Abstract
An investigation was conducted into the isolation of plasma membrane vesicles from primary roots of corn (Zea mays L., WF9 × M14) by sucrose density gradient centrifugation. Identification of plasma membranes in cell fractions was by specific staining with the periodic-chromic-phosphotungstic acid procedure. Plasma membrane vesicles were rich in K+-stimulated ATPase activity at pH 6.5, and equilibrated in linear gradients of sucrose at a peak density of about 1.165 g/cc. It was necessary to remove mitochondria (equilibrium density of 1.18 g/cc) from the homogenate before density gradient centrifugation to minimize mitochondrial contamination of the plasma membrane fraction. Endoplasmic reticulum (NADH-cytochrome c reductase) and Golgi apparatus (latent IDPase) had equilibrium densities in sucrose of about 1.10 g/cc and 1.12 to 1.15 g/cc, respectively. A correlation (r = 0.975) was observed between K+-stimulated ATPase activity at pH 6.5 and the content of plasma membranes in various cell fractions. ATPase activity at pH 9 and cytochrome c oxidase activity were also correlated.
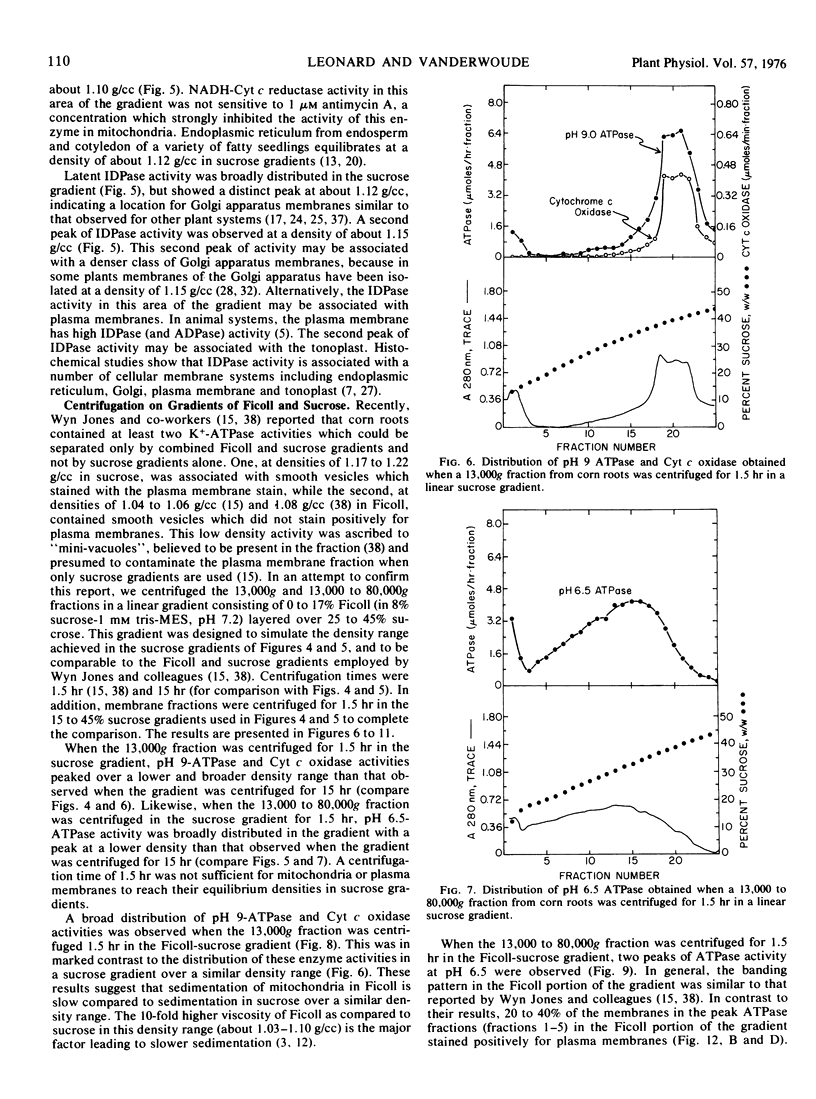
A major peak of ATPase activity at pH 6.5 was observed at low density in Ficoll after nonequilibrium centrifugation in a combination Ficoll-sucrose gradient. Twenty to forty percent of the vesicles in this ATPase fraction stained positively for plasma membranes, and with equilibrium centrifugation the major portion of the ATPase activity shifted to densities in sucrose which were characteristic of plasma membranes. All major vesicular ATPase activities observed in Ficoll or sucrose contained substantial amounts of plasma membranes. For unknown reasons, mitochondria and plasma membranes equilibrated over a broader density range and at lower peak densities in sucrose as a result of equilibrium centrifugation through Ficoll.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudhuin P. Morphometry of subcellular fractions. Methods Enzymol. 1974;32:3–20. doi: 10.1016/0076-6879(74)32004-6. [DOI] [PubMed] [Google Scholar]
- Donaldson R. P., Tolbert N. E., Schnarrenberger C. A comparison of microbody membranes with microsomes and mitochondria from plant and animal tissue. Arch Biochem Biophys. 1972 Sep;152(1):199–215. doi: 10.1016/0003-9861(72)90208-1. [DOI] [PubMed] [Google Scholar]
- Emmelot P., Bos C. J., van Hoeven R. P., van Blitterswijk W. J. Isolation of plasma membranes from rat and mouse livers and hepatomas. Methods Enzymol. 1974;31:75–90. doi: 10.1016/0076-6879(74)31008-7. [DOI] [PubMed] [Google Scholar]
- Fuhrmann G. F., Wehrli E., Boehm C. Preparation and identification of yeast plasma membrane vesicles. Biochim Biophys Acta. 1974 Sep 23;363(3):295–310. doi: 10.1016/0005-2736(74)90070-4. [DOI] [PubMed] [Google Scholar]
- Goff C. W. Localization of nucleoside diphosphatase in the onion root tip. Protoplasma. 1973;78(4):397–416. doi: 10.1007/BF01275775. [DOI] [PubMed] [Google Scholar]
- Hardin J. W., Cherry J. H., Morré D. J., Lembi C. A. Enhancement of RNA polymerase activity by a factor released by auxin from plasma membrane. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3146–3150. doi: 10.1073/pnas.69.11.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T., Bracker C. E., Keenan T. W. Purification of an ion-stimulated adenosine triphosphatase from plant roots: association with plasma membranes. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3307–3311. doi: 10.1073/pnas.69.11.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Holtz R. B., Stewart P. S., Patton S. Isolation and characterization of membranes from the cultivated mushroom. Plant Physiol. 1972 Nov;50(5):541–546. doi: 10.1104/pp.50.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. H. Comparative studies of glyoxysomes from various Fatty seedlings. Plant Physiol. 1975 May;55(5):870–874. doi: 10.1104/pp.55.5.