer’s protocol. Paired-end sequencing (2 × 150 base pairs) was performed on the Illumina MiSeq desktop sequencer.
Data were imported into the CLC Genomics Workbench 6.0 (Aarhus, Denmark). Reads were trimmed for low-quality bases, ambiguous bases, and adaptor sequence followed by mapping to Hg19. After duplicate-read removal, variants were called with the probabilistic variant detector requiring a read coverage of at least 30 and a probability of 90. Variants were uploaded to the Cartagenia Bench Lab NGS (Leuven, Belgium) and filtered using the following criteria: all variants were filtered against ESP6500 and 1000 genomes discarding all variants present in >10% in any of these cohorts. Furthermore, variants seen in >5% of samples in our in-house database were also excluded. Potential splice-site variants were kept, along with all exonic variants that were not synonymous.
Next-generation sequencing revealed no variation in known ErS-related genes but elucidated 2 variations in the ankyrin-2 gene (ANK2). In the first case, an ANK2 missense variant was found at a highly conserved position (c.9854T>C, p.Ile3285Thr, NM_001148.4), and 3 in silico programs (SIFT, PolyPhen2, and Mutation Taster) predict it to be disease causing. In case 2, an ANK2 missense variant was found (c.11791G>A, p.Glu3931Lys, NM_001148.4) that has been shown to reduce the function of ANK2 in neonatal mouse cardiomyocytes.9 Frequencies for the reported variants in the Exome Aggregation Consortium (ExAC) database are 0.008228 (c.9854T>C, p.Ile3285Thr, NM_001148.4) in case 1, and 0 (c.11791G>A, p.Glu3931Lys, NM_001148.4) in case 2.
Discussion
In case 2, the ECGs (Figure 3, Figure 3) were recorded while the patient was receiving postresuscitation hypothermia treatment for 24 hours, with a target temperature of 33°C. The large J waves in the precordial leads (Figure 3A) could resemble Osborn waves, which is the most striking feature of hypothermia.10 The ECG shown in Figure 3A was recorded 4 hours after hypothermia treatment was started, and only 1 hour before the patient developed VF. The ECG demonstrated prominent J waves in both the lateral and inferior leads. The ECG shown in Figure 3B was recorded after 12 hours of hypothermia treatment and after the patient had experienced VF and isoproterenol infusion had been started. In this instance, the patient’s ECG was normalized, and the arrhythmias were completely suppressed due to intravenous isoproterenol infusion. These observations were made during continued hypothermia treatment. Isoproterenol was discontinued after the patient had regained normal body temperature. Although Osborn waves mainly are apparent during severe hypothermia (<30°C), similar electrocardiographic changes and VF storm have been reported in a mildly hypothermic patient (33°C).11 Therefore, it cannot be ruled out that even mild hypothermia could induce an ER pattern and the development of incessant malignant ventricular arrhythmias in predisposed patients with possible hereditary cardiac ion channelopathies.
In both cases, the arrhythmias were completely refractory to well-known antiarrhythmic drugs such as amiodarone, beta blockers, magnesium channel blockers, and sodium channel blockers. In contrast, the repeated VF episodes responded instantly to intravenous isoproterenol infusion in both patients. Furthermore, quinidine appeared to be highly efficient, and it seems that J-wave amplitude correlated with the ventricular arrhythmia susceptibility and quinidine sulfate levels.12 In an experimental study, it was demonstrated that an outward shift in the balance of current, either by an increased potassium current or impaired calcium current, would mimic the ER pattern.13 It was shown that vagal stimulation induced VF and that administration of isoproterenol and quinidine reversed the arrhythmias.13 In both our cases, the arrhythmias were primarily seen during night and early morning with enhanced vagal stimulation. It seems that ErS is refractory to several known and well-established antiarrhythmic drugs and catheter ablation.12
In the group of channelopathies, ErS is among the most recent additions, and the underlying disease-causing mechanisms are still poorly understood. Several genetic variations in different ionic channels have been identified as the underlying cause. However, genetic variation in the ANK2 molecule has not previously been associated with ErS. Although the ANK2 variant (c.9854T>C, p.Ile3285Thr, NM_001148.4), found in case 1, causes an amino acid change in a highly conserved position, and in silico programs predict it to be disease associated, functional studies are needed to clarify its pathophysiological role. The variant discovered in case 2 (c.11791G>A, p.Glu3931Lys, NM_001148.4) has previously been found in 2 unrelated probands, one of whom experienced recurring ventricular tachycardia and the other idiopathic VF.9 Functional studies suggest that this variant could be disease causing.9
ANK2 plays a key role in cell physiology: organization of cardiac proteins and regulation of cardiac electrophysiological genesis. Humans with ANK2 mutations display varying degrees of cardiac dysfunction including bradycardia, sinus arrhythmia, idiopathic VF, polymorphic ventricular tachycardia, and an increased risk of sudden death. A loss-of-function mutation in ankyrin-2 has initially been described as type 4 long QT syndrome. However, a prolonged rate-corrected QT interval is not a consistent feature with ANK2 mutations, indicating that dysfunction of ankyrin-2 represents a clinical entity distinct from classic long QT syndromes.14 This is supported by the 2 present cases without QT prolongation.
