Abstract
Despite numerous publications on the appropriate use of blood and blood products, few specifically consider the role of transfusion in the management of HIV. This review is a synthesis of conditions encountered in the management of HIV-infected patients where the transfusion of blood or blood products may be indicated. A consistent message emerging from the review is that the principles of transfusion medicine do not differ between HIV-negative and -positive patients. The aim of the review is to provide clinicians with a practical and succinct overview of the haematological abnormalities and clinical circumstances most commonly encountered in the HIV setting, while focusing on the rational and appropriate use of blood and blood products for HIV patients. Important ethical considerations in dealing with both the collection and transfusion blood and blood products in the HIV era have also been addressed.
‘Blood transfusion is like marriage: it should not be entered upon lightly, unadvisedly or wantonly, or more often than is absolutely necessary.’1
Blood services have been at the forefront of raising awareness of the HIV pandemic. The emergence and recognition of HIV as a transfusion transmissible infection (TTI) in the early 1980s had a profound impact on blood services worldwide. There has been concerted international effort, expending considerable resources, to prevent transmission of TTIs through blood products and to provide safe blood products for transfusion. One means of reducing the risk of TTIs and properly managing haematological symptoms, especially in immunocompromised individuals, is appropriate clinical use of blood. This is contingent upon an appreciation of the risks of unnecessary transfusion. Clinicians should consider non-transfusion options such as haematinics in the management of anaemia. The decision to transfuse should be based on an individual patient’s co-morbid and clinical status, rather than on laboratory indices only. Specific and careful consideration of each patient is therefore required when HIV-related anaemia or associated conditions raise the possibility of transfusion.
There have been numerous requests from members of the Southern African HIV Clinicians Society for guidance and direction on blood transfusion in HIV-infected patients. While the role of blood transfusion in the management of haematological conditions such as anaemia and thrombocytopaenia does not differ substantially between HIV-negative and HIV-positive patients, it is the authors’ experience that there is a general need to promote rational transfusion practice. The objective of this review is not to provide a novel approach to the management of haematological conditions; rather, it is intended to provide a practical and succinct review on the rational use of blood transfusion in the management of haematological abnormalities, focusing on conditions either unique to, or more frequently encountered in, HIV-infected patients. This review should be read in conjunction with published national and international clinical guidelines.
(For a review of the ethical and legal considerations of transfusion and HIV, with a specific focus on South Africa, please refer to Appendix 1.)
Cytopaenias in HIV
Clinically significant cytopaenias (anaemia, thrombocytopaenia, neutropaenia) are common in persons with HIV.2 Many factors may contribute to the development of cytopaenias in HIV, including the virus itself that can infect progenitor cells directly, cytokine effects, reticulin fibrosis, altered immune function with auto-antibody production, micro-nutrient deficiency (folate, vitamin B12, iron), co-infection with other agents both opportunistic (e.g. TB, mycobacterium avium complex (MAC), cytomegalovirus (CMV), Ebstein-Barr virus) and conventional (e.g. bacteria, parvovirus), bone marrow infiltration by malignancy, and anaemia of chronic disease. Numerous drugs used in the management of HIV – including anti-retroviral therapy (ART) (e.g. AZT) and prophylactic therapies (e.g. co-trimoxazole) both may cause and exacerbate cytopaenias in HIV. In addition, bone marrow suppression in HIV occurs due to the effect of cytokines as well as reduced erythropoietin production and function.3-5
The decision to transfuse a patient is a clinical decision that needs to be individualised, informed by the patient’s clinical status, the laboratory findings where available, and available resources. This decision may incorporate the patient’s socio-economic circumstances: a patient with borderline decompensated anaemia, living in a remote area with poor access to follow-up care, might warrant transfusion; whilst the same patient might otherwise be managed conservatively in an urban setting with access to care.6 In general, indications for transfusion in HIV-positive patients are the same as for HIV-negative patients. HIV-positive patients may, however, have compromised bone marrow function and require additional haematological support until such time that ART results in improved bone marrow function.
Bone marrow involvement
Bone marrow infiltration can result in anaemia through destruction of the haematopoietic environment. Infiltration is reflected by pancytopaenia and a reticulocyte production index (RPI) <1% and sometimes by a leucoerythroblastic reaction where immature red and white blood cells are visible on the peripheral blood smear. Ideally, evidence of a leucoerythroblastic reaction requires consideration of a bone marrow biopsy and specialist input. Common infiltrative processes in the setting of HIV include granulomatous infection (e.g. mycobacterial and fungal), lymphoproliferative disorders and fibrosis. Management will be determined by the underlying cause, with transfusion limited to patients who are symptomatic.
Dysplastic changes in the bone marrow are common, and may occur at any stage of HIV infection. Where available, bone marrow biopsy may be informative in investigating various diagnoses e.g. neoplasia (lymphoproliferative or myeloproliferative disorder) and opportunistic infections such as TB. Special stains and cultures for fungi, viruses and mycobacteria may help to resolve the cause of unexplained fever or source of infection.7 Although the same indications for bone marrow biopsy apply independently of HIV status, certain indications (e.g. pancytopaenia, pyrexia of unknown origin (PUO), and lymphadenopathy) feature more prominently in patients with HIV. Similarly, certain diagnoses are more prevalent in the HIV population (e.g. immune thrombocytopaenia, disseminated mycobacterial infection, disseminated fungal infection).
A bone marrow biopsy is an invasive procedure; it may be painful and is not without risk. The latter includes infection, bleeding and haematoma. It should not be a first step in investigation and, regarding haematological disorders, should ideally be accompanied, where available, by additional testing i.e. bone marrow aspiration, cytogenetics and immunophenotyping (e.g. flow cytometry) to provide maximal diagnostic yield. Availability of this testing is often confined to tertiary academic and private referral laboratories. Bone marrow investigations may be helpful in the following situations:
bone marrow failure (pancytopaenia and reticulocyte production index (RPI) <1%)
cytopaenias unresponsive to treatment
investigation of opportunistic infection
pyrexia of unknown origin (PUO) after initial investigations fail to identify the cause
exclusion/staging of malignancy
diagnosis of specific pathology, such as pure red cell aplasia
atypical and/or abnormal blood cells noted in the peripheral blood smear.
Specimen adequacy is important for identifying a focal process (e.g. granuloma, metastatic carcinoma or lymphoid infiltrate). Both the focal nature of the pathology and increased marrow fibrosis (a component of granuloma formation) increases the risk of a non-diagnostic specimen.
Anaemia
Anaemia is not a diagnosis, and management should focus on investigation and treatment of the underlying cause, independent of HIV status. Anaemia refers to a reduced red cell mass as reflected by a decreased haematocrit or haemoglobin level. It is a clinical sign that reflects an underlying disease process that requires appropriate investigation and management that is specific to the underlying process. Anaemia is common to a diverse array of pathologies with similarly broad therapeutic options. Consequently, generic treatment (e.g. blood transfusion or haematinics) without knowledge of the specific cause is considered bad practice. As an example, iron-deficiency anaemia can be due to dietary deficiency (a simple problem of insufficient intake). However, it can also be due to chronic blood loss (e.g. menorrhagia, helminth infestation, visceral malignancy). Simply treating with iron supplements without a root-cause analysis ignores the differential diagnosis and may delay time-sensitive treatment of the actual cause (e.g. colonic carcinoma).
Anaemia may occur at any stage of HIV infection, and 63 - 95% of infected persons will develop anaemia during the course of their illness; furthermore, the incidence of anaemia increases with disease progression.2 The presence of anaemia is an independent, yet reversible, predictor of mortality.6,8,9 Table 1 lists some of the causes of anaemia in HIV; it is by no means complete. The main aetiologies for HIV-related anaemia are dyserythropoiesis (anaemia of chronic disease), infections and drugs.2-5 In addition, anaemia may be compounded by co-morbid haematinic deficiency (iron, folate and vitamin B12), suggesting a need for early replacement in the management of anaemia in HIV. Zidovudine (AZT), especially when used as a single agent, is the drug historically most frequently implicated in HIV-related anaemia; a dose-dependent macrocytic anaemia is characteristic.10 With lower doses, and in combination therapy, the haematological adverse events occur less frequently. Anaemia at baseline should not preclude the use of Zidovudine in patients initiating ART in resource-limited settings.11
Table 1. Anaemia and HIV-infection.
| Decreased production | Increased loss and/or destruction |
|---|---|
| Deficiencies | Haemolysis |
| Erythropoietin | Autoimmune haemolytic anaemia |
| Iron | Thrombotic thrombocytopaenic |
| Folate | purpura (TTP) |
| Vitamin B12 | Disseminated intravascular |
| Drugs | coagulation (DIC) |
| Zidovudine | Infections: Malaria |
| Co-trimoxazole | Pre-existing conditions |
| Anti-mycobacterial therapy | Glucose-6-phosphate |
| Amphotericin B | dehydrogenase deficiency |
| Ganciclovir | Sickle cell disease |
| Dapsone | Thalassaemia |
| Chemotherapy | Gastrointestinal bleeding |
| Infections | Infections (CMV, Candida, parasites) |
| HIV | Kaposi’s sarcoma |
| Cytomegalovirus (CMV) | GIT lymphoma |
| Epstein-Barr Virus (EBV) | Hypersplenism |
| Parvovirus B19 | Infection |
| Mycobacterium tuberculosis (MTB) | Haemophagocytosis |
| Mycobacterium avium complex (MAC) | Lymphoma |
| Histoplasma capsulatum | Idiopathic |
| Neoplasia | |
| Hodgkin’s disease | |
| Non-Hodgkin’s lymphoma | |
| Kaposi’s sarcoma | |
| Miscellaneous | |
| Anaemia of chronic disease | |
| Pure red cell aplasia (PRCA) | |
| Hypoplastic/aplastic anaemia | |
| Haemophagocytic syndrome | |
| Secondary myelodysplastic syndrome |
The following revised haemoglobin (Hb) and haematocrit (Hct) levels are based on recommendations published by Lawrie et al.12 in 2009, in which the normal ranges for Hb were found to be: 13.4 - 17.5 g/dl in males and 11.6 - 16.4 g/dl in females. Lower values may be accepted as normal in selected settings (e.g. evaluation during pregnancy or at sea level). There is significant variability in the definition of anaemia in relation to Hb level. Although anaemia may be strictly defined as a Hb level <13.4 g/dl for males and <11.6 g/dl in females, investigating all patients with Hb levels that approximate these values are both impractical and of questionable value in resource limited settings.
A baseline full blood count (FBC) with a differential count should be performed on all newly diagnosed HIV-positive patients. Clinicians are advised to monitor the patient’s Hb level regularly in accordance with local guidelines. All patients with anaemia should be investigated for the underlying cause(s) and treated appropriately. While reduced red blood cell (RBC) production frequently underlies HIV-related anaemia, it is important to exclude other causes e.g. haemolysis or blood loss.
The reticulocyte count and corrected reticulocyte production index (RPI) will guide one with regard to the underlying aetiology (e.g. decreased production or increased destruction). This discrimination is important as management differs accordingly. The reticulocyte count is a useful index of bone marrow function, and should increase in response to any blood loss (e.g. haemorrhage or haemolysis). Failure to increase appropriately may reflect a primary production problem that can affect multiple cell lines (e.g. aplastic anaemia) or be specific to one cell line (e.g. Parvovirus B19-related pure red cell aplasia (PRCA)). The reticulocyte count is normally 0.6 - 1.83%,13 but different textbooks and laboratories use different reference ranges. With anaemia, the corrected reticulocyte count (cc) is adjusted for the lower Hct. A reticulocyte production index of 1 - 2% indicates an appropriate marrow production; 3% or more indicates haemolysis; and levels <1% suggest reduced production.
Mean corpuscular volume (MCV) changes with age. From age 1 to 8 years, the lower limit of the MCV can be roughly calculated using the formula: age in years + 70 fl. In adults, MCV is typically 80 - 100 fl. An MCV less than this range suggests microcytosis, while greater levels suggest macrocytosis.
