Summary
It is now known that cells do not always die by apoptosis, but can also undergo a process known as programmed cell necrosis, or necroptosis. In a study recently published in Science Advances, investigators reported that this proinflammatory process is active in human atherosclerosis, may promote growth of the necrotic core, and may serve as a novel molecular imaging and translational therapeutic target. These findings represent a major step in our goal to reduce coronary disease and stroke.
Key Words: atherosclerosis, efferocytosis, necroptosis
Graphical abstract
It has been estimated that the human body turns over billions of cells per day. Most of these cells die via a noninflammatory process known as programmed cell death, or apoptosis. Under physiological conditions, these cells are recognized and cleared via a highly efficient process known as programmed cell removal, or efferocytosis. This process occurs in what has been described as an immunologically-silent manner, with the vast majority of apoptotic cells being phagocytosed before they have a chance to undergo secondary necrosis. For these reasons, investigators have long wondered why necrotic debris accumulates during atherogenesis, and studies have investigated whether efferocytosis may be defective as plaques develop 1, 2, 3.
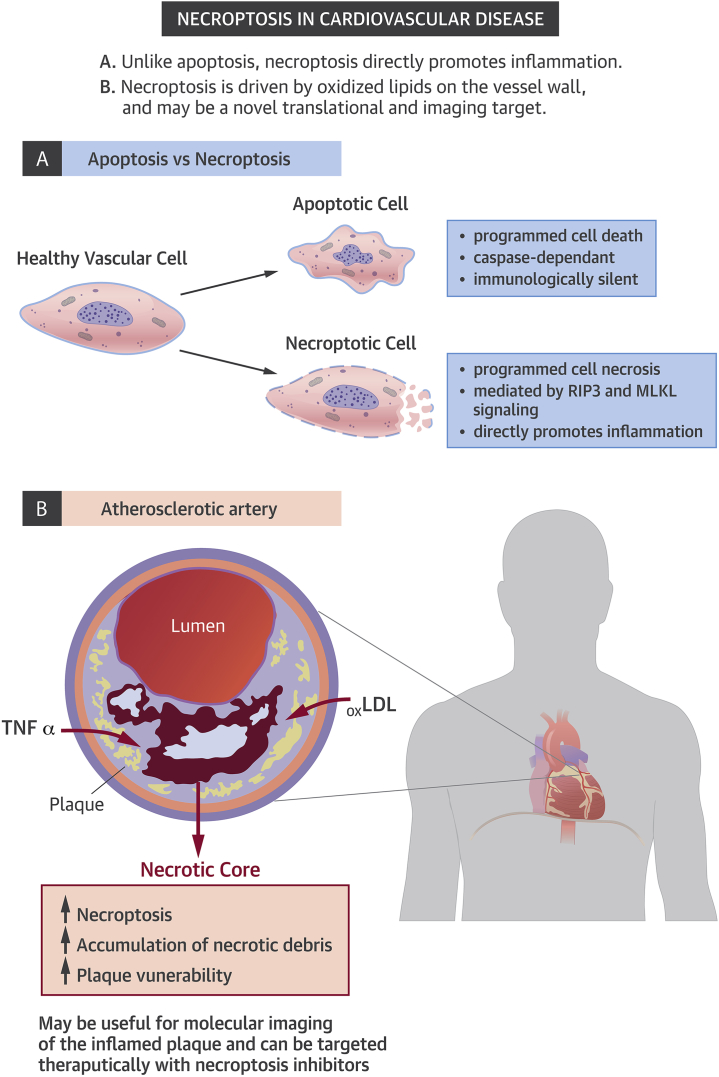
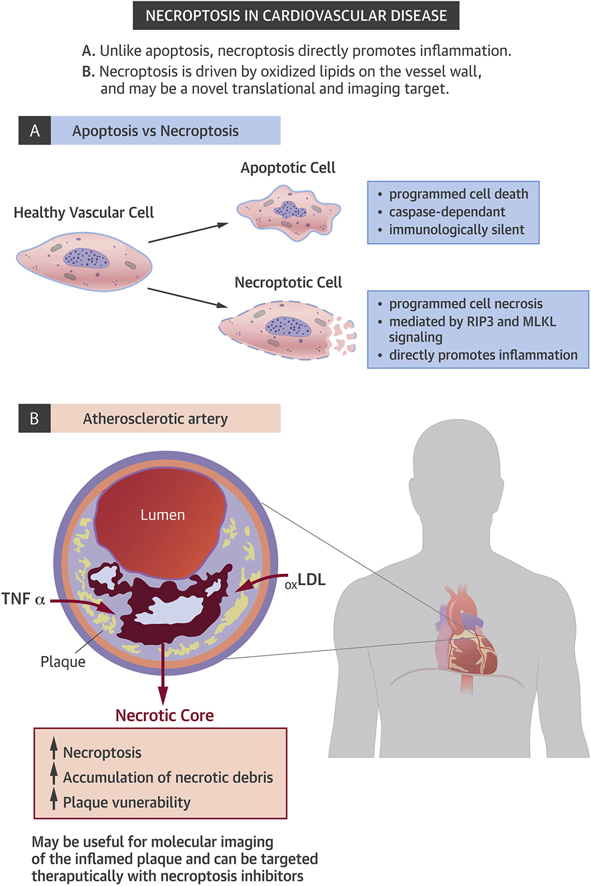
It is now known, however, that apoptosis is not the only way in which a cell can commit suicide. A process known as programmed cell necrosis, or “necroptosis,” has recently been described (4). Unlike apoptosis, necroptosis appears to result in an inflammatory form of cell death, which may have evolved as a host defense against viral infection or other forms of cellular injury. This process occurs when proapoptotic caspase enzymes are exhausted or inhibited, and leads to the breakdown of plasma membranes, which causes the release of previously sequestered proinflammatory intracellular contents. Emerging studies have suggested that necroptosis may play a role in a number of inflammatory conditions and human diseases. In a study recently published by Karunakaran et al. (5) in Science Advances, the authors asked whether this process may also be active in human atherosclerotic disease, and if it might serve as a novel translational target (Figure 1).