Conclusion
In patients with idiopathic VF storm with an intermittent ER pattern, the underlying cellular arrhythmogenic mechanism could be due to variations in ANK2. However, study of more cases with similar ER pattern and proven disease-causing ANK2 variants are needed to confirm this hypothesis. Further research could resolve the underlying mechanism and the vital function of the ANK2 molecule in the heart.
Acknowledgments
Department of Cardiology and MOMA, Aarhus University Hospital.
Footnotes
Conflicts of interest: The authors report no conflicts of interest.
References
- 1.Priori SG, Wilde AA, Horie M. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. doi: 10.1016/j.hrthm.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Mizusawa Y, Bezzina CR. Early repolarization pattern: its ECG characteristics, arrhythmogeneity and heritability. J Interv Card Electrophysiol. 2014;39:185–192. doi: 10.1007/s10840-013-9870-y. [DOI] [PubMed] [Google Scholar]
- 3.Haïssaguerre M, Derval N, Sacher F. Sudden cardiac arrest associated with early repolarization. N Engl J Med. 2008;358:2016–2023. doi: 10.1056/NEJMoa071968. [DOI] [PubMed] [Google Scholar]
- 4.Tikkanen JT, Anttonen O, Junttila MJ, Aro AL, Kerola T, Rissanen HA, Reunanen A, Huikuri HV. Long-term outcome associated with early repolarization on electrocardiography. N Engl J Med. 2009;361:2529–2537. doi: 10.1056/NEJMoa0907589. [DOI] [PubMed] [Google Scholar]
- 5.Haïssaguerre M, Chatel S, Sacher F. Ventricular fibrillation with prominent early repolarization associated with a rare variant of KCNJ8/KATP channel. J Cardiovasc Electrophysiol. 2009;20:93–98. doi: 10.1111/j.1540-8167.2008.01326.x. [DOI] [PubMed] [Google Scholar]
- 6.Medeiros-Domingo A, Tan BH, Crotti L. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K. Heart Rhythm. 2010:1466–1471. doi: 10.1016/j.hrthm.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burashnikov E, Pfeiffer R, Barajas-Martínez H. Mutations in the cardiac L-type calcium channel associated with inherited J-wave syndromes and sudden cardiac death. Heart Rhythm. 2010;7:1872–1882. doi: 10.1016/j.hrthm.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe H, Nogami A, Ohkubo K. Electrocardiographic characteristics and SCN5A mutations in idiopathic ventricular fibrillation associated with early repolarization. Circ Arrhythm Electrophysiol. 2011;4:874–881. doi: 10.1161/CIRCEP.111.963983. [DOI] [PubMed] [Google Scholar]
- 9.Mohler PJ, Splawski I, Napolitano C, Bottelli G, Sharpe L, Timothy K, Priori SG, Keating MT, Bennett V. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci USA. 2004;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborn JJ. Experimental hypothermia: respiratory and blood pH changes in relation to cardiac function. Am J Physiol. 1953175:389–398. [DOI] [PubMed]
- 11.Lassnig E, Maurer E, Nömeyer R, Eber B. Osborn waves and incessant ventricular fibrillation during therapeutic hypothermia. Resuscitation 2010;81:500–501. [DOI] [PubMed]
- 12.Sacher F, Derval N, Horlitz M, Haïssaguerre M. J wave elevation to monitor quinidine efficacy in early repolarization syndrome. J Electrocardiol. 2014;47:223–225. doi: 10.1016/j.jelectrocard.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Koncz I, Gurabi Z, Patocskai B, Panama BK, Szél T, Hu D, Barajas-Martínez H, Antzelevitch C. Mechanisms underlying the development of the electrocardiographic and arrhythmic manifestations of early repolarization syndrome. J Mol Cell Cardiol. 2014;68:20–28. doi: 10.1016/j.yjmcc.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol. 2009;47:203–209. doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]