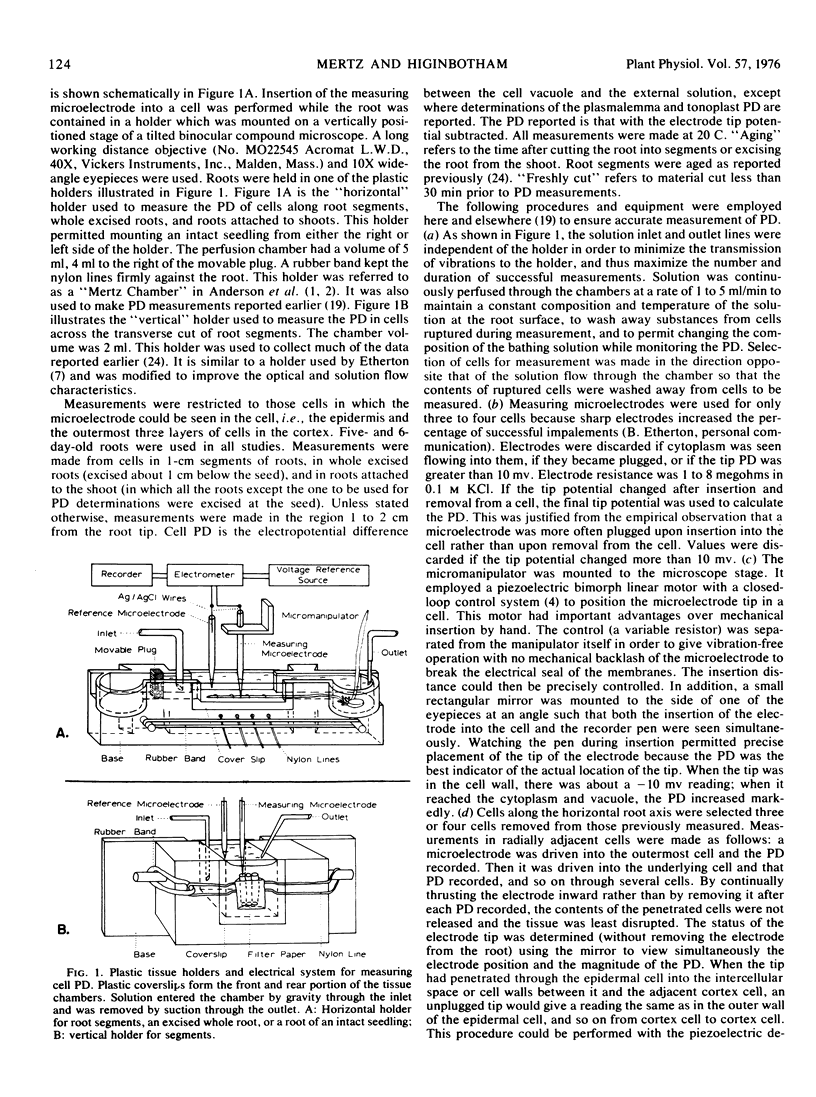
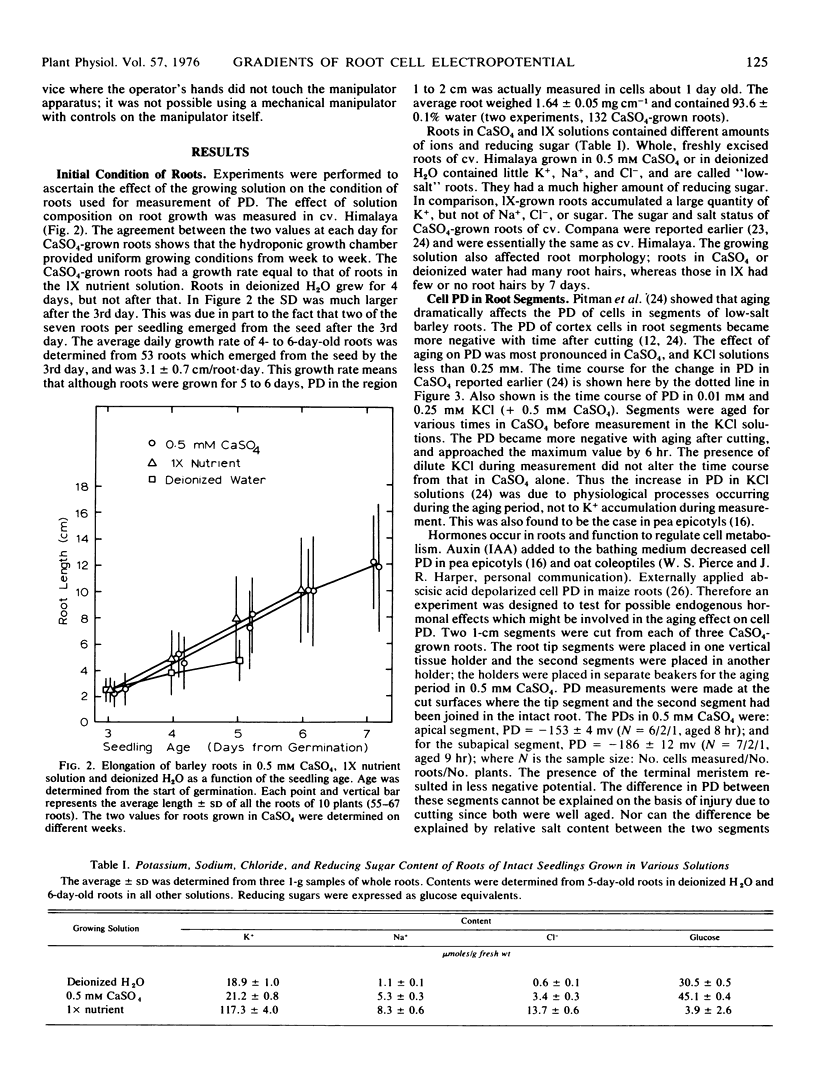
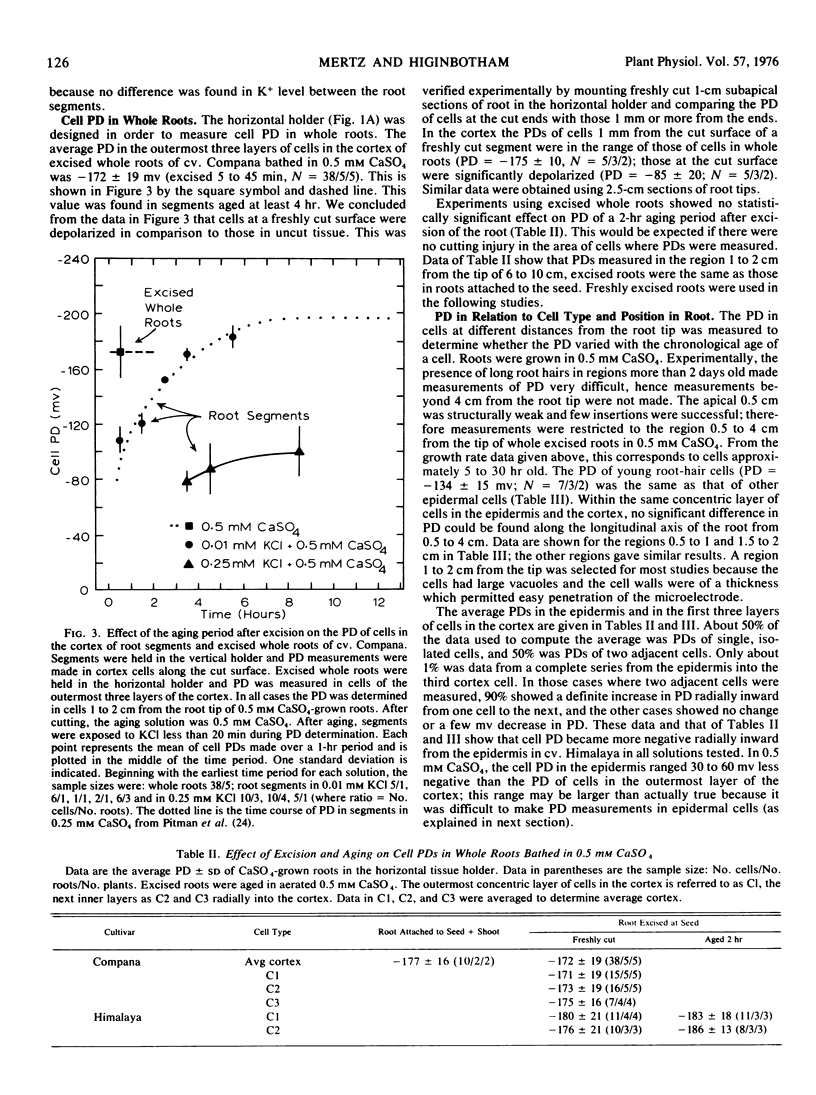
Abstract
Transmembrane electropotential difference (PD) was measured in whole roots of barley (Hordeum vulgare L. cvs. Compana and Himalaya). Seedlings were grown 4 to 5 days in aerated 0.5 mm CaSO4 or a nutrient solution. Measurements of PD were made with roots bathed in CaSO4, KCl + CaSO4, or the nutrient solution. The following results were found. (a) There was a radial PD gradient with epidermal cells being 10 to 58 millivolts less negative than cells in the third layer of the cortex (outside to inside). There was no longitudinal PD gradient in the region 0.5 to 4 cm from the root tip, nor was there any difference between the PD of young root hairs and other epidermal cells. (b) Cell PD in excised whole roots was not detectably different from that found in roots attached to the shoot, and was unchanged for 2 hours from excision. (c) In 1-centimeter sections of root, cell PD at the freshly cut surface was depolarized by 90 millivolts from that in the intact root; cells farther than 1 millimeter from the cut surface were not depolarized. The PD of cells at the cut surface became more negative upon aging the segment in 0.5 mm CaSO4, eventually becoming greater by -25 millivolts than that in cells of intact roots. Cells in segments to which the root tips were attached had less negative PDs after aging than those in subapical segments, indicating a possible hormonal effect. PDs in aged, excised segments are not equivalent to those in intact roots. (d) Creeping of cytoplasm over electrode tips inserted into the vacuole gave measurements of vacuole-to-cytoplasm PD of + 9 millivolts in 0.5 mm CaSO4 and + 35 millivolts in 1 mm KCl + 0.5 mm CaSO4. Most of the cell PD was across the plasmalemma. (e) The reducing sugar content of roots in CaSO4 solution was greater than that of roots in the nutrient solution in which ion uptake, particularly K+ occurred.