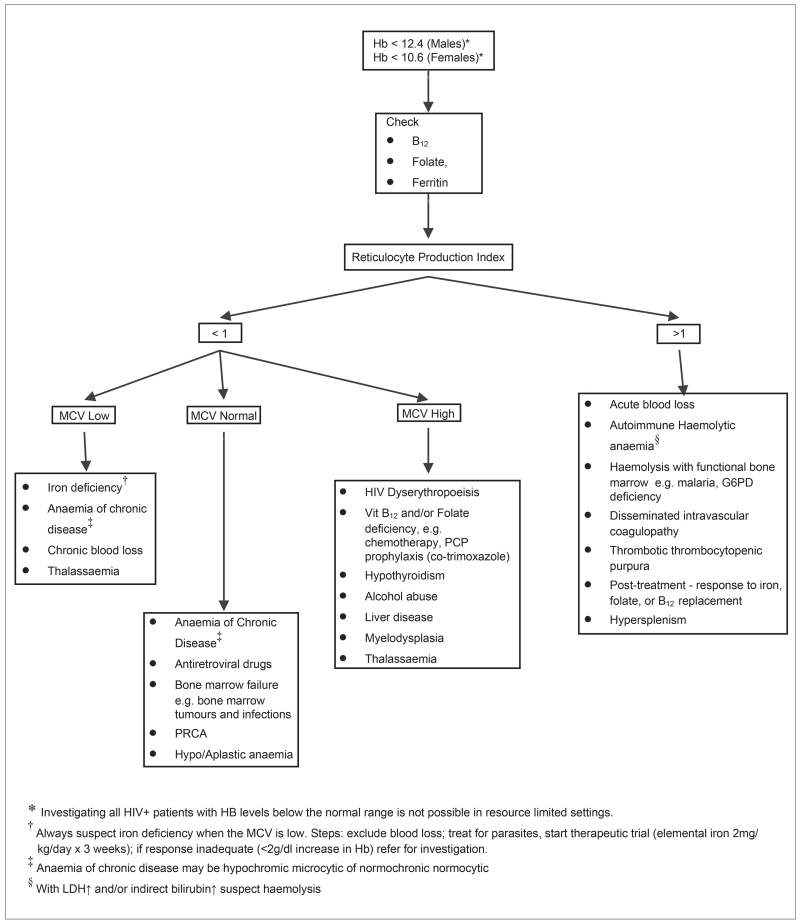
Fig. 1 provides a practical investigative approach to the causes of anaemia in the HIV positive patient.
Fig.1.
Diagnostic algorithm for anaemia in HIV infection.
Iron deficiency is the most common cause of microcytic anaemia. Iron deficiency is not a definitive diagnosis; rather, it reflects an underlying pathology that must be identified and treated appropriately and specifically. The mechanisms for iron deficiency include reduced intake (e.g. nutritional deficiency and inflammatory bowel disease) as well as increased loss (either acute haemorrhage or chronic, e.g. menorrhagia, helminthic infestation, gastrointestinal bleeding). Iron deficiency requires iron supplementation administered orally or parenterally. The latter may incur risk of, among others, anaphylaxis, and should only be reserved for severe cases of iron deficiency where oral supplementation is not tolerated (e.g. inflammatory bowel disorders, malabsorption). Patients should respond to iron replacement therapy within 2 months if the underlying cause has been addressed. Failure to respond within this period should prompt review of initial diagnosis with attention to patient adherence. Adherence is a recognised problem of iron supplementation, given gastrointestinal side effects. Excessive iron therapy can be deleterious and should not be given as a routine supplement in the absence of a clinical indication.14
In proven iron deficiency, one should – especially in children – consider administration of an anti-helminthic agent, in conjunction with iron therapy, in view of the high prevalence of co-morbid helminthic infestation in low-resource settings. Helminthic infestation (e.g. hookworm) is known both to cause and exacerbate underlying iron deficiency.
Transfusion should not be a first line of intervention in iron deficiency; oral iron is generally effective in managing stable patients. Transfusion is reserved for patients with decompensated anaemia (e.g. patients with signs of hypoxia, angina, cerebrovascular compromise, heart failure). Transfusion with red cell concentrate (‘packed cells’) can ameliorate symptoms in these patients. Each unit of packed red cells contains (replaces) approximately 250 mg of iron.
Macrocytic anaemia is often attributed to deficiencies in Vitamin B12 (cobalamin) and/or folate. In stable patients with proven vitamin B12 deficiency (pernicious anaemia), the patient should be treated with injectable vitamin B12 for at least 3 days before starting folate therapy. This is essential to avoid permanent neurological complications. In all other cases, start supplementation with folate and vitamin B12. The response to vitamin B12 occurs within 48 to 72 hours. Serum potassium levels can fall during initial therapy for severe Vitamin B12 or folate deficiencies owing to increased utilisation of potassium by new haematopoietic cells; potassium levels should be monitored during therapy, with potassium supplementation where indicated.15,16
Blood transfusion should be avoided as patients with macrocytic anaemia generally tolerate extremely low haemoglobin levels very well. Transfusion can lead to circulatory overload especially in critically ill patients who require stringent maintenance of fluid volume status. In severely symptomatic anaemic patients, a maximum of 1 - 2 units of red cell concentrate may be administering slowly (over 4 hours per unit), followed by intravenous furosemide (20 - 40 mg), or be given in an isovolemic manner (i.e. removing 250 ml of the patient’s whole blood, which has a low haematocrit, and replacing with 250 ml of packed RBCs, which has a high haematocrit).
Anaemia of chronic disease (ACD) is common in HIV; the mechanism is complex and is caused, in part, by the release of cytokines, resulting in iron blockade and dyserythropoiesis. In ACD, haemoglobin levels seldom drop below 7 g/dl. Additional causes should be considered if haemoglobin levels fall below 7 g/dl. ACD does not respond to haematinics and requires treatment of the underlying condition (e.g. ART in the setting of HIV). Where treatment is not possible and the patient remains symptomatic, judicious use of erythropoietin can be considered.17,18
Autoimmune haemolytic anaemia is more common in HIV-infected patients than in HIV-negative patients. The direct anti-globulin test (DAT) (Coombs test) is positive in up to 20 - 40% of HIV-positive patients;19 few patients, however, demonstrate signs of haemolysis.20 The presence of a high reticulocyte count, unconjugated hyperbilirubinaemia, elevated LDH, spherocytes on the peripheral smear, and falling haemoglobin, suggests haemolysis and should prompt further investigation. Primary management of AIHA is similar to that of the HIV-negative patient (i.e. treatment of the underlying cause (e.g. ART), corticosteroids, IVIG, and immunosuppressive therapy).21 These therapies generally require specialist input and/or support. AIHA can complicate compatibility testing and the rapid access to blood for transfusion. Red cell auto-antibodies can mask clinically significant allo-antibodies that have developed owing to prior antigen exposure (e.g. pregnancy and/or previous blood transfusion). In non-urgent cases, a full serological investigation should be done to resolve the specificity of the auto-antibody as well as to exclude any co-existing allo-antibodies. Once an allo-antibody has been excluded, it is generally acceptable to administer red cell transfusions to patients whose DAT is positive and where the indirect anti-globulin test (IAT) phase of the crossmatch is also positive; a haemolytic transfusion reaction in these patients is unlikely.21 These patients do, however, warrant slow transfusion and careful monitoring.
Importantly, the reticulocyte count and peripheral smear must be requested before a transfusion, since the value of the count as well as the appearance of the peripheral blood morphology and the MCV may change after a transfusion and not truly reflect the patient’s haematological status.
Thrombocytopaenia
Thrombocytopaenia occurs commonly (often as one of the presenting symptoms), increases with disease progression, and is associated with shortened survival in HIV-positive patients.22-25 The most common cause of primary HIV-associated thrombocytopaenia is immune-mediated destruction.25 This is frequently attributed to high levels of auto-antibodies directed against platelet-associated antigens. HIV can also infect megakaryocytes directly, given megakaryocyte expression of CD4 and CXCR4 receptors which are known docking points for HIV.25 Consequently, platelet production and lifespan are reduced in the HIV-positive patient. Additional causes of reduced platelet survival and decreased production are listed in Table 2.
Table 2. Thrombocytopaenia and HIV infection.
| Decreased production | Increased loss/destruction/sequestration |
|---|---|
| Drugs | Immune thrombocytopaenia (secondary – HIV associated) |
| AZT | |
| Co-trimoxazole | Thrombotic thrombocytopenic purpura (TTP) |
| Fluconazole | |
| Ganciclovir | Disseminated intravascular coagulation (DIC) |
| Acyclovir | |
| Rifabutin | Haemolytic uraemic syndrome (HUS) |
| Clarithromycin | Hypersplenism |
| Didanosine | Infection |
| Chemotherapy | Haemophagocytosis |
| Deficiencies | Drugs |
| Vitamin B12 | Interferon |
| Folate | Saquinavir |
| Infections | Secondary anti-phospholipid syndrome |
| HIV | |
| CMV | |
| MTB | |
| MAC | |
| Histoplasma capsulatum | |
| Neoplasia | |
| Hodgkin’s disease | |
| Non-Hodgkin’s lymphoma | |
| Miscellaneous | |
| Hypoplastic/aplastic anaemia | |
| Haemophagocytic syndrome | |
| Secondary myelodysplastic syndrome |
As with anaemia, thrombocytopaenia is associated with a poor prognosis, while the treatment of the cause of the thrombocytopaenia confers improved survival.26-28 Most patients with a platelet count >30 ×109/l do not require treatment but do warrant investigation to elucidate the underlying cause.
Patients do not generally bleed spontaneously at platelet counts >10 - 20×109/l. Prophylactic platelet transfusion is seldom indicated. Some exceptions include: prematurity or neonatal thrombocytopaenia; intracranial pathology or risk of intra-cranial haemorrhage; functional platelet disorders independent of platelet count. Therefore, platelet counts <100×109/l may warrant investigation as to the underlying cause with appropriate management, but do not necessarily require platelet transfusions in the absence of bleeding. A unit of platelet concentrate containing >3×1011 platelets usually results in an increment in the platelet count, in an adult, of ~ 30×109/l. In the absence of factors that shorten the lifespan of transfused platelets (such as fever, splenomegaly and/or anti-platelet antibodies), the lifespan of transfused platelets is ~3 - 4 days.
Immune mediated thrombocytopaenias
Immune thrombocytopaenia (ITP) can occur at any stage of HIV but most commonly in early disease. Auto-antibodies directed against platelet antigens are readily found in most people with HIV, but they are not necessarily clinically significant. Laboratory confirmation of ITP is complex; consequently, ITP is a diagnosis of exclusion. First line therapy is oral prednisone (1 mg/kg each day). Prednisone at this dosage does not appear to significantly affect viral replication; it may, however, promote Kaposi sarcoma growth.29 If there is no response to steroids after one week, prednisone dosage can be increased to 2 mg/kg per day. Patients should not receive high-dose steroids for more than 2 weeks without referring the patient to a specialist unit. Patients on steroids require proton pump inhibitor (PPI) or H2-receptor antagonist prophylaxis for the prevention of peptic ulcer disease related to steroid therapy. If the platelet count drops below 30×109/l, IVIg therapy, with or without steroids, should be considered (exception: patients with haemophilia or patients on anticoagulation therapy, where the lowest accepted platelet count is usually 50×109/l). Response to therapy should be monitored, and ART needs to be initiated or continued. A high index of suspicion of concomitant TB should be maintained. Tranexamic acid and progesterone should be considered in female ITP patients presenting with a platelet count <50×109/l and genito-urinary bleeding – remembering, though, that tranexamic acid in patients with haematuria may result in blood clots and urinary colic. It is prudent to give patients prophylactic pneumococcal vaccination in anticipation of potential need for splenectomy. It is recommended that refractory patients be referred to a tertiary unit for further management.