Figure 1.
Necroptosis in Cardiovascular Disease
LDL = low-density lipoprotein; TNF = tumor necrosis factor.
The authors began by showing that the critical pronecroptotic factors RIP3 and MLKL are present and activated in human atherosclerotic plaque. Moreover, they found that the factors are preferentially up-regulated in subjects with unstable compared with stable disease (e.g., in carotid surgery patients who presented with stroke or TIA as opposed to those with incidentally-detected stenoses). Together, these data provide the first evidence that necroptotic pathways are associated with human vascular disease and may correlate with lesion vulnerability.
Next, the authors explain the mechanism by which necroptosis is activated in cardiovascular disease by showing that oxidized low-density lipoprotein directly stimulates pronecroptotic signaling cascades, including phosphorylation of the canonical factor RIP3. This pathway is shown to involve reactive oxygen species and damage-associated molecular patterns, which in turn render these cells resistant to efferocytic clearance. These findings are important as they link causal pro-atherosclerotic factors that are known to be up-regulated in the diseased vessel wall (such as dyslipidemia and oxidative stress) to signaling that promotes necroptosis, beyond their effects on apoptosis.
In vivo, a novel radiotracer developed with a factor that targets the necroptotic pathway was shown to specifically localize to the atherosclerotic plaque in mouse models of vascular disease. Finally, the authors confirmed that an orally-available necroptosis inhibitor known as necrostatin-1 promoted features of plaque stability in atheroprone Apoe−/− mice who were fed a high-fat diet, and even reduced plaque size in animals with established lesions. These findings are of interest because the therapy appears to be nontoxic and is able to reduce the size of the necrotic core without having an effect on plasma lipid levels.
Translational Relevance
Although great strides have been made with risk factor-reducing approaches such as lipid lowering, antiplatelet, and antihypertensive therapies, there is a need to understand the root cause and pathobiology of atherosclerosis to develop novel translational therapies for myocardial infarction and stroke. The finding that necroptosis is active in the vessel wall of humans and may promote the growth of the necrotic core represents an important step forward. Of interest, there is currently an ongoing study examining the molecular triggers of necroptosis in human neutrophils of healthy volunteers (NCT02385331).
Many studies have shown methods to either diagnose or prevent atherosclerosis in mice. Very few can do both at the same time, and even fewer have shown efficacy in established lesions, which is essential in the clinical setting. Perhaps even more importantly, the pathway identified in the current study represents a readily “druggable” target, as opposed to a candidate gene that may prove difficult to modulate in vivo without off-target effects. The exciting study by Karunakaran et al. (5) may allow for the development of an orally-available “theranostic” for imaging and treating vulnerable plaques that contain necrotic tissue cores, which would represent a significant breakthrough in the treatment of atherosclerotic cardiovascular disease.
Footnotes
This work was supported by the National Institutes of Health (R01HL12522401 and R01HL12337001). Dr. Leeper is a co-founder of and declares an equity interest in Forty Seven, Inc.
References
- 1.Thorp E., Tabas I. Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J Leukoc Biol. 2009;86:1089–1095. doi: 10.1189/jlb.0209115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kojima Y., Downing K., Kundu R. Cyclin-dependent kinase inhibitor 2B regulates efferocytosis and atherosclerosis. J Clin Invest. 2014;124:1083–1097. doi: 10.1172/JCI70391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kojima Y., Volkmer J.P., McKenna K. CD47-blocking antibodies restore phagocytosis and prevent atherosclerosis. Nature. 2016;536:86–90. doi: 10.1038/nature18935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pasparakis M., Vandenabeele P. Necroptosis and its role in inflammation. Nature. 2015;517:311–320. doi: 10.1038/nature14191. [DOI] [PubMed] [Google Scholar]
- 5.Karunakaran D., Geoffrion M., Wei L. Targeting macrophage necroptosis for therapeutic and diagnostic interventions in atherosclerosis. Science Advances. 2016;2 doi: 10.1126/sciadv.1600224. [DOI] [PMC free article] [PubMed] [Google Scholar]