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. P., Hendrix D. L., Higinbotham N. Higher plant cell membrane resistance by a single intracellular electrode method. Plant Physiol. 1974 Jan;53(1):122–124. doi: 10.1104/pp.53.1.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson W. P., Hendrix D. L., Higinbotham N. The effect of cyanide and carbon monoxide on the electrical potential and resistance of cell membranes. Plant Physiol. 1974 Nov;54(5):712–716. doi: 10.1104/pp.54.5.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor G. I. Micropositioner in a closed-loop control system. IEEE Trans Biomed Eng. 1973 Mar;20(2):114–119. doi: 10.1109/TBME.1973.324172. [DOI] [PubMed] [Google Scholar]
- Davis R. F. Membrane electrical potentials in the cortex and stele of corn roots. Plant Physiol. 1972 Mar;49(3):451–452. doi: 10.1104/pp.49.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETHERTON B., HIGINBOTHAM N. Transmembrane potential measurements of cells of higher plants as related to salt uptake. Science. 1960 Feb 12;131(3398):409–410. doi: 10.1126/science.131.3398.409. [DOI] [PubMed] [Google Scholar]
- Ecklund P. R., Moore T. C. Correlations of Growth Rate and De-etiolation with Rate of Ent-Kaurene Biosynthesis in Pea (Pisum sativum L.). Plant Physiol. 1974 Jan;53(1):5–10. doi: 10.1104/pp.53.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Effect of Indole-3-acetic Acid on Membrane Potentials of Oat Coleoptile Cells. Plant Physiol. 1970 Apr;45(4):527–528. doi: 10.1104/pp.45.