Abstract
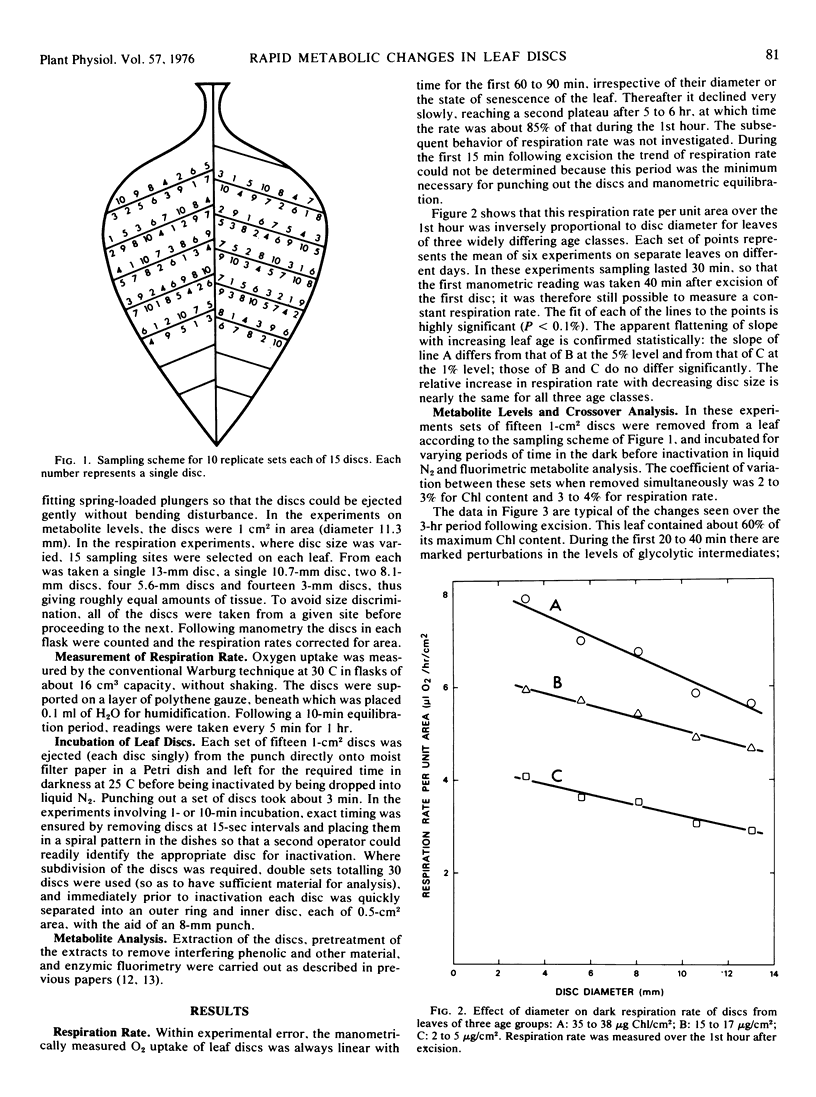
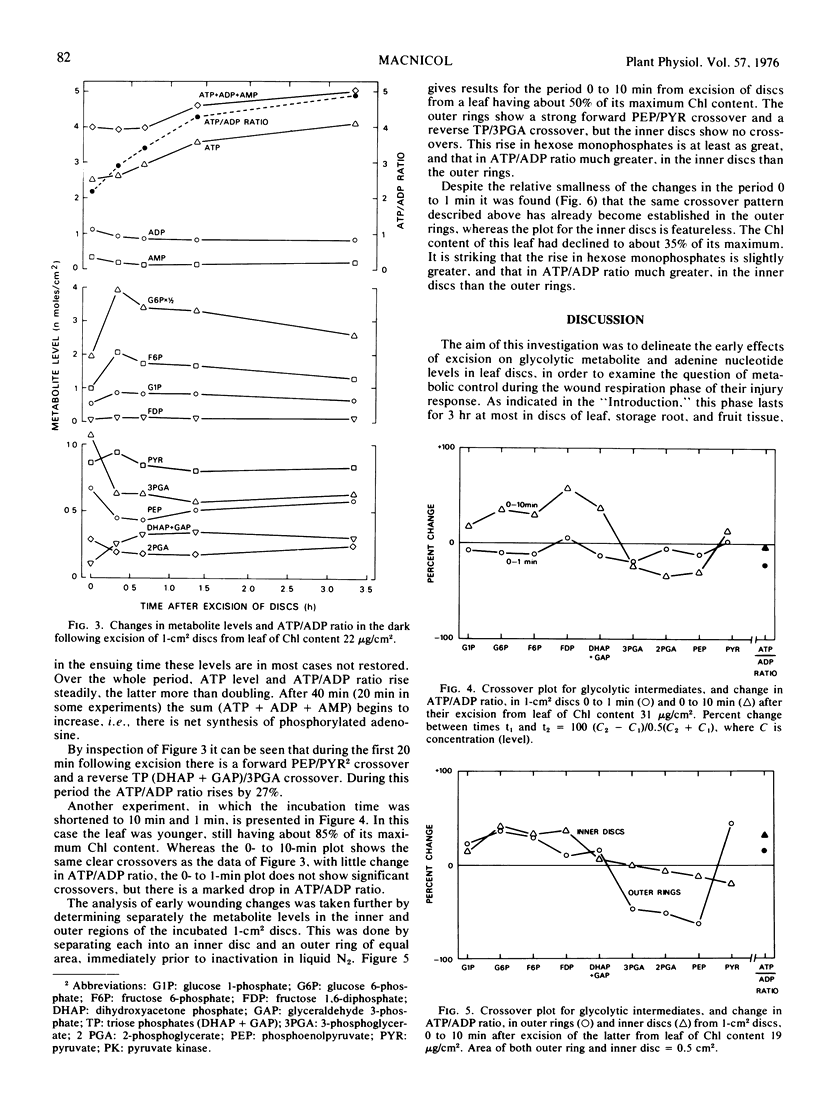
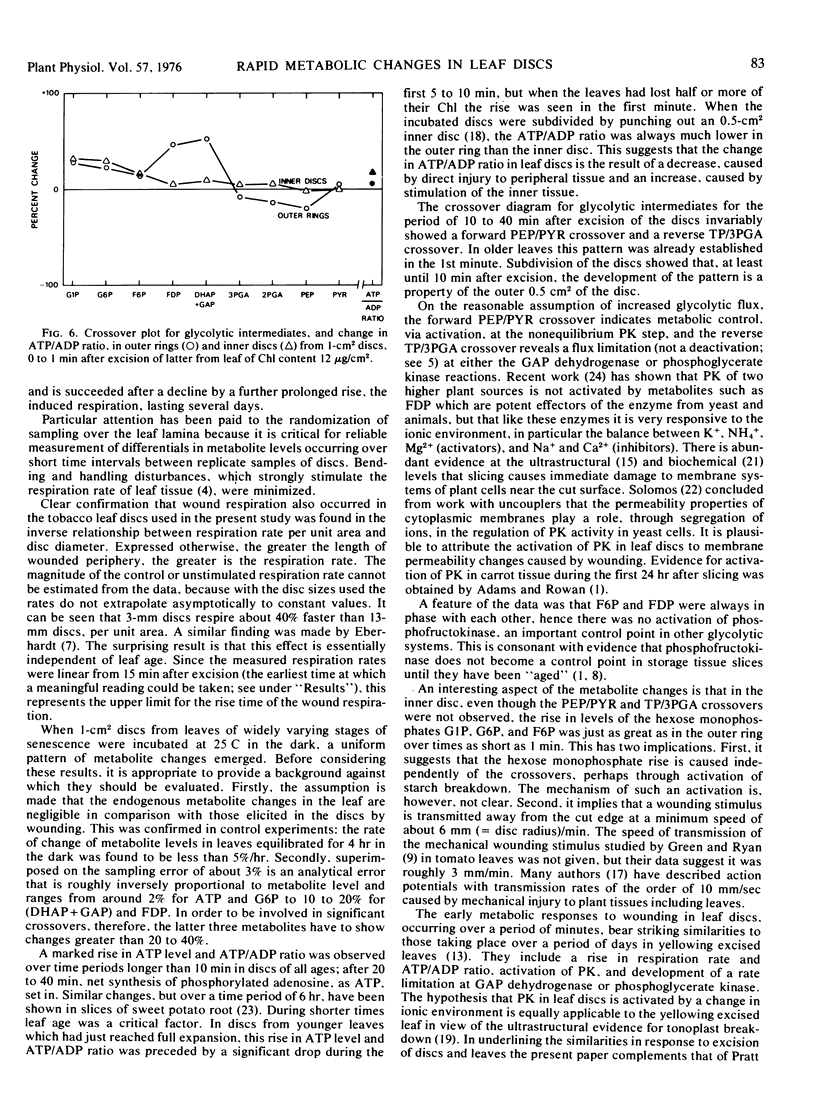
The dark respiration rate of discs from fully expanded tobacco leaves (Nicotiana tabacum) increased linearly with decreasing diameter, the relative increase being independent of leaf age. The wound respiration responsible for this situation reached a plateau within 15 minutes of excision. Metabolite analysis gave evidence for two independent effects, also unrelated to age. The first was a forward crossover between phosphoenolpyruvate and pyruvate which was found as early as 1 minute after excision and persisted for up to 40 minutes. It was attributed to activation of pyruvate kinase by a changed ionic balance resulting from membrane damage, was accompanied by a reverse crossover between triose phosphates and 3-phosphoglycerate, and was localized in the outer region of the discs. The second effect was a rapid rise in hexose monophosphate and ATP levels throughout the discs. After 