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung D. W., Hanson J. B. Respiratory activation of 2,4-dinitrophenol-stimulated ATPase activity in plant mitochondria. Arch Biochem Biophys. 1973 Sep;158(1):139–148. doi: 10.1016/0003-9861(73)90606-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh R. A., Williamson F. A., Jones R. G. Presence of Two Different Membrane-bound, KCl-stimulated Adenosine Triphosphatase Activities in Maize Roots. Plant Physiol. 1975 Apr;55(4):678–685. doi: 10.1104/pp.55.4.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hansen D., Hodges T. K. Membrane-bound Adenosine Triphosphatase Activities of Oat Roots. Plant Physiol. 1973 Apr;51(4):749–754. doi: 10.1104/pp.51.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hanson J. B. Increased Membrane-bound Adenosine Triphosphatase Activity Accompanying Development of Enhanced Solute Uptake in Washed Corn Root Tissue. Plant Physiol. 1972 Mar;49(3):436–440. doi: 10.1104/pp.49.3.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard R. T., Hodges T. K. Characterization of Plasma Membrane-associated Adenosine Triphosphase Activity of Oat Roots. Plant Physiol. 1973 Jul;52(1):6–12. doi: 10.1104/pp.52.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord J. M., Kagawa T., Moore T. S., Beevers H. Endoplasmic reticulum as the site of lecithin formation in castor bean endosperm. J Cell Biol. 1973 Jun;57(3):659–667. doi: 10.1083/jcb.57.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore T. S., Beevers H. Isolation and characterization of organelles from soybean suspension cultures. Plant Physiol. 1974 Feb;53(2):261–265. doi: 10.1104/pp.53.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patni N. J., Billmire E., Aaronson S. Isolation of the Ochromanas danica plasma membrane and identification of several membrane enzymes. Biochim Biophys Acta. 1974 Dec 24;373(3):347–355. doi: 10.1016/0005-2736(74)90014-5. [DOI] [PubMed] [Google Scholar]
- Roland J. C., Lembi C. A., Morré D. J. Phosphotungstic acid-chromic acid as a selective electron-dense stain for plasma membranes of plant cells. Stain Technol. 1972 Jul;47(4):195–200. doi: 10.3109/10520297209116484. [DOI] [PubMed] [Google Scholar]
- Schibeci A., Rattray J. B., Kidby D. K. Isolation and identification of yeast plasma membrane. Biochim Biophys Acta. 1973 Jun 7;311(1):15–25. doi: 10.1016/0005-2736(73)90250-2. [DOI] [PubMed] [Google Scholar]
- Shore G., Maclachlan G. A. The site of cellulose synthesis. Hormone treatment alters the intracellular location of alkali-insoluble beta-1,4-glucan (cellulose) synthetase activities. J Cell Biol. 1975 Mar;64(3):557–571. doi: 10.1083/jcb.64.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitz T. O., Kent A. B., Hopkins H. A., Schmidt R. R. Equilibrium density-gradient procedure for selection of synchronous cells from asynchronous cultures. Science. 1970 Jun 5;168(3936):1231–1232. doi: 10.1126/science.168.3936.1231. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Volcani B. E. Isolation and characterization of plasma and smooth membranes of the marine diatom Nitzschia alba. Arch Biochem Biophys. 1974 Jul;163(1):29–45. doi: 10.1016/0003-9861(74)90451-2. [DOI] [PubMed] [Google Scholar]
- Sullivan C. W., Volcani B. E. Synergistically stimulated (Na+,K+)-adenosine triphosphatase from plasma membrane of a marine diatom. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4376–4380. doi: 10.1073/pnas.71.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Der Woude W. J., Lembi C. A., Morré D. J. beta-Glucan Synthetases of Plasma Membrane and Golgi Apparatus from Onion Stem. Plant Physiol. 1974 Sep;54(3):333–340. doi: 10.1104/pp.54.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanDerWoude W. J., Lembi C. A., Morré D. J. Auxin (2,4-D) stimulation (in vivo and in vitro) of polysaccharide synthesis in plasma membrane fragments isolated from onion stems. Biochem Biophys Res Commun. 1972 Jan 14;46(1):245–253. doi: 10.1016/0006-291x(72)90656-0. [DOI] [PubMed] [Google Scholar]