Thrombotic thrombocytopaenic purpura (TTP) is a medical emergency with a high mortality rate. Despite being frequently encountered in HIV, the diagnosis is often missed. With timely and appropriate management, the prognosis is significantly improved. TTP should be considered in all patients presenting with signs of micro-angiopathic haemolytic anaemia, thrombocytopaenia, fever, renal and liver dysfunction, and fluctuating neurological signs. Where possible, patients should be referred urgently to a tertiary facility for further aggressive management. Therapeutic plasma exchange (TPE) is ideal as it allows large volume plasma transfusions. If TPE is not available, fresh frozen plasma (FFP) or cryo-poor plasma at ~30 ml/kg per day should be infused in divided doses. TTP associated with HIV has been shown to respond well to FFP infusion alone and is appropriate in resource-limited settings without TPE.5,30 Prednisone therapy at 1 mg/kg per day is also recommended. Tranexamic acid should be avoided. Platelet transfusions are not routinely given to patients with TTP as they may potentiate thrombotic events and complicate monitoring therapeutic response.
Neutropaenia
The incidence of neutropaenia similarly increases with HIV disease progression.31,32 The aetiology of neutropaenia is often multi-factorial and attributable to conditions considered under the pathogenesis discussed above.
Transfusion medicine best practice
Transfusion is only one element of the patient’s management.
The decision to prescribe blood products should be based on individual patient needs, informed by best practice as well as the transfusing institution or national guidelines (e.g. Clinical Guidelines for the use of Blood Products in South Africa).33 Blood should only be transfused when clinically indicated and where the benefits outweigh the recognised risks.
Appropriate management of chronic anaemia through investigation and treatment of the underlying cause may help to reduce the need for blood transfusion.
Blood loss should be minimised to reduce the need for transfusion.
A patient with acute blood loss should receive effective resuscitation with intravenous replacement fluids, oxygen and immediate measures to stop further blood loss, while the need for transfusion is being assessed.
An appropriately trained healthcare worker must monitor the transfused patient and respond immediately and appropriately if any adverse event occurs.
To avoid wastage and unnecessary risk, the clinician should prescribe the minimum effective volume of blood and blood products necessary to stabilise the patient. Routine transfusion to predefined haemoglobin (Hb) levels should be avoided.
Blood should be transfused or discarded within 6 hours of breaking the seal on the blood bag to prevent risks associated with bacterial contamination.
Special considerations
Rate of transfusion
The rate of transfusion depends on the indication for the transfusion, patient’s co-morbidities and prior response to transfusion, if known. For example, those with acute haemorrhagic shock require rapid rates of transfusion as part of urgent resuscitation management. In contrast, patients with longstanding chronic anaemia should not be transfused rapidly (rate should not exceed 2 ml per minute), given adaptation to their longstanding anaemia where rapid transfusion can precipitate cardio-respiratory failure. Particular caution needs to be taken for patients at age extremes (e.g. neonates, children, and age ≥60) and those with co-morbid disease (e.g. renal and cardio-pulmonary disease). These patient groups are at particular risk of volume overload, also referred to as transfusion associated circulatory overload (TACO). Precautions to minimise the risk of volume overload include spacing of transfusions where possible, small volume infusion, and/or low dose furosemide following transfusion (where not contraindicated).
Filters
Red blood cells, whole blood, FFP and cryoprecipitate must be administered through a standard blood administration set. These sets have 170 - 240 μm mesh filters to prevent the transfusion of clots or coagulation debris. The filter should be covered (‘primed’) with blood to ensure that the full filtering area is used. Platelets should be transfused with a platelet giving set (a standard filter may be used in an emergency). Standard filters incur significantly greater platelet loss owing to adhesion to the comparatively larger surface area, larger chamber and longer tubing.
Administration sets should be changed:
following reported transfusion reactions; this prevents further introduction of potentially harmful blood entering the patient. This is particularly important in the setting of suspected septic transfusion reactions where bacteria from the implicated unit can similarly contaminate the original administration set.
between red cells and other blood products
between red cell units of different ABO groups (e.g. group O and group A red cells administered consecutively)
prior to infusing other fluids (e.g. Dextran, Ringers lactate)
every 12 - 24 hours (or according to the package insert/instruction) in patients requiring on-going transfusion.
Temperature of the blood
Blood warmers are not indicated for routine blood transfusion; cold blood transfused at a slow rate is unlikely to adversely affect the patient. Selected indications for blood warming include:
massive transfusion >50 ml/kg/hr
infants transfused at >15 ml/kg/hr
neonates receiving exchange transfusion or large volume transfusion
patients with high titre cold haemagglutinins reactive in vitro at temperatures >30°C.
In these select settings, large volume or rapid infusion of cold blood can precipitate cardiac arrhythmias and impair haemostasis. Blood warmers should be designated only for transfusion use, and require a temperature-monitoring device that has been properly maintained in line with the manufacturer’s instructions.
Improper or excessive warming can induce haemolysis of the red cells, with consequent renal failure and even death. Blood must never be placed in a microwave or oven. Rapid warming in a water bath is ineffective as only the outer cells are warmed and, at temperatures >37°C, may cause damage to these cells. Moreover, this practice risks bacterial contamination of the transfusion ports.
Patient identification
Near-miss or actual misdirected transfusions remains the greatest risk of a haemolytic reaction to any patient receiving a blood transfusion. It is the responsibility of each person involved in the transfusion of the patient to avoid identification errors. This begins with the prescribing doctor and extends to all staff involved in administering the transfusion. Patient identification should be repeated at every step of the transfusion process to ensure that errors do not occur. Where possible, the patient should be positively identified in the presence of 2 staff members. The patient needs to state his or her name, which should be an identical match with that on the chart and with the name on the blood packs for transfusion. Where the patient is unable to state his or her name, confirm the identity by using at least 2 or, if possible, 3 identification points, e.g. full name, hospital number, date of birth and/or identification number. Scrupulous attention to detail can help to prevent serious harm. Clerical error (i.e. through patient or blood product misidentification) is the foremost reason for severe haemolytic transfusion reactions.
Paediatric patients
Anaemia in paediatric patients is very common in Africa.34 In Ghana and Malawi, for example, more than 50% of children <5 years have a haemoglobin level <11 g/dl, and 20 - 47% of those admitted to hospital, reportedly receive blood transfusions.35-37 Furthermore, 6 - 13% of patients admitted with severe anaemia (Hb<5 g/dl) die – many before transfusion is possible. Anaemia in southern Africa disproportionately affects the young, given pervasive malnutrition, and helminthic and infectious diseases (specifically HIV and malaria, both endemic to southern Africa). This has a major adverse effect on childhood morbidity and mortality, particularly where resource constraints and the relatively high cost of blood transfusion limit availability of safe blood.
Both anaemia and thrombocytopaenia are common among South African HIV-positive children. Of those referred with cytopaenias, ~35% are anaemic (Hb <11 g/dl), ~12.5% have thrombocytopaenia, ~35% have combined anaemia and thrombocytopaenia, and ~10% are pancytopenic.38 The need for blood transfusion places a significant burden on transfusion inventories and hospital budgets.
Critically ill paediatric patients and neonates have special transfusion requirements. Neonates specifically, require small-volume transfusions that are relatively fresh (collected within the previous 14 days) and, when feasible, leucodepleted and/or CMV-negative. Neonates are particularly susceptible to volume overload, and measures should be adopted to counteract the risk e.g. small-volume and slow rates of transfusion. Given the risks of transfusion, both the decision to transfuse and the required volume should be carefully considered and individualised.39
In stable HIV-infected anaemic children, it is reasonable to consider a Hb <6 g/dl as the transfusion trigger.40,41 A threshold Hb of 7 - 8 g/dl is recommended in haemodynamically stable, critically ill patients (e.g. trauma, intensive supportive care, or surgery).42-44 Higher thresholds are required in premature infants and children who are actively bleeding.45 The recommended volume of transfusion is 10 ml/kg for red cell concentrate and, in severe malnutrition or heart failure, 5 ml/kg. For each 1 g/dl rise in Hb desired, 3 - 5 ml/kg are needed. A paediatric unit of red cell concentrate contains 60 - 80 ml of red cells, compared with an adult unit of approximately 265 ml red blood cells. The calculated transfusion volume needed should be rounded off to the nearest volume of bag available, to not waste this scarce resource.
Appropriate clinical use of plasma products33
Plasma products produced by component processing laboratories at the blood service centres through physical separation techniques are referred to as plasma components. Products derived from large pools of plasma by chemico-physical processing techniques (fractionation) are referred to as plasma derivatives.
Fresh frozen plasma (FFP) refers to plasma that is separated from anticoagulated whole blood and frozen within 18 hours of donation. FFP contains all coagulation factors at normal physiological levels. The indications for transfusion of FFP are outlined in Table 3 and are identical to those for HIV-negative patients. FFP should be transfused judiciously. In addition to infectious risk, it is associated with transfusion-related acute lung injury (TRALI), which has significant morbidity and mortality. Inappropriate uses of FFP include volume expansion and nutritional supplementation.
Table 3. Clinical indications for FFP.
| Indications |
|---|
| Multiple coagulation factor deficiencies e.g. |
| DIC |
| Massive blood transfusion |
| Liver disease |
| Active or ongoing bleeding with abnormal coagulation tests |
| Replacement of inherited single factor deficiencies, where single factor concentrate is not available |
| Thrombotic thrombocytopaenic purpura |
| Reversal of warfarin with active bleeding where prothrombin complex concentrate (PCC) is not available |
| Vitamin K deficiency associated with active bleeding |
| Scoline apnoea |
Cryoprecipitate is the cold insoluble fraction of FFP and contains Factor VIII and von Willebrand Factor (100 IU per unit), fibrinogen (150 - 250 mg per unit), fibronectin, and Factor XIII. It is indicated primarily for the treatment of hypofibrinogenaemia (acquired or congenital) as found mainly in DIC states. Owing to the relative low levels of Factor VIII and von Willebrand Factor, it is not indicated in von Willebrand ’s Disease or in Haemophillia A.
Freeze dried plasma (FDP) is produced from pooled fresh human plasma that has been subjected to a pathogen inactivation procedure that inactivates lipid-enveloped viruses. FDP has the same indications and dosage as FFP.
Intravenous immunoglobulin (IVIg) is prepared by a fractionation process which inactivates lipid-enveloped viruses. The two principal indications for IVIg are antibody replacement therapy and immunomodulation. In the setting of HIV, IVIg is primarily used in the management of ITP and PRCA.
Intramuscular hyperimmune immunoglobulin is produced by a method of fractionation similar to that of intravenous preparations. Most preparations have a high titre of antibodies for passive immune prophylaxis against selected infections (e.g. chicken pox, hepatitis B, rabies etc).
Oncology patients
Independent of HIV status, the oncology population is a major user of blood and blood products. Certain malignancies are AIDS-defining conditions (e.g. high-grade B-cell non-Hodgkin’s lymphoma, Kaposi’s sarcoma and carcinoma of the cervix). However, HIV-positive patients are susceptible to the full range of malignancies encountered in HIV-negative patients, such as breast cancer, colorectal cancer etc. With the advent of HAART, as HIV-positive patients live longer, they will be at risk of the same spectrum of malignancies as seen in negative individuals.
General transfusion principles apply to the HIV-positive oncology patient. The decision to transfuse should be individualised to the patient’s needs; this can be challenging in patients with uncertain outcomes. For example, transfusion in patients with poor expected short-term outcomes may be inappropriate if it is unlikely to change the outcome. However, transfusion has a role in palliative care where it can be used to maintain or improve quality of life in terminally ill patients. Adjunctive therapy with haematinics, erythropoietin and other alternatives to transfusion should be considered in patients who require active management of their anaemia.