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherton B. Relationship of Cell Transmembrane Electropotential to Potassium and Sodium Accumulation Ratios in Oat and Pea Seedlings. Plant Physiol. 1963 Sep;38(5):581–585. doi: 10.1104/pp.38.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D. Influence of Phenolic Acids on Ion Uptake: IV. Depolarization of Membrane Potentials. Plant Physiol. 1974 Dec;54(6):855–858. doi: 10.1104/pp.54.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higinbotham N., Etherton B., Foster R. J. Effect of External K, NH(4), Na, Ca, Mg, and H Ions on the Cell Transmembrane Electropotential of Avena Coleoptile. Plant Physiol. 1964 Mar;39(2):196–203. doi: 10.1104/pp.39.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Increase in electrogenic membrane potential with washing of corn root tissue. Plant Physiol. 1974 Nov;54(5):799–801. doi: 10.1104/pp.54.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie E. A. The active transport of ions in plant cells. Q Rev Biophys. 1970 Aug;3(3):251–294. doi: 10.1017/s0033583500004741. [DOI] [PubMed] [Google Scholar]
- Macklon A. E., Higinbotham N. Electropotential in excised pea epicotyls. Plant Physiol. 1968 Jun;43(6):888–892. doi: 10.1104/pp.43.6.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Adaptation of barley roots to low oxygen supply and its relation to potassium and sodium uptake. Plant Physiol. 1969 Sep;44(9):1233–1240. doi: 10.1104/pp.44.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G., Mertz S. M., Graves J. S., Pierce W. S., Higinbotham N. Electrical potential differences in cells of barley roots and their relation to ion uptake. Plant Physiol. 1971 Jan;47(1):76–80. doi: 10.1104/pp.47.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]


