Introduction
KEY TEACHING POINTS
|
The implantation of transvenous leads for pacemakers and defibrillators is traditionally performed using fluoroscopic guidance. When cardiac resynchronization therapy (CRT) is indicated, iodine contrast medium (ICM) infusion for coronary sinus (CS) branch visualization is also needed.
Adverse reactions to ICM during cardiac catheterization and coronary interventions are common.1 Chronic kidney disease (CKD) is frequently present in a population with symptomatic heart failure.2 Contrast-induced acute kidney injury is a serious and frequent procedural complication of CRT implantation with a significant negative influence on long-term survival.3
The feasibility of cardiac resynchronization therapy-defibrillator (CRT-D) implantation guided by an advanced electroanatomic mapping system (MS), without contrast liquid infusion, is already reported in the literature.4 Moreover, using the MS to find the latest left ventricle activation site, it is possible to optimize the left ventricle (LV) lead position.5
Case report
An 87-year-old woman with nonischemic cardiomyopathy, left branch bundle block, left ventricular ejection fraction 42%, CKD (glomerular filtration rate = 25 mL/min/1.73 m2), and chronic atrial fibrillation was selected for cardiac resynchronization therapy-pacemaker (CRT-P) implantation. She experienced 2 hospitalizations owing to heart failure worsening in the previous 6 months, and also owing to fast ventricular rate, despite optimal therapy. Allergy to ICM was reported in her clinical history.
The implantation was performed using the EnSite Velocity NavX system, version 4.0.1 (St Jude Medical, St Paul, MN). The EnSite electroanatomic MS can be used to create geometric models of the cardiac chambers and allows confirmation of the catheters’ location in the 3-dimensional space in real time, minimizing the use of fluoroscopy. The MS allows visualizing and mapping by continuous acquisition of signals from all catheters, leads, and unipolar guidewires.
A decapolar diagnostic electrophysiological catheter with a very soft tip (Livewire 5F; St Jude Medical), connected to the EnSite box (CathLink) of the MS, was introduced via the delivery system through the left subclavian vein and was used to create the right ventricular (RV) septal anatomy and to cannulate the CS without fluoroscopy.
The bipolar RV lead (Isoflex 1948; St Jude Medical) was delivered via the left cephalic vein; it was connected to the MS by means of pacing threshold cables with crocodile clips (401748; St Jude Medical) and positioned in the RV apex, under continuous MS monitoring.
The delivery system (Cardiac Positioning System, CPS; St Jude Medical) was pushed over the decapolar lead into the CS. The decapolar lead was then removed and the VisionWire (0.014” insulated unipolar guidewire; Biotronik, Berlin, Germany) was connected to the MS by means of the above-mentioned threshold cables and introduced into the CS to find all the available collateral branches and to create the electroanatomic mapping. This guidewire has an insulated body and conductive distal tip and this feature makes it appear like a unipolar catheter in the EnSite mapping system. The guidewire allowed us to deeply explore the branches, to acquire the local intracardiac signal, and to deliver, over the wire, the permanent LV lead to the target site. The CS branches were detected without use of contrast liquid infusion and fluoroscopy.
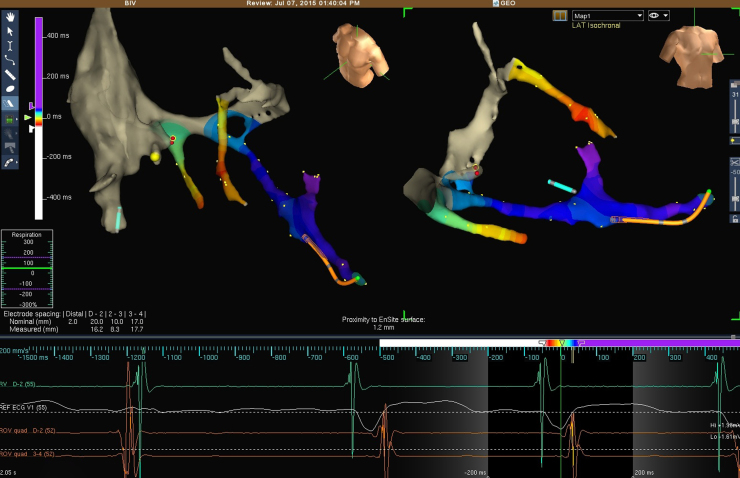
Three LV veins were cannulated with the guidewire and analyzed: a posterior vein (middle), a posterolateral vein, and an anterior vein. During the entire CS exploration, the local activation time map was acquired in order to investigate the local electrical delay from the onset of QRS. The optimal position of the CS lead (Quartet 1458Q; St Jude Medical) was chosen following the criterion of the most delayed site, during spontaneous activation of the LV (Figure 1).