Transfusion thresholds can differ to accommodate planned therapies. For example, radiotherapy requires a higher tissue oxygenation to be effective; therefore these patients require a higher haemoglobin level, and a target of 10 g/dl should be used. Similarly, maintenance of higher haemoglobin levels has been shown to improve radiotherapy outcomes in head and neck cancer.46
Surgical patients
Perioperative indications for blood transfusion are the same for both HIV-positive and -negative patients. However, HIV-positive patients are more likely to be anaemic and consequently more likely to require transfusion pre-operatively. All reasonable measures must be taken to treat and correct anaemia and its causes before surgery. Estimating transfusion needs and communicating with the hospital blood bank is especially important. Algorithms to help predict need for transfusion may be useful47 but, in practice, if the patient presents at surgery with a haemoglobin level <7 - 8 g/dl, and the predicted blood loss is >500 ml, then perioperative transfusion should be anticipated and planned according to the patient’s condition.48
A study of Jehovah’s Witness patients undergoing surgery who refused transfusion noted that mortality in elective surgery depended on estimated blood loss rather than on preoperative haemoglobin levels. Furthermore, the study found that elective surgery could be performed safely in patients with a preoperative haemoglobin level as low as 6 g/dl if estimated blood loss was maintained below 500 ml.49 Other studies in Jehovah’s Witness patients have demonstrated a significant increase in mortality and morbidity in patients with a postoperative haemoglobin level <7 g/dl and 8 g/dl respectively.50
It has been shown that the risks of surgery and anaesthesia in HIV-positive patients is similar to that of surgery in immunocompromised or malnourished patients, with increased risk with disease progression.51 As for any patient, the preoperative physiological status (including an assessment of nutritional reserve) is considered the best predictor of surgical morbidity and mortality.52 HIV-associated thrombocytopaenia can potentially increase the risk of bleeding, but regional anaesthesia is not contraindicated.52,53
Haemophiliac patients
In the late 1970s and early 1980s, patients with haemophilia (PWH) were infected with HIV through the use of contaminated blood products, specifically clotting factor concentrates, FFP and cryoprecipitate. This tragedy has since lead to a complete revision of transfusion practice, policy and procedures. With robust donor selection, sensitive laboratory testing and good manufacturing practice for the production of fractionated factor concentrates, the current risk of transfusion-transmitted HIV is very low. However, development of thrombocytopaenia, lymphadenopathy or splenomegaly in PWH still warrants testing for HIV infection.
PWH who are found to be HIV-positive can develop thrombocytopaenia consequent to their HIV disease. Therefore, the development of purpura or increased mucosal bleeding in an HIV-positive PWH may be due to thrombocytopaenia rather than factor deficiency. Investigation and identification of the cause is important: factor administration alone may not be sufficient, and therapy may need to be directed toward the HIV thrombocytopaenia (as a primary cause). Management is similar to that of patients without haemophilia, and includes steroids, platelet transfusions and, in the HIV-positive patients, the initiation of HAART.
Obstetric patients
Regarding blood transfusion, the management of HIV-positive obstetric patients should not differ from that of HIV-negative obstetric patients. Transfusion should be used sparingly in obstetrics. Pregnancy may, however, be associated with sudden massive blood loss – obstetric haemorrhage remains the third most common cause of maternal mortality in South Africa.54 Hospital maternity sections frequently rank among the highest in terms of demand for blood and blood products, along with trauma and ICU.55
There is a high prevalence of underlying anaemia in pregnancy owing to various causes. This anaemia must be anticipated, recognised and treated to lessen the risk associated with blood loss at delivery and to lessen the requirements for transfusion. Specific physiological changes in the haematological system occur in pregnancy, including haemodilution (there is an increase in plasma volume by 45 - 50% that reaches a maximum at about 34 weeks’ gestation and exceeds the 18 - 30% increase in the red cell mass).56 In the anaemic patient on oral haematinics, a static Hb may represent a response to the anaemia masked by haemodilution. Plasma volume increases more in multiple pregnancies.
The fetus must also be considered in terms of the potential effects of the anaemia. Folic acid deficiency (a cause of pregnancy-related anaemia) has been associated with neural tube defects. Periconceptual folic acid supplementation is recommended.57
Pregnancy is also associated with specific disorders that affect the haematological system, increasing the need for transfusion. These include HELLP syndrome (haemolysis, elevated liver enzymes and low platelets) associated with pre-eclampsia/eclampsia and pregnancy-associated thrombocytopaenia.
Although these guidelines contain recommendations for complex investigations and discuss current blood product use, it is also recognised that a large proportion of pregnant patients in South Africa and elsewhere in Africa are cared for at primary care centres where only basic haematological investigation may be available. In parts of southern Africa, the available blood supply does not meet the clinical demand, and clinicians face a chronic shortage of blood. In all circumstances, much can be achieved by the prompt use of standard obstetric protocols to minimise blood loss. Where possible, the more complex patient who requires specialised antenatal or intrapartum care should be transferred to better-resourced settings.
The prevalence and cause of anaemia in pregnancy varies throughout Africa. In Malawi and Zimbabwe (and in the Limpopo and northern KwaZulu-Natal provinces of South Africa), for example, malaria and/or gastrointestinal parasite infection may be a cause of anaemia in addition to underlying nutritional deficiencies. Should a patient in such areas become infected with HIV, these underlying causes of anaemia will persist and require treatment in accordance with the principles mentioned before.58 Anaemia should be identified, appropriately investigated, and treated early in pregnancy. Other haematological complications such as bleeding tendencies and cardiac failure warrant urgent admission or referral to a secondary/tertiary centre. Routine obstetric assessment includes the gestational age, which indicates the time available to treat a low Hb. Risk factors for obstetric haemorrhage may indicate referral to a secondary/tertiary centre for delivery, where transfusion, if required, will be available.
According to the South African National Prevention of Mother to Child Transmission of HIV (PMTCT) Clinical Guidelines (2010),59 all HIV-positive pregnant women should receive iron and folate supplementation. In South Africa, folate should be available at the treatment dose of 5 mg and not the prophylactic dose. In countries where folate fortification of food takes place, folate deficiency is rare. The recommended daily dosage for iron is 60/65 mg elemental iron in the 2nd trimester and 120 mg elemental iron, in divided doses, in the 3rd trimester. If there is intolerance, replacement can be deferred since, in the 1st trimester, oral iron may cause increased nausea and vomiting. If calcium is given antenatally, it must not be given at the same time as the iron, as it blocks absorption.60,61 There is an exception: Patients with a known haemolytic anaemia – e.g. thalassaemia or sickle cell anaemia (particularly from malaria-endemic areas) – should not be given iron routinely.
Anaemia must be recognised and managed promptly before the time of likely delivery. The time intervals for assessment of response are therefore shorter than in the non-pregnant patient. A review after only 3 months is not appropriate.
For treatment, dosage: 200 - 250 mg elemental iron daily in divided doses, i.e. one tablet of iron sulphate or iron fumarate 3 times daily. If vitamin C is available, it should be given with iron, also in divided doses.61 Iron taken with food increases tolerance.
- There is an increased absorption of iron in pregnancy. If the anaemia is a result of iron deficiency, an Hb increase of up to 0.7 g/dl per week may result. The lower the starting Hb, the more rapid the anticipated response.
-
2.1With an inadequate (<0.5 mg/dl per week) or absent response, a full blood count (FBC) and reticulocyte production index (RPI) should be performed. The RPI is frequently >2 in the early response to iron deficiency anaemia in pregnancy.
-
2.2The FBC may direct further investigation of the anaemia as mentioned above.
-
2.3In iron deficiency anaemia responding to iron but with a delayed Hb response, the red cell distribution width (RDW) may also increase, usually to >20%. The increase in RDW is not valuable if a recent transfusion or drugs have been given that cause macrocytosis e.g. AZT62 causing an artificial increase in RDW.
-
2.4If the MCV is high (>110 fl), Vitamin B12 deficiency, though rare, should be considered, especially if there is oral ulceration or neurological symptoms.
-
2.1
Intravenous iron is only indicated, remote from term, if iron deficiency anaemia, proven on FBC and iron studies, is associated with intolerance of oral iron or there is no improvement of the anaemia despite apparent compliance.63 Intravenous iron should only be given where there is no other reason for immediate transfusion.
According to the National PMTCT Guidelines (2010), the newly diagnosed HIV-positive pregnant patient who has a CD4 count >350 cells/mm3 or WHO Staging 1 or 2 should, after 14 weeks’ gestation, commence Zidovudine (AZT), if consenting. If CD4 count <350 cells/mm3, or WHO Stage 3 or 4, full ART should be offered.59 AZT is associated with macrocytosis,62 but rarely associated with severe anaemia as a result of a pure red cell aplasia.64 If there is adequate time remaining in the pregnancy, patients who are treatment-naïve, do not qualify for full ART, and have an Hb <10g/dl, should receive a full course of haematinics and their response observed. Their response should be re-assessed after 2 weeks and, if there is a response, AZT be commenced. If the patient’s Hb is <8g/dl, the South African PMTCT Guidelines suggest withholding AZT.59 If, however, there is a rapid response of the anaemia to iron and folate, AZT can be given. If the Hb is <8g/dl and there is no response to haematinics, further investigation is warranted. In certain patients, there may be a case for considering full ART. These include cases where delivery is approaching and where the Hb remains low or is falling. Patients with a falling Hb on AZT (or AZT-containing regimen) should be investigated for other causes of anaemia, usually nutritional or infective, but autoimmune haemolytic anaemia should also be considered. Haematinics should be commenced if not yet initiated and the patient examined and investigated further for additional underlying pathology. If a pure red cell aplasia is confirmed, AZT should be stopped. In such cases, patients usually respond within a week. Blood transfusion may be required if the patient is symptomatic or if the Hb falls below 6g/dl with no response to treatment.
Many patients tolerate very low levels of Hb (5 - 6 g/dl). If there are no medical or obstetric complications, and it is early in pregnancy (<34 weeks), oral replacement with haematinics should be used and the patient reassessed after 1 - 2 weeks. Where patients are in cardiac failure, have worsening anaemia with no response to haematinics, or where delivery is imminent, transfusion should be considered. Transfuse one unit of red cell concentrate and reassess. There is no need to transfuse if the Hb >7 g/dl and there are no obstetric complications. A higher Hb (>8 g/dl) should be targeted in patients at increased risk of obstetric haemorrhage, e.g. previous PPH, multiple pregnancy and placenta previa (where a Hb >9g/dl should be targeted).
Routine obstetric intervention may prevent transfusion. Appropriate observation is critical
Timely caesarean section for antepartum haemorrhage, controlled delivery of the placenta in vaginal delivery, and the recognition and treatment of uterine atony may prevent the need for transfusion. If a transfusion is not to be given to a patient in whom the peripartum loss has been considerable (>500 ml in normal delivery, >1000 ml in caesarean section), the cause of bleeding must be controlled and the condition of the patient considered. Close observation is essential; any further blood loss must be accurately recorded and action taken if necessary. Blood loss is frequently underestimated.63 In patients with physiological compromise (both HIV-negative or -positive), transfusion should be considered earlier than in otherwise healthy counterparts.
If blood products are required and not available at the point of care, the patient should be transferred to a unit with access to a blood bank as soon as possible. The patient should be oxygenated, kept warm, with adequate intravenous (IV) support and with adequate measures to control further haemorrhage.
There is no need to give blood to reach a particular Hb level. Transfusion practice depends on the availability and proximity of blood products in the case of an emergency; the American and British Anaesthetic Task Forces in Obstetrics recommend neither routine type and screen, nor crossmatch of patients who undergo a routine normal delivery or routine caesarean section if these services are readily available.63,65 Despite these recommendations, patients at risk for greater than average blood loss (e.g. caesarean section for placenta previa) should have blood crossmatched and should, wherever possible, give birth in a place where further blood products are readily available.54
Malaria
The risk of severe malaria appears to be greater in HIV-positive (non-immune) patients than in HIV-negative patients.66 In addition, HIV-positive patients are at a significantly higher risk of developing severe anaemia. Pregnant women co-infected with HIV and malaria are at greater risk of complicated disease than women with either malaria or HIV infection alone. Peripartum complications include severe anaemia,67 thereby increasing the need for blood transfusion.
