Abstract
Anthranilate synthetase, phosphoribosyltransferase, phosphoribosyl anthranilate isomerase, and indoleglycerol phosphate synthetase were examined in partially purified extracts of the monocotyledon, Zea mays and the dicotyledon, Pisum sativum. The plant extracts were chromatographed on DEAE-cellulose and Sephadex G150. The molecular weights of the enzymes were determined and found to be similar to those observed for many bacteria. None of the plant tryptophan enzyme activities was aggregated in vitro as is also the case with most bacteria. This is in contrast with the complex aggregation patterns observed in other eucaryotic organisms that have been examined (fungi and Euglena gracilis). The tryptophan enzymes from peas and corn were generally similar but some differences in stability were observed.
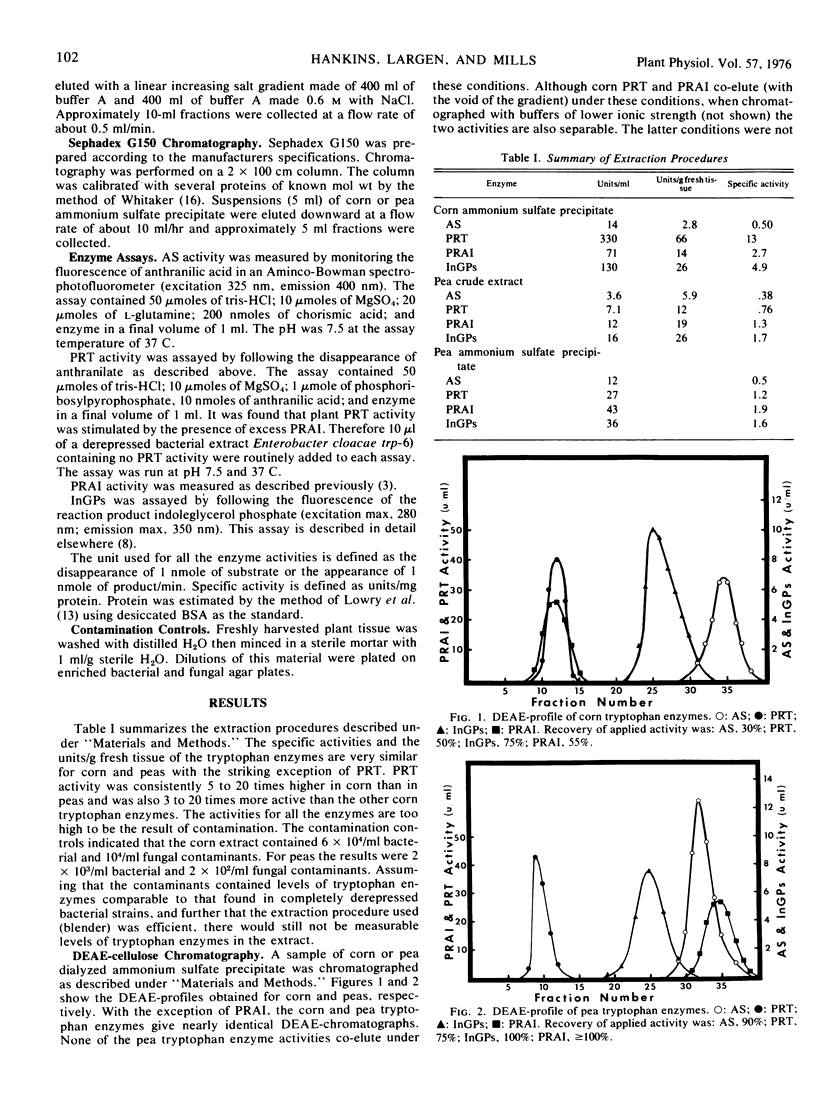
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belser W. L., Murphy J. B., Delmer D. P., Mills S. E. End product control of tryptophan biosynthesis in extracts and intact cells of the higher plant Nicotiana tabacum var. Wisconsin 38. Biochim Biophys Acta. 1971 Apr 20;237(1):1–10. doi: 10.1016/0304-4165(71)90023-7. [DOI] [PubMed] [Google Scholar]
- Crawford I. P., Gunsalus I. C. Inducibility of tryptophan synthetase in Pseudomonas putida. Proc Natl Acad Sci U S A. 1966 Aug;56(2):717–724. doi: 10.1073/pnas.56.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer D. P., Mills S. Tryptophan synthase from Nicotiana tabacum. Biochim Biophys Acta. 1968 Oct 8;167(2):431–443. doi: 10.1016/0005-2744(68)90223-4. [DOI] [PubMed] [Google Scholar]
- Enatsu T., Crawford I. P. Enzymes of the tryptophan synthetic pathway in Pseudomonas putida. J Bacteriol. 1968 Jan;95(1):107–112. doi: 10.1128/jb.95.1.107-112.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hütter R., DeMoss J. A. Organization of the tryptophan pathway: a phylogenetic study of the fungi. J Bacteriol. 1967 Dec;94(6):1896–1907. doi: 10.1128/jb.94.6.1896-1907.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Largen M., Belser W. L. Tryptophan biosynthetic pathway in the Enterobacteriaceae: some physical properties of the enzymes. J Bacteriol. 1975 Jan;121(1):239–249. doi: 10.1128/jb.121.1.239-249.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchett W. H., DeMoss J. A. The subunit structure of tryptophan synthase from Neurospora crassa. J Biol Chem. 1975 Apr 25;250(8):2941–2946. [PubMed] [Google Scholar]
- Widholm J. M. Anthranilate synthetase from 5-methyltryptophan-susceptible and -resistant cultured Daucus carota cells. Biochim Biophys Acta. 1972 Aug 18;279(1):48–57. doi: 10.1016/0304-4165(72)90240-1. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. Cultured Nicotiana tabacum cells with an altered anthranilate synthetase which is less sensitive to feedback inhibition. Biochim Biophys Acta. 1972 Jan 28;261(1):52–58. doi: 10.1016/0304-4165(72)90312-1. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. Measurement of the five enzymes which convert chorismate to tryptophan in cultured Daucus carota cell extracts. Biochim Biophys Acta. 1973 Sep 14;320(2):217–226. doi: 10.1016/0304-4165(73)90301-2. [DOI] [PubMed] [Google Scholar]
- Widholm J. M. Tryptophan biosynthesis in Nicotiana tabacum and Daucus carota cell cultures: site of action of inhibitory tryptophan analogs. Biochim Biophys Acta. 1972 Jan 28;261(1):44–51. doi: 10.1016/0304-4165(72)90311-x. [DOI] [PubMed] [Google Scholar]