Figure 1.
Final activation map of coronary sinus tributary veins during spontaneous rhythm and final position of right (RV) and left ventricular (LV) leads in 2 views (corresponding to left and right anterior oblique views) (top). In the color range, blue and purple represent the latest activated sites with respect to the onset of QRS. Electrical signals from RV apical lead, surface lead, and distal and proximal dipoles of quadripolar LV lead, respectively (bottom).
The most delayed activation was recorded in a small vein, a tributary of the posterolateral branch. It was not possible to position the lead in this site owing to its small size.
The adjacent site, in the mid portion of the posterolateral vein, was selected as the target site, with electrical delay of 105 ms from the onset of QRS (Figure 1). Here, the electrical parameters (pacing threshold, sensing, and impedance) and the lead stability were optimal.
The LV and RV leads were connected to the CRT-P device (Quadra Allure MP PM3262; St Jude Medical). The atrial port was plugged owing to chronic atrial fibrillation. The distal dipole of the quadripolar lead was chosen for the LV pacing because it corresponded to the latest activated site.
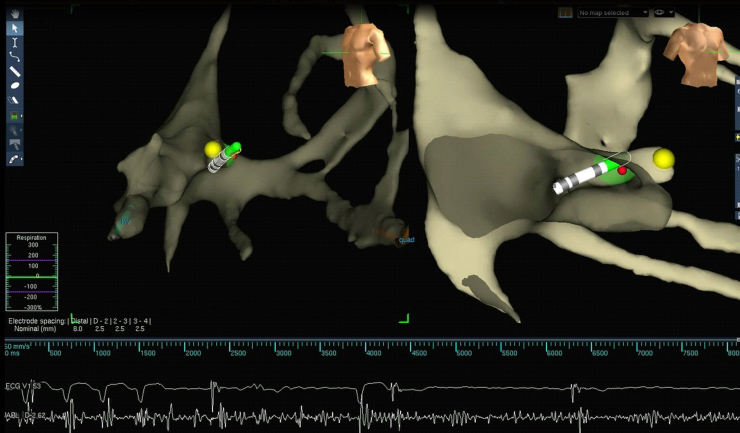
At the end of the implantation procedure, an ablation catheter (Safire 4 mm; St Jude Medical) was inserted through the femoral vein and guided to the atrioventricular (AV) node by means of the previously created anatomic map. Sixty seconds of radiofrequency energy at 50 watts was delivered for effective AV node ablation (Figure 2). No additional fluoroscopy was used for this final step.
Figure 2.
Atrioventricular (AV) node ablation. Yellow dot is the tag of His bundle. AV block during radiofrequency ablation.
The procedure was completed in 120 minutes, with less than 4 minutes of fluoroscopy. No ICM was used.
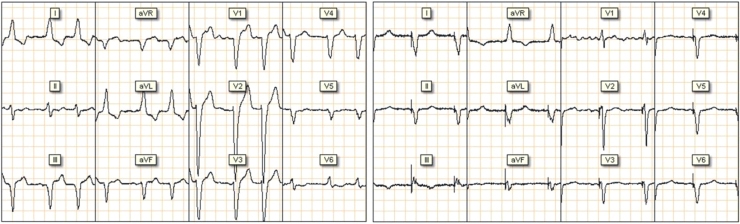
The comparison of electrocardiogram signals pre- and post-procedure (Figure 3) showed QRS narrowing from 160 ms to 145 ms.
Figure 3.
Pre (left side) and post (right side) pacemaker implantation electrocardiogram.
Discussion
In our experience of CRT implantations we usually use fluoroscopy guide and contrast medium infusion to find and visualize the CS and its branches. Taking advantage of our experience in using the electroanatomic mapping systems for electrophysiology procedures, for the first time in our center we used MS in CRT device implantation for LV lead positioning in order to avoid the infusion of contrast medium in a CKD and ICM-allergic patient. The method described by Del Greco et al4 for CRT-D implantation guided by electroanatomic MS is reproducible and feasible. The device implantation was completed without complications, with a procedural time comparable to a traditional approach and with a reduced fluoroscopy time.6 The use of contrast medium was avoided, thus minimizing the risk of complications related to worsening of kidney disease and the patient’s allergy. We believe that, besides the non-use of ICM and the fluoroscopy reduction, the benefits derived by this technique are to be able to leave a clear trace of each vessel and to go back quickly and easily into it. Moreover, the MS allows one to draw not only an anatomic map (collateral CS branches) but also a picture of electrical activation in order to choose the right vessel and the right site within it (latest activated site7). The MS was extremely useful in guiding the LV catheter placement. The lead was positioned in the most delayed of all the available vessels to obtain the greatest increase in cardiac contractility.8 This approach allowed achieving a complete map of the CS vessels’ electrical activation, without the need for single-point recording.8, 9 In our experience, the use of the previously acquired anatomic map allowed a safe and effective AV node ablation.