Severely anaemic patients may benefit from transfusion early in the course of acute malaria but, once stable and in process of recovery, the benefit of transfusion68 is limited. However, persistent worsening anaemia is a recognised complication in the weeks following clearance of parasitemia.69 Clinicians should therefore monitor HIV-positive patients for at least 3 months following malaria treatment. Paediatric patients are at greatest risk. One study of HIV-1 and Plasmodium falciparum co-infected children aged 3 - 36 months demonstrated significantly worse anaemia (Hb <6.0 g/dl) and a nearly 10 times greater mortality within 3 months post treatment, compared with an HIV-negative cohort.70
HIV-malaria co-infected patients are particularly prone to invasive bacterial infection (IBI); this underscores the need for good transfusion practice and vigilance against bacterial contamination.71 Improper handling of blood and blood products further increases the risk of Gram-positive bacteraemia. Broad-spectrum antibiotics (such as a third generation cephalosporin) should be routinely administered to HIV-malaria co-infected patients with severe malaria, to provide cover against both Gram-positive and Gram-negative bacteria; this should be instituted from the time of admission.72 Aggressive initial management of malaria is essential. Blood transfusion has a role in management, but should not delay initiation of anti-malarial therapy.
Haemoglobinopathies
There is no evidence to suggest that patients with haemoglobinopathies who are HIV-positive should be managed differently to those who are HIV-negative.
Massive transfusions
The principles of management of patients requiring massive blood transfusion are the same for HIV-positive and HIV-negative patients.
Leukodepleted blood
The routine use of leukodepleted blood in HIV-positive patients is not recommended. Even though HIV/AIDS patients are immunosuppressed, there is no substantive data supporting improved outcomes in patients who routinely receive leukodepleted blood components. Currently, the indications for the transfusion of leukodepleted blood products are the same for HIV-positive and HIV-negative patients. While the use of leukodepleted blood products may reduce the risk of transmission of leucocyte-associated pathogens such as CMV and HTLV, the Viral Activation Transfusion Study (VATS) demonstrated no clinical benefit for HIV-positive persons, who received white-blood-cell-reduced transfusions.73-75 The indications for use of leukodepleted products include:
- prevention of alloimmunisation:
- patients on chronic transfusion regimens, such as aplastic anaemia or sickle cell anaemia
- organ and stem cell transplant patients
- haem-oncology patients
- patients at risk for CMV infection such as:
- transplant patients receiving immunosuppressant drugs
- infants <1 year old
prevention of febrile non-haemolytic transfusion reactions
other
patients undergoing cardiac surgery.
Note: Where indicated and if available, prestorage leukodepleted products obtained from blood services are preferable to bedside leukodepletion; prestorage leukodepletion removes leucocytes prior to release of cytokines, which are responsible for adverse effects such as febrile non-haemolytic reactions.
Random-donor platelet concentrates are prepared from buffy coats and are not usually leukodepleted. Single donor (apheresis) platelet concentrates are routinely leukodepleted; the indications for this product are similar to those mentioned above for leukodepleted products.
Irradiated blood products
HIV-positive patients do not routinely require blood products to be irradiated. Blood products are irradiated to prevent transfusion-associated graft v. host disease (TA-GvHD). It has been postulated that, in patients with HIV infection, depletion of CD4 cells increases the number of donor cells needed to induce TA-GvHD. In HIV and AIDS, there has to date only been one reported case of TA-GvHD despite widespread use of blood transfusions in patients with profound HIV-associated immune suppression. The indications for irradiated blood products are the same for HIV-positive as for HIV-negative patients. Specific indications for irradiation include:
blood donations from blood relatives
HLA-matched platelet concentrates
recipients of allogeneic bone marrow transplant
Hodgkin’s disease
intrauterine transfusions
patients (haematological/non-haematological disorders) receiving Fludarabine therapy.
Note: Please refer to the Clinical Guidelines for the use of Blood Products in South Africa or your national guidelines for additional information.
Blood conservation strategies
Blood conservation (restrictive transfusion practice) is clinically effective in most patient subsets. In particular, the TRICC study76 showed that restrictive transfusion practice applied to critically ill adult patients was at least as effective and potentially superior to that of liberal transfusion practice in terms of lower morbidity and mortality.76 This was also shown in a major randomised control trial (PICU study) of stable, critically ill children.43
Low-cost and relatively simple preventative measures can be employed to minimise blood use. One example is that of judicious screening for anaemia with early intervention. All cases of clinically significant anaemia should be investigated, and the underlying cause addressed and appropriately managed. Early intervention is particularly important for surgical candidates where timely management of anaemia can help to minimise periopertaive transfusion.
Clinicians should employ alternatives to blood transfusion wherever appropriate e.g. haematinic therapy in chronic anaemia or the use of crystalloids/colloids to restore blood volume in resuscitation. Where indicated and where available, erythropoietin is another measure to be considered in patients refractory to standard therapy.6,9,77 Erythropoietin has been shown to benefit HIV-positive patients. In the critical care setting, excessive phlebotomy can exacerbate underlying anaemia; this can be avoided through considered testing, confined to that which directly influences patient management.
Good surgical and anaesthetic techniques, with particular attention to haemostasis and keeping patients warm, are essential transfusion conservation principles. Suitable alternatives or adjuncts to transfusion should be considered, e.g. anti-fibrinolytics and fibrin sealants. Bleeding, when it occurs, should be managed aggressively, avoiding a passive watch-and-wait approach. The use of medication that can impede haemostasis (e.g. anticoagulants, anti-platelet agents and non-steroidal anti-inflammatories) should be prescribed cautiously in the chronic patient at risk for bleeding, and stopped in the bleeding patient. The latter may require specific reversal of anticoagulation if bleeding does not stop with conservative management.
Pre-surgical autologous blood donation obtained from HIV-positive donors is not routinely available and should not be accepted. HIV-infected blood products pose significant risk, both to blood service staff as well as to other patients. Risk to other patients can occur through administrative or clerical error resulting in mis-transfusion (unintentional transfusion to the incorrect recipient).
Finally: acute normovolemic haemodilution and cell salvage are two intraoperative blood conservation techniques that can be used in HIV-positive surgical patients. These techniques should be considered particularly where significant blood loss is anticipated.
Lookback programmes
The Blood Service’s Transfusion Transmissible Infection (TTI) Lookback Programme aims to trace all patients who are identified as recipients of blood from donors who test positive for a transfusion-transmissible infection on a subsequent blood donation, where the initial (index) donation might possibly have been donated in a window period. In such a ‘donor-triggered’ lookback investigation, the recipient/s of the previous TTI negative units is/are identified and their treating doctor notified. As far as possible, the patient must be recalled, counselled and tested for the relevant viral marker, and the result reported to the blood service. Despite diligent donor selection and laboratory screening, suspicion may arise that a patient might have been infected with HIV, HBV or HCV through blood transfusion. In such instances, the attending clinician should contact the blood service promptly to initiate a ‘recipient-triggered’ lookback investigation, a formal procedure designed to trace and confirm the status of the implicated blood donor(s).
Haemovigilance programme
Haemovigilance is the process through which information related to the transfusion of blood and blood products is monitored and centrally reported. It is a system to detect, gather and analyse information on untoward and unexpected effects of the transfusion of blood and blood products. Such programmes aim to improve blood systems and blood safety through the early detection and comprehensive reporting of untoward effects of blood transfusion e.g. transfusion reactions, transfusion-transmitted infections, etc. Ideally, a haemovigilance programme is integrated into blood transfusion practice to maximise the safety of not only the blood supply, but also all aspects of laboratory and clinical blood transfusion practice. In some countries such as Namibia and South Africa, data reported to the National Haemovigilance Programme are analysed and the results published in an Annual Haemovigilance Report. It is important that medical practitioners who transfuse blood and blood products report all adverse transfusion events to the blood service.
Laboratory testing of donated blood
Serological tests are performed on every blood donation to determine the donor’s ABO group and Rh type and to detect irregular blood group antibodies. Every blood donation is tested for HIV, hepatitis B, hepatitis C and syphilis, using serological techniques. Nucleic acid testing (NAT) is used in combination with serological testing in a few well-resourced African countries. Clinicians should consider the following options when ordering blood for their patients:
Type and screen
The clinician should select this option if their patient has a low probability of needing a transfusion or in, for example, certain elective surgical procedures where the extent of blood loss is unpredictable. The blood specimen submitted to the blood bank will be tested to determine the patient’s ABO group and Rh type and will be tested to ensure that the patient does not have irregular blood group antibodies (a ‘rare blood type’) that could delay finding compatible blood. The specimen will be held for approximately 96 hours, depending on blood bank policy. Blood will only be crossmatched when requested by the attending doctor. The ‘type and screen’ expedites crossmatching and dispatch of blood from the blood bank should transfusion be required. If irregular antibodies are detected, the requesting doctor will be notified. The presence of irregular antibodies can delay procurement of compatible blood, and patient management needs to be changed accordingly. This could, for instance, necessitate delaying surgery.
• A full crossmatch refers to full compatibility testing between a patient’s blood sample (intended recipient) and a given donor (unit of blood). This includes the type and screen as described above as well as confirmatory blood grouping on the intended donor unit. In addition, the patient’s serum is ‘crossmatched’ with the red blood cells of the donor to ensure serological compatibility. Incompatibility between patient and donor is reflected by in vitro agglutination.
• In contrast, an emergency crossmatch refers to partial compatibility testing, given the urgent need to transfuse. Blood is issued after performing the ABO group, Rh type and antibody screen only. Further testing is completed after the unit has been issued.
Providing sufficient clinical detail, including the HIV status of the recipient, to the hospital blood bank staff, will expedite the crossmatching process and the timely availability of compatible blood. The direct antiglobulin test (DAT) is, for example, positive in up to 40% of HIV-positive patients. This will manifest as a positive crossmatch. Knowing the patient’s HIV status will therefore assist the blood bank’s medical and technical staff in interpreting the compatibility test results and, as indicated, expedite the release of compatible blood.
Adverse events associated with blood transfusion33
Evaluation of benefits and risks of transfusions should precede the decision to transfuse. All blood products carry risk of adverse effects. These include transfusion reactions, transfusion transmissible infections, alloimmunisation and immune modulation. The attending doctor must be familiar with best practice recommendations regarding transfusion practice, and is also responsible for obtaining and documenting informed consent.
Transfusion reactions are the most common hazard of blood transfusion, occurring with variable frequency depending on the type of reaction. Transfusion reactions fall broadly into the following categories:
haemolytic – acute
haemolytic – delayed (DHTR)
febrile non-haemolytic
allergic
anaphylactic
reactions due to bacterial contamination
reactions due to ‘citrate toxicity’
reactions due to circulatory overload (TACO)
transfusion-associated acute lung injury (TRALI)
transfusion-associated graft versus host disease (TA-GvHD).
Signs and symptoms suggestive of a transfusion reaction include:
chills/rigors
fever/sweating
tachycardia/bradycardia
dyspnoea/bronchospasm
hypertension/hypotension
urticaria/pruritus
chest/flank pain
nausea/vomiting
haemoglobinuria
oliguria/anuria
restlessness
jaundice.
If a transfusion reaction is suspected, the transfusion must be stopped immediately pending further evaluation. The administration set must be changed and venous access should be maintained with normal saline unless it is a simple urticarial reaction. If the latter is the case, the transfusion can continue with symptoms or after use of an antihistamine (such as diphenhydramine 12.5 - 25mg for an adult patient).
The following additional steps should be taken:
Both a member of the medical staff as well as the blood bank must be contacted immediately.
The medical management of the transfusion reaction will depend on the type and severity of the reaction.
The patient’s temperature, pulse, respirations and blood pressure must be recorded.
All clerical and identity checks must be repeated to ensure that the correct blood product was transfused to the intended patient. Clerical error is the foremost reason for major acute haemolytic transfusion reaction, i.e. blood given to the wrong patient.
If a case of misdirected transfusion is noted, immediate steps must be taken to locate the units originally intended for the patient, as they may be in the process of being transfused to another incorrect patient.
Send a fresh blood specimen for compatibility testing to the blood bank.
All empty and non-transfused blood units should be returned to the blood bank.