1 to 10 minutes the ATP/ADP ratio rose strongly for at least 3 hours; after 20 to 40 minutes there was net synthesis of adenine nucleotide as ATP. These results indicate that extrapolation from leaf discs to intact leaves is highly inadvisable.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. B., Rowan K. S. Glycolytic control of respiration during aging of carrot root tissue. Plant Physiol. 1970 Apr;45(4):490–494. doi: 10.1104/pp.45.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B., HOLMES W., HIGGINS J., CONNELLY C. M. Localization of interaction sites in multi-component transfer systems: theorems derived from analogues. Nature. 1958 Nov 1;182(4644):1190–1193. doi: 10.1038/1821190a0. [DOI] [PubMed] [Google Scholar]
- Green T. R., Ryan C. A. Wound-Induced Proteinase Inhibitor in Plant Leaves: A Possible Defense Mechanism against Insects. Science. 1972 Feb 18;175(4023):776–777. doi: 10.1126/science.175.4023.776. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H., WHALEY W. G., LEECH J. H. Cell ultrastructure responses to mechanical injury. A preliminary report. J Ultrastruct Res. 1960 Dec;4:473–481. doi: 10.1016/s0022-5320(60)80034-2. [DOI] [PubMed] [Google Scholar]
- Macnicol P. K. Analysis of adenine nucleotides and metabolic intermediates in mature and senescent leaf tissue. Anal Biochem. 1972 Feb;45(2):624–633. doi: 10.1016/0003-2697(72)90224-2. [DOI] [PubMed] [Google Scholar]
- Macnicol P. K. Metabolic Regulation in the Senescing Tobacco Leaf: II. Changes in Glycolytic Metabolite Levels in the Detached Leaf. Plant Physiol. 1973 Apr;51(4):798–801. doi: 10.1104/pp.51.4.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T. Observations on yeast pyruvate kinase activity in vivo. Biochem Biophys Res Commun. 1970 Sep 10;40(5):1076–1083. doi: 10.1016/0006-291x(70)90904-6. [DOI] [PubMed] [Google Scholar]
- Tomlinson J. D., Turner J. F. Pyruvate kinase of higher plants. Biochim Biophys Acta. 1973 Nov 2;329(1):128–139. doi: 10.1016/0304-4165(73)90015-9. [DOI] [PubMed] [Google Scholar]


