KEY TEACHING POINTS
|
Introduction
Ventricular premature depolarizations (VPDs) frequently occur de novo after a myocardial infarction (MI) and have been associated with arrhythmogenic sudden cardiac death.1, 2, 3 The understanding of the mechanisms underlying the initiation of the VPDs and its relationship to ventricular fibrillation (VF) in these patients is deficient.4 The Purkinje network has been implicated in the initiation of monomorphic VPDs that can trigger VF in patients with no structural heart disease.5, 6, 7, 8 In addition, in the setting of ischemic cardiomyopathy, VPDs from the border zone of the scar have been shown to be a potential trigger for VF. Interestingly, Purkinje potentials are commonly recorded at the site of origin of the triggering VPDs.5 We present a case of a patient with postinfarction monomorphic VPDs exiting from different sites of the Purkinje network and initiating VF.
Case report
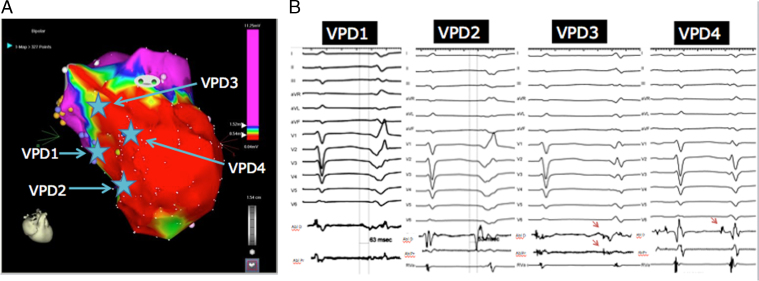
A 47-year-old man was admitted with a late presenting anterior wall MI in cardiogenic shock. A coronary angiogram showed occlusion of the proximal left anterior descending artery, for which he underwent angioplasty and insertion of a stent. An intraaortic balloon pump was placed and he was maintained on inotropes with a good initial clinical response. However, within 12 hours of presentation he developed multiple episodes of VF that required external defibrillation. A left ventricular assist device was subsequently inserted, but the patient continued to be in VF storm despite achieving optimal hemodynamic support. The patient was therefore brought to the electrophysiology laboratory for mapping and catheter ablation of the triggering VPD. It was noted that all VF episodes were preceded by a monomorphic VPD, occurring with a coupling interval of 356 ms in quadrigeminy, despite ongoing therapy with beta blockers, amiodarone, and lidocaine (Figure 1). Electroanatomic mapping demonstrated the presence of significant voltage abnormalities consistent with a large anterior, anteroapical, and anteroseptal infarct (Figure 2A). The clinical VPD had an earliest activation at the inferior basal border zone of the septal scar (63 ms pre-QRS). This site was characterized by early very low-amplitude fractionated potentials. Ablation at that site eliminated the presenting VPD; however, a variant of the clinical VPD with similar coupling interval was seen with a more apical exit site (earliest activation 53 ms pre-QRS). Owing to their anatomic location, it was thought that both origins were along the posterior fascicle (Figure 2A). Ablation at the earliest site of activation of the second VPD changed the exit of the VPD to a more superior septal site, possibly at the distal anterior fascicle, as suggested by the presence of Purkinje potentials (coupling interval 430 ms). Ablation at the earliest site (54 ms pre-QRS) revealed a fourth VPD (coupling interval 365 ms) that was mapped to the parahisian area on the left ventricle (58 ms pre-QRS). The morphologies of all VPDs with the earliest mapped electrograms are shown in Figure 2B and their location on the electroanatomic map in Figure 2A. Given the location of the last VPD near the His bundle region, and since all the VPDs seemed to arise from areas along the course of the conduction system, we elected to empirically target the Purkinje network, applying radiofrequency lesions in a linear fashion longitudinal and perpendicular to both fascicles (Figure 3). Following the above ablation strategy, there were no more VPDs seen. The patient was extubated the following day and was discharged home with a left ventricular assist device and internal cardioverter-defibrillator, with no recurrence of VF for a follow-up period of 10 months. He eventually received a cardiac transplantation for intractable heart failure.
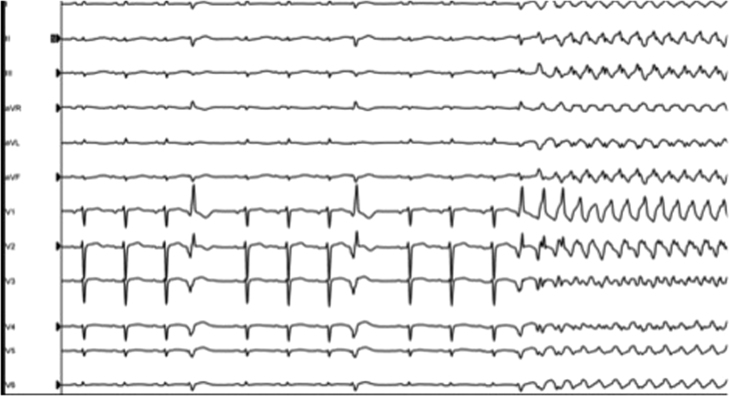
Figure 1.