Popular misconceptions
An HIV-infected patient doesn’t warrant transfusion as the prognosis is poor anyway
Anecdotal reports of patients dying following transfusion led to the unjustifiable practice of withholding blood transfusion from HIV-positive patients. At the start of the HIV pandemic, patients presented with advanced disease and generally had little prospect of effective management. Today, with highly effective ART and prophylaxis, HIV is a chronic manageable disease with an excellent outcome. Transfusion best practice and a rational approach to the management of anaemia apply, independent of HIV status.
Moribund HIV+ patients require rapid correction of their anaemia
Again, best transfusion practice applies to the HIV-positive patient. Rapid correction of chronic anaemia increases the risk of transfusion-associated circulatory overload (TACO) and cardiac decompensation as would be encountered in any patient with severe chronic anaemia (see section on transfusion rates). HIV-positive patients with acute haemorrhage may require resuscitation and rapid transfusion, similarly to HIV-negative patients.
If a patient needs only one unit of blood, he/she does not need blood at all
The indication for transfusion is based on clinical symptoms and signs, and not on laboratory indices. Patients should be evaluated after each unit transfused. A single unit transfusion, in the right circumstances, may be sufficient to stabilise the patient. ‘Topping up’ the patient with additional blood after the indication for transfusion has been addressed, may confer additional unnecessary risk.
Table 4. Products, services and glossary.
| RED CELL PRODUCTS: STORE AT 1°C - 6°C. | ||||
|---|---|---|---|---|
| Definitions, products and services described below refer to those available in South Africa and may not be available in other countries and regions. | ||||
| Product | Average vol. | Average unit price incl. VAT (2012) | Characteristics | Major indications |
| Whole blood leucodepleted (<5 days old) |
485 ml | R2 499.00 | WBC: <5×l06/unit Leucocyte depleted at the time of processing. |
Indicated for neonatal exchange transfusion. |
| Red cell concentrate in additive solution |
300 ml | R1 369.00 | Buffy coat removed WBC: <2.4×l09/unit |
To increase tissue oxygenation owing to reduced haemoglobin concentration. |
| Red cell concentrate (leucodepleted) | 260 ml | R2 237.00 | WBC: <5×l06/unit Leucocyte depleted at the time of processing. |
See indications for leucodepleted products. |
| Red cell concentrate in additive solution (<5 days old) |
300 ml | R 1 484.00 | Buffy coat removed WBC: <2.4×l09/unit |
|
| Red cell concentrate (leucodepleted) (<5 days old) |
260 ml | R2 237.00 | WBC: <5×l06/unit Leucocyte depleted at the time of processing. |
Suitable for neonatal exchange transfusion. See indications for leucodepleted products. |
| Red cell concentrate paediatric leucodepleted |
75 ml | R1 265.00 | WBC: <5×l06/unit Leucocyte depleted at the time of processing. |
For paediatric use. |
|
| ||||
| PLATELET PRODUCTS: USE IMMEDIATELY AFTER ISSUE; DO NOT REFRIGERATE. | ||||
| Product | Average vol. | Average unit price incl. VAT (2012) | Characteristics | Major indications |
|
| ||||
| Platelet concentrate pooled non- leucodepleted |
250 ml | R5 769.00 | Platelets: ≥2.4×lO11/unit WBC: <5×108 /unit Prepared from buffy coat of 5 whole blood donations. Not leucodepleted. |
Clinically significant thrombocytopaenia or platelet function abnormalities. |
| Platelet concentrate leucodepleted (apheresis) |
200 ml | R7 936.00 | Platelets: ≥2.4×lO11/unit WBC: <5×l06/unit Prepared from a single donor by apheresis. If unavailable, leucodepleted pooled platelets will be supplied. |
See indications for leucodepleted products. |
| Platelet concentrate paediatric leucodepleted |
R1 741.18 | Platelets: ≥ 5.5×1010/unit WBC: <5×l06 /unit Prepared from a single donor by apheresis. |
For paediatric use. | |
|
| ||||
| PLASMA PRODUCTS - MUST BE TRANSFUSED IMMEDIATELY AFTER ISSUE. | ||||
| Product | Average vol. | Average unit price | Characteristics | Major Indications |
|
| ||||
| Cryoprecipitate | 30 ml | R774.00 | Fibrinogen content – >300 mg/unit | 1. Hypofibrinogenaemia 2. Factor XIII deficiency |
| Fresh frozen plasma – paediatric | 130 ml | R637.00 | Contains physiological levels of most clotting factors. NB: Freeze-dried plasma is used as an alternative. |
|
| FFP – cryo-poor | 250 ml | R883.79 | FFP from which the cryoprecipitate has been removed. Limited availability. |
May be indicated for TTP. |
| SPECIAL REQUESTS: CONTACT THE BLOOD BANK – ADVANCE NOTICE IS REQUIRED. | |||
|---|---|---|---|
| Service/procedure | Average unit cost |
Characteristics | Major indications |
| Irradiated products | R293.00 | For the prevention of transfusion-associated graft - versus-host disease. |
|
| HLA-matched platelet concentrate | R1 073.00 | HLA-matched single-donor apheresis platelet concentrate. |
Prevention and management of platelet refractoriness. |
| Autologous programmes | R150.00 | The collection, normal testing and processing of a patient’s own blood for him- or herself. |
For use in certain limited elective surgical cases in suitable patients |
| Directed programmes | R181.00 | Programme where family members or friends donate for a specific patient. Chosen donors have to meet the same criteria as normal donors and must have a compatible blood group. Blood donated by first-line blood relatives requires irradiation to prevent transfusion associated graft v. host disease. |
For use in certain limited cases. The blood must be tested and processed as usual, requiring 3 - 5 days before the unit is available for transfusion. |
| Washed products | R1 051.00 | The product is suspended in isotonic saline and centrifuged; the saline from the first saline ‘wash’ is removed, and the red cells re-suspended in isotonic saline. |
As washed cells are manipulated in an open system, with a possibility of bacterial contamination, they must be transfused within 24 hours of preparation. |
| Cryo-preserved cells | ~R7 400.00 | The storage of frozen rare donations for use locally and internationally. |
As washed cells are manipulated in an open system, with a possibility of bacterial contamination, they must be transfused within 24 hours of preparation. |
| Leucocyte depleted (leucodepleted) products |
Included in cost of product |
Filtered under laboratory conditions. This ensures optimal removal of leucocytes to minimise cytokine release. Leucocyte depletion will result in a leucocyte count <5×l06 per unit and usually <1×l06 per unit. |
|
| TYPES OF CROSSMATCH | |||
| Test | Time-frame | Average cost | Comments |
| Type and screen | N/A | R272.00 | The specimen will be grouped and tested to ensure that it does not contain antibodies that could delay finding compatible blood. The specimen will be held for 96 hours. Blood will only be crossmatched when requested by the attending doctor. |
| Standard crossmatch | Within 2 hours | R609.00 | Crossmatched products will be held in reserve for 24 hours unless otherwise indicated by the attending doctor. Crossmatched products not collected, will incur the fee. |
| Emergency crossmatch | 20 - 30 minutes | R114.00 | Blood issued on emergency or without a compatibility test is transfused at the attending doctor’s own responsibility. There are risks involved in emergency procedures – use them only for emergencies. |
| Uncrossmatched blood | 5-10 minutes | R136.00 | Blood issued on emergency or without a compatibility test is transfused at the attending doctor’s own responsibility. There are risks involved in emergency procedures – use them only for emergencies. |
| GENERAL | |||
| Blood on returnable basis (BRB) | Blood is transported in a temperature-controlled hamper. Provided the blood is returned within 10 hours of issue, remains sealed in the hamper, and the temperature of the hamper does not exceed 10°C, the fee for the blood will fall away. However, the service and laboratory test charge will be levied. |
||
| Informed consent | As with any treatment, the patient has the right to decide whether or not to accept the treatment. As far as possible, the patient should understand the benefits, risks and alternatives to transfusion as explained by the prescribing doctor. It is recommended that transfusion transmissible infections and receiving of incorrect products be mentioned specifically. Informed consent is a process which must be acknowledged and documented. |
||
| Blood administration set | For the infusion of whole blood and red cell concentrate. | ||
| Platelet administration set | For the infusion of platelets. | ||
| Blood pack without anticoagulant | For therapeutic venesections. | ||
| Blood pack with anticoagulant | For blood salvage and subsequent autologous reinfusion. | ||
| Voluntary donor | A person who donated blood or a blood components without compensation. | ||
| Recipient | A person who receives blood or blood products. | ||
| Look-back programme | A formal process for the identification of donors and recipients who test positive for viral markers after having donated or receiving, respectively, a unit which at the time of issue tested negative. In cases where the recipient tests positive, the donor/s of the original unit/s are traced and tested to confirm whether the infection was transmitted via the transfusion. In cases where the donor tests positive on a subsequent unit, the recipients of the previous unit are traced and tested to confirm whether or not the infection was transmitted through transfusion of blood or blood products |
||
| Haemovigilance | A system to detect, gather and analyse information on untoward and unexpected effects of the transfusion of blood and blood products | ||
Appendix 1: Legal and human rights considerations
The use of blood and blood products raises two broad categories of legal and human rights considerations: the rights of donors and the rights of recipients. Much of the debate regarding blood, blood products and HIV has historically focused on the rights of people to be protected from infection; very little focus has been placed on the rights of people living with HIV insofar as access to blood and blood products is concerned. This appendix expands on both categories of rights bearers – including the legal requirements of informed consent – as well as the issue of confidentiality as it relates to the communication of information on a patient’s HIV status.
Informed consent
South African law has recognised the concept of informed consent for many years.1,2, The right to informed consent has been fleshed out by way of case law,3 regulatory council guidelines,4 the Patients’ Rights Charter, and legislation.5 In particular, the National Health Act 61 of 2003 (NHA) has codified the law on informed consent and defines it as consent for the provision of a specific health service; the Act further stipulates that informed consent may only be provided by a person with legal capacity to do so, and that the person providing consent has been adequately informed.6
The law on informed consent is based on the notion that a patient – referred to in the NHA as a user – has the right to participate in any decision affecting his or her personal health and treatment.7 If, however, the circumstances prevent the user from making the decision himself or herself, the NHA provides guidance on how to proceed.8
Confidentiality
The communication of any information pertaining to a patient’s HIV status, whether positive or negative, is subject to the confidentiality provisions in the NHA.9 Section 14 of that statute makes it clear that ‘[a]ll information concerning a user, including information relating to his or her health status ... is confidential.’ This guarantee is subject to the provisions of Section 15, which deals with access to health records. Simply put, Section 15(1) ensures that the guarantee of confidentiality should not stand in the way of running an efficient and effective blood service, including the appropriate handling of blood and blood products.
The rights of donors
In considering the rights of donors, two key questions arise: (i) Is there a right to donate blood?; and (ii) can blood services be used as a testing facility to determine an individual’s HIV status?
Is there a right to donate blood?
There is no right to donate blood. On the contrary, blood services are constitutionally obliged to take all reasonable steps to ensure a safe supply of blood and blood products. By necessity, this implies refusal to accept as donors those at high risk of carrying a transfusion-transmissible pathogen (such as HIV). However, the manner in which donors are treated may not result in the violation of fundamental constitutional rights and values. The need to protect public health cannot be done in a manner that unreasonably and unjustifiably limits rights. If it did, potential donors would have legal recourse to vindicate their rights.
Can blood services be used as a testing site to test for HIV?
While potential donors should be discouraged from using blood donation services for the purpose of establishing their HIV status, there is nothing in the law that can be used to prevent this from happening. Both donor education and evidence-based self-exclusion questionnaires serve as appropriate means to discourage and defer those at risk of HIV infection from donating blood. Provision of alternative HIV counselling and testing services also help to prevent the blood collection centre from being used as a default testing site. This relies on the premise that alternative testing services, of high quality, rendered in a non-discriminatory way, are available outside of HIV-specific service points and are either free or affordable at point of delivery.
The rights of recipients
In considering the rights of recipients, two key issues arise: (i) the right to have access to safe blood and blood products; and (ii) the rights of terminally ill patients.