Clinical ventricular premature depolarization (VPD) in quadrigeminy at the beginning of the electrophysiology study, initiating ventricular fibrillation. Small differences in the morphology of each VPD are noted. Although these differences could be due to different timing in the respiratory cycle and/or changes in heart filling, we cannot rule out temporary changes in ventricular refractoriness or slight changes in exit.
Figure 2.
A: Endocardial bipolar electroanatomic map of the left ventricle showing large anterior, anteroseptal, and apical scar at the distribution of the left anterior descending artery infarct (voltage cutoffs 0.5–1.5 mV). The site of earliest mapping of each ventricular premature depolarization (VPD) is shown in the map. B: Morphology, activation times at earliest site, and coupling intervals of different VPDs. For VPDs 1 and 2 the timing from the onset of local electrogram to QRS is shown with vertical lines. Purkinje potentials preceded the local electrograms at earliest sites for VPDs 3 and 4 and are shown with red arrows. Abl = ablation catheter; D = distal; P = proximal; RVA = right ventricular apical catheter.
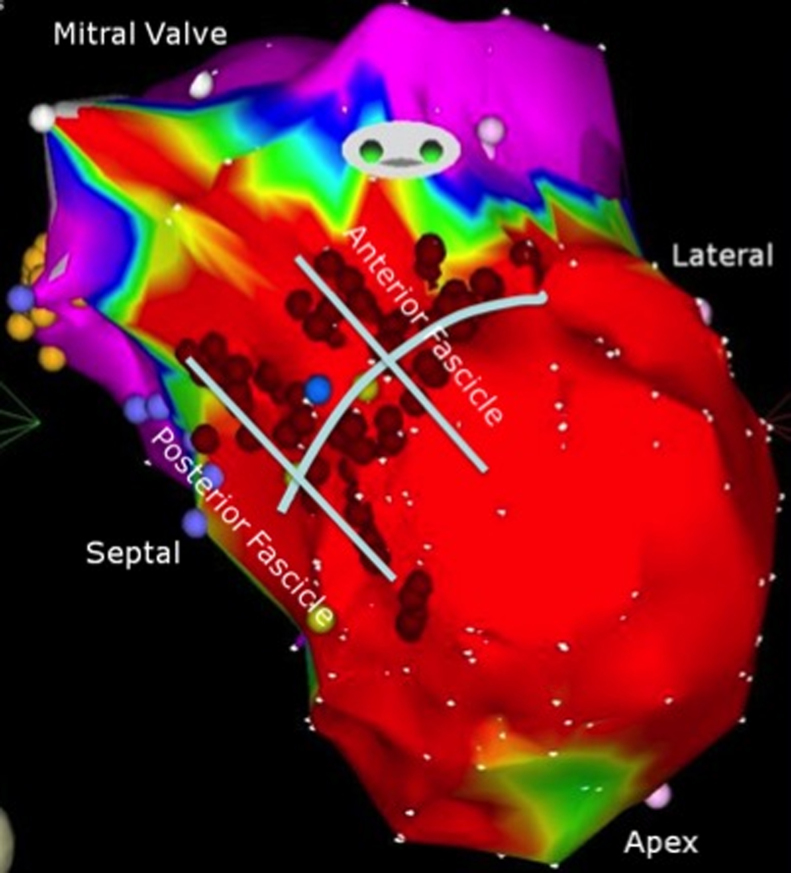
Figure 3.
Endocardial bipolar electroanatomic map of the left ventricle showing the final lesion set targeting the anatomic location of the anterior and posterior fascicles (voltage cutoffs 0.5–1.5 mV).
Discussion
VPD-triggered VF post MI has been previously described in the literature.1, 2, 3, 4, 5 The observed characteristics of the triggering VPDs include varying frequency, a coupling interval that is not particularly short (391 ± 48 ms), and a site of origin that is typically at the scar border and characterized by the presence of Purkinje potentials. Our initial triggering VPD shared the above characteristics. The etiology of the enhanced electrical activity of the Purkinje network post MI is unclear. One possible mechanism is related to its relative resistance to ischemia. Purkinje fibers within the scar are more likely to survive post MI and maintain their inherited automaticity due to its subendocardial location and exposure to cavitary blood.4, 9 In addition, the heterogeneity in electrical dispersion of repolarization due to differences in both fixed (fibrosis/scarring) and functional (hibernating/stunned myocardium with slow conduction) barriers in the scar create the necessary milieu for the development of polymorphic ventricular tachycardia and VF.4, 9, 10, 11, 12, 13 It is therefore not a surprise that several studies have provided evidence that ablation of these triggers was able to eliminate further arrhythmias, similar to our case study.4, 5, 6, 7, 8, 14
In our case, the clinical VPD had a right bundle branch pattern and despite rightward axis and negative forces across the precordial leads, it had earliest activation at the mid to proximal inferior septum. This suggests a possible course of the VPD via the scar to a more apical and lateral exit. Following ablation of the clinical VPD, we were able to eliminate sequential VPDs that, although they had earliest activation at anatomically distant sites, were all characterized by the presence of Purkinje potentials, suggesting that they were all part of the Purkinje network. The facts that these VPDs appeared in sequence after each ablation, had similar coupling intervals, and possibly originated from the Purkinje network support the notion that they were different exits of a more generalized activation of the Purkinje network, rather than a result of focal activation. This is further supported by the fact that only after a more extensive empiric ablation targeting both the anterior and posterior fascicles, all VPDs were eliminated. Nevertheless, the clinical benefit of targeting all subsequent VPDs after the clinical VPD was abolished, although possible, cannot be proven by this report.