Right to have access to blood and blood products
The right to have access to health services, which includes access to blood and blood products, is guaranteed in Section 27 of the Constitution. The central issue is whether access to blood and blood products can be denied solely on the basis of HIV status. This raises concerns of health rights as outlined in Section 27; it also breeches the constitutional guarantee of equality and protection against unfair discrimination. In this regard, two Constitutional Court decisions are relevant.10,11, Read together, the cases are clear: given that HIV infection is a chronic manageable condition for those with access to appropriate treatment and care, it would not be reasonable to limit access on the basis of HIV status alone.12
The right of access to safe and adequate blood and blood products obligates blood services to take all reasonable measures to ensure that such products are indeed available and safe for use; such measures do not require a blood service that is 100% safe, as the technology to ensure this does not exist. Where people have been exposed to unsafe blood and/or blood products despite blood services having acted reasonably, the latter cannot be held liable for any resultant harm. On the other hand, donors who have acted unreasonably – such as by misrepresenting their actual risk of infection in self-exclusion questionnaires – may indeed be sued for damages.13 In addition to civil liability, such a donor may also be criminally liable.
Rights of terminally ill patients
The right to have access to health care services does not impose an obligation on the state – and those who provide public services – to ensure that everyone receives every health service they need. Instead, it is an obligation to ‘take reasonable legislative and other measures, within … available resources, to achieve the progressive realisation of [the right]’.14 This was made clear in Soobramoney v Minister of Health, KwaZulu-Natal.15 In its judgment on Mr Soobramoney’s appeal, the Constitutional Court adjudicated the claim on the basis of the State’s positive obligations under Section 26(2), holding that the guidelines according to which access was limited were reasonable and had been applied ‘fairly and rationally’.16 In the result, the State had complied with its Section 27(2) obligations.
The primary responsibilities of the physician are to assist the patient in maintaining an optimal quality of life by controlling symptoms and addressing psychosocial needs, as well as enabling the patient to die with dignity and in comfort.17 It is considered ethically justifiable to discontinue life-sustaining treatment if the patient has the ability to make that decision, fully understands its consequences, and states that they no longer wish to continue treatment. A decision to withhold or withdraw life-prolonging treatment should be only be made by the senior clinician in charge of a patient’s care, informed by the patient’s views or those closest to him or her.18
There may be circumstances when withholding treatment, even if it is not requested by the patient, may be permissible. This may apply, for example, in cases akin to that of Soobramoney. Ordinarily, it would not be justifiable to discontinue life-sustaining treatment for cost reasons alone. That said, there may be cases in which the costs expended on one terminally ill patient could be better used on another patient with an improved outlook.19 In such circumstances, a health care facility may have the right to limit access to life-sustaining interventions, provided that such a limitation is based on reasonable national admission criteria developed by expert professional bodies, such as the HPCSA.20
APPENDIX 1 CITATIONS
- 1.1923 CPD 128.
- 2.Meerkotter Anneke. The Rights and Duties of Users of the Health Care System. In: Hassim Adila, et al., editors. Health & Democracy: A Guide to Human Rights, Health Law and Policy in Post-Apartheid South Africa. Siber Ink; Cape Town: 2007. p. 249. [Google Scholar]
- 3.See, for example, Castell v de Greef 1994 (4) SA 320 (C); C v Minister of Correctional Services 1996 (4) SA 292 (T).
- 4.Such as the Health Professions Council Guideline for Good Practice in Medicine, Dentistry and Medical Sciences
- 5.In particular, consider the Children’s Act 38 of 2005 and the National Health Act 61 of 2003.
- 6.Above note 2 at page 252.
- 7.This is codified in section 8 of the NHA.
- 8.See generally sections 7 to 9 of the NHA (in Chapter 2 entitled Rights and Duties of Users and Health Care Personnel). See also sections 129 and 130 of the Children’s Act.
- 9.As is the case with the provisions of the NHA dealing with informed consent, the NHA also codifies the common law – decisions of the courts – on confidentiality. This is underpinned by the constitutional guarantee of privacy.
- 10.2001 (1) SA 1 CC.
- 11.2004 (6) SA 505 (CC).
- 12.This position is supported by the ISBT Code of Ethics for Blood Donation and Transfusion which states that ‘blood is a public resource and access should not be restricted’ (see above note 10 at paragraph 10).
- 13.In Canadian Blood Services/Société Canadienne du Sang v Freeman [2010] ONSC 4885), a gay man who knowingly provided false information on a self-exclusion questionnaire was successfully sued by the blood services for negligent misrepresentation. His blood sample, which was tested and resulted in a false negative for syphilis, subsequently tainted an entire unit of blood. It is likely that the case, if decided under our Constitution, would have been decided differently. However, the principle of negligent (material) misrepresentation would stand.
- 14.Section 27(2) of the Constitution.
- 15.1998 (1) SA 765 (CC).
- 16.At paragraph 25.
- 17.At principle 5.
- 18.At section 3.
- 19.See section 9.1.
- 20.At section 9.2. See also paragraph 3 of the preamble to the World Medical Association (WMA) Declaration on Terminal Illness, first adopted by the 35th World Assembly (October 1983) and revised by the WMA General Assembly in October 2006. That preamble recognises that many palliative and life-sustaining measures require technologies and/or financial resources that are simply not available globally. The declaration is available at http://www.wma.net/en/30publications/10policies/i2/.
Footnotes
Dr Vis Poovalingam, who had a critical role in motivating for this review, is acknowledged for her valuable contribution.
Disclaimer
Specific recommendations provided in this document are intended only as a guide to clinical therapy, based on expert consensus and best current evidence. Treatment decisions for patients should be made by their responsible clinicians, with due consideration for individual circumstances. The most current peer-reviewed literature, reference text books and local guidelines should always be consulted.
REFERENCES AND RECOMMENDED READING
- 1.Beal RW. The rational use of blood. Australian And New Zealand Journal of Surgery. 1976;46:309–313. doi: 10.1111/j.1445-2197.1976.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 2.Evans RH, Scadden DT. Haematological aspects of HIV infection. Baillière’s Best Practice & Research Clinical Haematology. 2000;13:215–230. doi: 10.1053/beha.1999.0069. [DOI] [PubMed] [Google Scholar]
- 3.Volberding PA, Baker KR, Levine AM. Human immunodeficiency virus hematology. Hematology/The Education Program Of The American Society Of Hematology American Society Of Hematology Education Program. 2003:294–313. doi: 10.1182/asheducation-2003.1.294. [DOI] [PubMed] [Google Scholar]
- 4.Claster S. Biology of anemia, differential diagnosis, and treatment options in human immunodeficiency virus infection. J Infect Dis. 2002;185(Suppl 2):S105–S109. doi: 10.1086/340202. [DOI] [PubMed] [Google Scholar]
- 5.Sloand E. Hematologic complications of HIV infection. AIDS Reviews. 2005;7:187–196. [PubMed] [Google Scholar]
- 6.Sullivan P. Associations of anemia, treatments for anemia, and survival in patients with human immunodeficiency virus infection. J Infect Dis. 2002;185(Suppl 2):S138–S142. doi: 10.1086/340203. [DOI] [PubMed] [Google Scholar]
- 7.van Schalkwyk WA, Opie J, Novitzky N. The diagnostic utility of bone marrow biopsies performed for the investigation of fever and/or cytopaenias in HIV-infected adults at Groote Schuur Hospital, Western Cape, South Africa. International Journal of Laboratory Hematology. 2011;33:258–266. doi: 10.1111/j.1751-553X.2010.01280.x. [DOI] [PubMed] [Google Scholar]
- 8.Mocroft A, Kirk O, Barton SE, et al. EuroSIDA study group Anaemia is an independent predictive marker for clinical prognosis in HIV-infected patients from across Europe. AIDS (London, England) 1999;13:943–950. doi: 10.1097/00002030-199905280-00010. [DOI] [PubMed] [Google Scholar]
- 9.Moore RD, Keruly JC, Chaisson RE. Anemia and survival in HIV infection. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1998;19:29–33. doi: 10.1097/00042560-199809010-00004. [DOI] [PubMed] [Google Scholar]
- 10.Dainiak N, Worthington M, Riordan MA, Kreczko S, Goldman L. 3′-Azido-3′-deoxythymidine (AZT) inhibits proliferation in vitro of human haematopoietic progenitor cells. Br J Haematol. 1988;69:299–304. doi: 10.1111/j.1365-2141.1988.tb02366.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiragga AN, Castelnuovo B, Nakanjako D, Manabe YC. Baseline severe anaemia should not preclude use of zidovudine in antiretroviral-eligible patients in resource-limited settings. Journal of The International AIDS Society. 2010;13:42. doi: 10.1186/1758-2652-13-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lawrie D, Coetzee LM, Becker P, Mahlangu J, Stevens W, Glencross DK. Local reference ranges for full blood count and CD4 lymphocyte count testing. S Afr Med J. 2009;99:243–248. [PubMed] [Google Scholar]
- 13.Lotspeich-Steininger C, Stiene-Martin E, Koepke J, editors. Clinical Hematology: Principles, Procedures, Correlations. Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- 14.Afacan YE, Hasan MS, Omene JA. Iron deficiency anemia in HIV infection: immunologic and virologic response. Journal of The National Medical Association. 2002;94:73–77. [PMC free article] [PubMed] [Google Scholar]
- 15.Carmel R. Treatment of severe pernicious anemia: no association with sudden death. Am J Clin Nutr. 1988:48. doi: 10.1093/ajcn/48.6.1443. [DOI] [PubMed] [Google Scholar]
- 16.McMullin MF, Cuthbert RJ. Transfusion in the management of patients with megaloblastic anaemia. Int J Clin Pract. 1999;53:104–106. [PubMed] [Google Scholar]
- 17.Volberding P, Anemia in HIV Working Group Consensus statement: anemia in HIV infection--current trends, treatment options, and practice strategies. Clin Ther. 2000;22:1004–1020. doi: 10.1016/s0149-2918(00)80081-8. [DOI] [PubMed] [Google Scholar]
- 18.Volberding PA, Levine AM, Dieterich D, Mildvan D, Mitsuyasu R, Saag M. Anemia in HIV infection: clinical impact and evidence-based management strategies. Clin Infect Dis. 2004;38:1454–1463. doi: 10.1086/383031. [DOI] [PubMed] [Google Scholar]
- 19.Telen MJ, Roberts KB, Bartlett JA. HIV-associated autoimmune hemolytic anemia: report of a case and review of the literature. J Acquir Immune Defic Syndr. 1990;3:933–937. [PubMed] [Google Scholar]
- 20.Saif MW. HIV-associated autoimmune hemolytic anemia: an update. AIDS Patient Care And Stds. 2001;15:217–224. doi: 10.1089/10872910151133783. [DOI] [PubMed] [Google Scholar]