Conclusions
Our case supports the notion that the Purkinje network has an indispensable role in the mechanism of polymorphic ventricular tachycardia and VF after an MI. Our case suggests that activation of the Purkinje network after an MI can be generalized, giving genesis of VPDs with different morphologies determined by preferential exit sites. Therefore, substrate ablation of the Purkinje network might have a role during VF storm in patients where the triggering VPD is infrequent, where it cannot be induced in the laboratory, or in a case of multiple morphology VPDs with septal origin.
References
- 1.Bogun F., Crawford T., Chalfoun N. Relationship of frequent postinfarction premature ventricular complexes to the reentry circuit of scar-related ventricular tachycardia. Heart Rhythm. 2008;5:367–374. doi: 10.1016/j.hrthm.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 2.Hallstrom A.P., Bigger J.T., Jr, Roden D. Prognostic significance of ventricular premature depolarizations measured 1 year after myocardial infarction in patients with early postinfarction asymptomatic ventricular arrhythmia. J Am Coll Cardiol. 1992;20:259–264. doi: 10.1016/0735-1097(92)90089-6. [DOI] [PubMed] [Google Scholar]
- 3.Ruberman W., Weinblatt E., Goldberg J.D., Frank C.W., Chaudhary B.S., Shapiro S. Ventricular premature complexes and sudden death after myocardial infarction. Circulation. 1981;64:297–305. doi: 10.1161/01.cir.64.2.297. [DOI] [PubMed] [Google Scholar]
- 4.Szumowski L., Sanders P., Walczak F. Mapping and ablation of polymorphic ventricular tachycardia after myocardial infarction. J Am Coll Cardiol. 2004;44:1700–1706. doi: 10.1016/j.jacc.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 5.Marrouche N.F., Verma A., Wazni O. Mode of initiation and ablation of ventricular fibrillation storms in patients with ischemic cardiomyopathy. J Am Coll Cardiol. 2004;43:1715–1720. doi: 10.1016/j.jacc.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Haissaguerre M., Extramiana F., Hocini M. Mapping and ablation of ventricular fibrillation associated with long-QT and Brugada syndromes. Circulation. 2003;108:925–928. doi: 10.1161/01.CIR.0000088781.99943.95. [DOI] [PubMed] [Google Scholar]
- 7.Haissaguerre M., Shoda M., Jais P. Mapping and ablation of idiopathic ventricular fibrillation. Circulation. 2002;106:962–967. doi: 10.1161/01.cir.0000027564.55739.b1. [DOI] [PubMed] [Google Scholar]
- 8.Haissaguerre M., Shah D.C., Jais P. Role of Purkinje conducting system in triggering of idiopathic ventricular fibrillation. Lancet. 2002;359:677–678. doi: 10.1016/S0140-6736(02)07807-8. [DOI] [PubMed] [Google Scholar]
- 9.Friedman P.L., Stewart J.R., Fenoglio J.J., Jr, Wit A.L. Survival of subendocardial Purkinje fibers after extensive myocardial infarction in dogs. Circ Res. 1973;33:597–611. doi: 10.1161/01.res.33.5.597. [DOI] [PubMed] [Google Scholar]
- 10.Arnar D.O., Bullinga J.R., Martins J.B. Role of the Purkinje system in spontaneous ventricular tachycardia during acute ischemia in a canine model. Circulation. 1997;96:2421–2429. doi: 10.1161/01.cir.96.7.2421. [DOI] [PubMed] [Google Scholar]
- 11.Berenfeld O., Jalife J. Purkinje-muscle reentry as a mechanism of polymorphic ventricular arrhythmias in a three-dimensional model of the ventricles. Circ Res. 1998;82:1063–1077. doi: 10.1161/01.res.82.10.1063. [DOI] [PubMed] [Google Scholar]
- 12.Chialvo D.R., Michaels D.C., Jalife J. Supernormal excitability as a mechanism of chaotic dynamics of activation in cardiac Purkinje fibers. Circ Res. 1990;66:525–545. doi: 10.1161/01.res.66.2.525. [DOI] [PubMed] [Google Scholar]
- 13.Kupersmith J., Li Z.Y., Maidonado C. Marked action potential prolongation as a source of injury current leading to border zone arrhythmogenesis. Am Heart J. 1994;127:1543–1553. doi: 10.1016/0002-8703(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 14.Bansch D., Oyang F., Antz M. Successful catheter ablation of electrical storm after myocardial infarction. Circulation. 2003;108:3011–3016. doi: 10.1161/01.CIR.0000103701.30662.5C. [DOI] [PubMed] [Google Scholar]