- 21.Salama A, Berghöfer H, Mueller-Eckhardt C. Red blood cell transfusion in warm-type autoimmune haemolytic anaemia. Lancet. 1992;340:1515–7. doi: 10.1016/0140-6736(92)92766-9. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PS, Hanson DL, Chu SY, Jones JL, Ciesielski CA. Surveillance for thrombocytopaenia in persons infected with HIV: results from the multistate Adult and Adolescent Spectrum of Disease Project. Journal Of Acquired Immune Deficiency Syndromes And Human Retrovirology. 1997;14:374–379. doi: 10.1097/00042560-199704010-00011. [DOI] [PubMed] [Google Scholar]
- 23.Coyle TE. Hematologic complications of human immunodeficiency virus infection and the acquired immunodeficiency syndrome. Med Clin North Am. 1997;81:449–470. doi: 10.1016/s0025-7125(05)70526-5. [DOI] [PubMed] [Google Scholar]
- 24.Passos AM, Treitinger A, Spada C. An overview of the mechanisms of HIV-related thrombocytopaenia. Acta Haematologica. 2010;124:13–18. doi: 10.1159/000313782. [DOI] [PubMed] [Google Scholar]
- 25.Scaradavou A. HIV-related thrombocytopaenia. Blood Reviews. 2002;16:73–76. doi: 10.1054/blre.2001.0188. [DOI] [PubMed] [Google Scholar]
- 26.Miguez-Burbano MJ, Jackson J, Jr, Hadrigan S. Thrombocytopaenia in HIV disease: clinical relevance, physiopathology and management. Current Medicinal Chemistry Cardiovascular and Hematological Agents. 2005;3:365–376. doi: 10.2174/156801605774322364. [DOI] [PubMed] [Google Scholar]
- 27.Burbano X, Miguez MJ, Lecusay R, et al. Thrombocytopaenia in HIV-infected drug users in the HAART era. Platelets. 2001;12:456–461. doi: 10.1080/09537100120093956. [DOI] [PubMed] [Google Scholar]
- 28.Ehmann WC, Rabkin CS, Eyster ME, Goedert JJ, Multicenter Hemophilia Cohort study Thrombocytopaenia in HIV-infected and uninfected hemophiliacs. Am J Hematol. 1997;54:296–300. doi: 10.1002/(sici)1096-8652(199704)54:4<296::aid-ajh6>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 29.Trattner A, Hodak E, David M, Sandbank M. The appearance of Kaposi sarcoma during corticosteroid therapy. Cancer. 1993;72:1779–1783. doi: 10.1002/1097-0142(19930901)72:5<1779::aid-cncr2820720543>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 30.Novitzky N, Thomson J, Abrahams L, du Toit C, McDonald A. Thrombotic thrombocytopenic purpura in patients with retroviral infection is highly responsive to plasma infusion therapy. Br J Haematol. 2005;128:373–379. doi: 10.1111/j.1365-2141.2004.05325.x. [DOI] [PubMed] [Google Scholar]
- 31.Moore RD, Keruly JC, Chaisson RE. Neutropaenia and bacterial infection in acquired immunodeficiency syndrome. Arch Intern Med. 1995;155:1965–1970. [PubMed] [Google Scholar]
- 32.Murphy MF, Metcalfe P, Waters AH, et al. Incidence and mechanism of neutropaenia and thrombocytopaenia in patients with human immunodeficiency virus infection. Br J Haematol. 1987;66:337–340. doi: 10.1111/j.1365-2141.1987.tb06920.x. [DOI] [PubMed] [Google Scholar]
- 33.Medical Directors of the South African Blood Transfusion Services . Clinical Guidelines for the use of Blood Products in South Africa. Fourth ed 2008. [Google Scholar]
- 34.World Health Organization . Guidelines for the Management of Common Illnesses with Limited Resources. 2005. Pocket book of hospital care for children. [PubMed] [Google Scholar]
- 35.Greenberg AE, Nguyen-Dinh P, Mann JM, et al. The association between malaria, blood transfusions, and HIV seropositivity in a pediatric population in Kinshasa, Zaire. JAMA. 1988;259:545–549. [PubMed] [Google Scholar]
- 36.English M, Ahmed M, Ngando C, Berkley J, Ross A. Blood transfusion for severe anaemia in children in a Kenyan hospital. Lancet. 2002;359:494–495. doi: 10.1016/S0140-6736(02)07666-3. [DOI] [PubMed] [Google Scholar]
- 37.Obonyo CO, Steyerberg EW, Oloo AJ, Habbema JD. Blood transfusions for severe malaria-related anemia in Africa: a decision analysis. Am J Trop Med Hyg. 1998;59:808–812. doi: 10.4269/ajtmh.1998.59.808. [DOI] [PubMed] [Google Scholar]
- 38.Thejpal R. Unravelling the Cytopaenias in HIV Infected Children. SAPA/IPA; Johannesburg: 2010. [Google Scholar]
- 39.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 40.Cheema B, Molyneux EM, Emmanuel JC, et al. Development and evaluation of a new paediatric blood transfusion protocol for Africa. Transfusion Medicine (Oxford, England) 2010;20:140–151. doi: 10.1111/j.1365-3148.2010.00989.x. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization . The Clinical Use of Blood in Medicine, Obstetrics, Paediatrics, Surgery & Anaesthesia, Trauma & Burns. World Health Orbanization; Geneva: 2001. [Google Scholar]
- 42.Hébert PC, Wells G, Tweeddale M, et al. Transfusion Requirements in Critical Care (TRICC) Investigators. the Canadian Critical Care Trials Group Does transfusion practice affect mortality in critically ill patients? Am J Respir Crit Care Med. 1997;155:1618–1623. doi: 10.1164/ajrccm.155.5.9154866. [DOI] [PubMed] [Google Scholar]
- 43.Lacroix J, Hébert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. New Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 44.Kirpalani H, Whyte RK, Andersen C, et al. The premature infants in need of transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 45.Gibson BES, Todd A, Roberts I, et al. Transfusion guidelines for neonates and older children. Br J Haematol. 2004;124:433–453. doi: 10.1111/j.1365-2141.2004.04815.x. [DOI] [PubMed] [Google Scholar]
- 46.Hu K, Harrison LB. Impact of anemia in patients with head and neck cancer treated with radiation therapy. Curr Treat Options Oncol. 2005;6:31–45. doi: 10.1007/s11864-005-0011-4. [DOI] [PubMed] [Google Scholar]
- 47.Fenton PM. Managing situations of acute blood loss with limited resources. Transfusion Alternatives in Transfusion Medicine. 2008;10:82–89. [Google Scholar]
- 48.Safe Transfusion Practice in Sub-Saharan Africa A Manual for Field Workers. 2010 http://www.safetransfusionmanual.org/2010/03/indications-surgical-patient.html.
- 49.Spence RK, Carson JA, Poses R, et al. Elective surgery without transfusion: influence of preoperative hemoglobin level and blood loss on mortality. Am J Surg. 1990;159:320–324. doi: 10.1016/s0002-9610(05)81227-9. [DOI] [PubMed] [Google Scholar]
- 50.Carson JL, Noveck H, Berlin JA, Gould SA. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 51.Nelson L, Fried M, Stewart K. The risks of surgery in HIV-infected patients. J Perioper Pract. 2007;17:470. doi: 10.1177/175045890701701003. [DOI] [PubMed] [Google Scholar]
- 52.Avidan MS, Jones N, Pozniak AL. The implications of HIV for the anaesthetist and the intensivist. Anaesthesia. 2000;55:344–354. doi: 10.1046/j.1365-2044.2000.01265.x. [DOI] [PubMed] [Google Scholar]
- 53.Prout J, Agarwal B. Anaesthesia and critical care for patients with HIV infection. Continuing Education in Anaesthesia. Critical Care & Pain. 2005;5:153–156. [Google Scholar]
- 54.Committee for Confidential Enquiry into Maternal Mortality in South Africa . Confidential Enquiry into Maternal Mortality in South Africa. Department of Health; Pretoria: 2006. Saving Mothers Report, 2002-2004. [Google Scholar]
- 55.Blood Transfusion Committee Statistics. Chris Hani Baragwanath Hospital Blood Transfusion Committee; Johannesburg: 2010. [Google Scholar]
- 56.Hytten F. Blood volume changes in normal pregnancy. Clinics In Haematology. 1985;14:601–612. [PubMed] [Google Scholar]
- 57.Rose NC, Mennuti MT. Periconceptional folate supplementation and neural tube defects. Clin Obstet Gynecol. 1994;37:605–620. doi: 10.1097/00003081-199409000-00013. [DOI] [PubMed] [Google Scholar]
- 58.van den Broek NR, Letsky EA. Etiology of anemia in pregnancy in south Malawi. Am J Clin Nutr. 2000;72:247S–256S. doi: 10.1093/ajcn/72.1.247S. [DOI] [PubMed] [Google Scholar]
- 59.Prevention of Mother to Child Transmission. South African National Aids Council; Pretoria: 2010. [Google Scholar]
- 60.Bendich A. Calcium supplementation and iron status of females. Nutrition. 2001;17:46–51. doi: 10.1016/s0899-9007(00)00482-2. [DOI] [PubMed] [Google Scholar]
- 61.Hallberg L, Brune M, Rossander L. Iron absorption in man: ascorbic acid and dose-dependent inhibition by phytate. Am J Clin Nutr. 1989;49:140–144. doi: 10.1093/ajcn/49.1.140. [DOI] [PubMed] [Google Scholar]
- 62.Snower D, Weil S. Changing aetiology of macrocytosis: zidovudine as a frequent causative factor. Am J Clin Pathol. 1993;99:57–60. doi: 10.1093/ajcp/99.1.57. [DOI] [PubMed] [Google Scholar]
- 63.Blood Transfusion in Obstetrics. Royal College of Obstetricians and Gynaecologists; London: 2007. [Google Scholar]
- 64.Balakrishnan A, Valsalan R, Sheshadri S, Pandit VR, Medep V, Agrawal RK. Zidovudine-induced reversible pure red cell aplasia. Indian Journal Of Pharmacology. 2010;42:189–191. doi: 10.4103/0253-7613.66845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Practice guidelines for obstetric anesthesia: an updated report by the American Society of Anesthesiologists Task Force on Obstetric Anesthesia. Anesthesiology. 2007;106:843–863. doi: 10.1097/01.anes.0000264744.63275.10. [DOI] [PubMed] [Google Scholar]
- 66.Cohen C, Karstaedt A, Frean J, et al. Increased prevalence of severe malaria in HIV-infected adults in South Africa. Clin Infect Dis. 2005;41:1631–1637. doi: 10.1086/498023. [DOI] [PubMed] [Google Scholar]
- 67.Malaria and HIV/AIDS Interactions and Implications: Conclusions of a Technical Consultation convened by WHO. World Health Organization; Geneva: 2004. [Google Scholar]
- 68.Meremikwu M, Smith HJ. Blood transfusion for treating malarial anaemia. Cochrane Database Of Systematic Reviews (Online) 2000:CD001475. doi: 10.1002/14651858.CD001475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tolentino K, Friedman JF. An update on anemia in less developed countries. Am J Trop Med Hyg. 2007;77:44–51. [PubMed] [Google Scholar]
- 70.Davenport GC, Ouma C, Hittner JB, et al. Hematological predictors of increased severe anemia in Kenyan children coinfected with Plasmodium falciparum and HIV-1. Am J Hematol. 2010;85:227–233. doi: 10.1002/ajh.21653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gwer S, Newton CRJC, Berkley JA. Over-diagnosis and co-morbidity of severe malaria in African children: a guide for clinicians. Am J Trop Med Hyg. 2007;77:6–13. [PMC free article] [PubMed] [Google Scholar]
- 72.Guidelines for the Treatment of Malaria 2009 . Department of Health; Pretoria: 2009. [Google Scholar]
- 73.Collier AC, Kalish LA, Busch MP, et al. Leukocyte-reduced red blood cell transfusions in patients with anemia and human immunodeficiency virus infection: the Viral Activation Transfusion Study: a randomized controlled trial. JAMA. 2001;285:1592–1601. doi: 10.1001/jama.285.12.1592. [DOI] [PubMed] [Google Scholar]
- 74.Drew WL, Chou S, Mohr BA, et al. Absence of activation of CMV by blood transfusion to HIV-infected, CMV-seropositive patients. Transfusion. 2003;43:1351–1357. doi: 10.1046/j.1537-2995.2003.00520.x. [DOI] [PubMed] [Google Scholar]
- 75.Buskin SE, Sullivan PS. Anemia and its treatment and outcomes in persons infected with human immunodeficiency virus. Transfusion. 2004;44:826–832. doi: 10.1111/j.1537-2995.2004.03359.x. [DOI] [PubMed] [Google Scholar]
- 76.Hébert PC, Wells G, Blajchman MA, et al. Canadian Critical Care Trials Group A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care Transfusion Requirements in Critical Care Investigators. New Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 77.Moore RD. Anemia and human immunodeficiency virus disease in the era of highly active antiretroviral therapy. Semin Hematol. 2000;37:18–23. doi: 10.1016/s0037-1963(00)90064-7. [DOI] [PubMed] [Google Scholar]