Using this approach in patients without contraindications to use of ICM, we cannot exclude the presence of further lateral vessels that are not selected with the wire. The guidewire allows drawing of the vessel but does not give information about its size in relation to the LV lead caliber. Another possible limitation is that this technique is feasible only with the EnSite, the unique mapping system that allows visualizing every catheter, lead, and unipolar guidewire.
Conclusion
This case confirmed that CRT implantation by means of electroanatomic mapping, in a patient with contraindications to ICM, was feasible, safe, and effective, and it did not require any specific learning curve. The approach also yielded a further benefit regarding choosing the most appropriate LV target site for better CRT response.
Footnotes
Stefano Indiani and Domenico Pacetta are employees of St Jude Medical, Italy. No funding was received.
References
- 1.Namasivayam S., Kalra M.K., Torres W.E., Small W.C. Adverse reactions to intravenous iodinated contrast media: a primer for radiologists. Emerg Radiol. 2006;12:210–215. doi: 10.1007/s10140-006-0488-6. [DOI] [PubMed] [Google Scholar]
- 2.Cleland J.G., Carubelli V., Castiello T., Yassin A., Pellicori P., Antony R. Renal dysfunction in acute and chronic heart failure: prevalence, incidence and prognosis. Heart Fail Rev. 2012;17(2):133–149. doi: 10.1007/s10741-012-9306-2. [DOI] [PubMed] [Google Scholar]
- 3.Kowalczyk J., Lenarczyk R., Kowalski O., Podoleckie T., Francuz P., Pruszkowska-Skrzep P., Szulik M., Mazurek M., Jedrzejczyk-Patej E., Sredniawa B., Kalarus Z. Contrast-induced acute kidney injury in patients undergoing cardiac resynchronization therapy-incidence and prognostic importance. Sub-analysis of data from randomized TRUST CRT trial. J Interv Card Electrophysiol. 2014;40(1):1–8. doi: 10.1007/s10840-014-9887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Del Greco M., Marini M., Bonmassari R. Implantation of a biventricular implantable cardioverter-defibrillator guided by an electroanatomic mapping system. Europace. 2012;14(1):107–111. doi: 10.1093/europace/eur250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rad M.M., Blaauw Y., Dinh T., Pison L., Crijns H., Prinzen F.W., Vernooy K. Left ventricular lead placement in the latest activated region guided by coronary venous electroanatomic mapping. Europace. 2015;17(1):84–93. doi: 10.1093/europace/euu221. [DOI] [PubMed] [Google Scholar]
- 6.Landolina M., Gasparini M., Lunati M. Long-term complication related to biventricular defibrillator implantation. Rate of surgical revisions and impact on survival: insight from the Italian Clinical Service Database. Circulation. 2011;123:2526–2535. doi: 10.1161/CIRCULATIONAHA.110.015024. [DOI] [PubMed] [Google Scholar]
- 7.Daubert J.C., Saxon L., Adamson P.B. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: implant and follow-up recommendations and management. Europace. 2012;14:1236–1286. doi: 10.1093/europace/eus222. [DOI] [PubMed] [Google Scholar]
- 8.Zanon F., Baracca E., Pastore G., Fraccaro C., Roncon L., Aggio S., Noventa F., Mazza A., Prinzen F. Determination of the longest intrapatient left ventricular electrical delay may predict acute hemodynamic improvement in patients after cardiac resynchronization therapy. Circ Arrhythm Electrophysiol. 2014;7:377–383. doi: 10.1161/CIRCEP.113.000850. [DOI] [PubMed] [Google Scholar]
- 9.Gold M.R., Birgersdotter-Green U., Singh J.P., Ellenbogen K.A., Yu Y., Meyer T.E., Seth M., Tchou P.J. The relationship between ventricular electrical delay and left ventricular remodelling with cardiac resynchronization therapy. Eur Heart J. 2011;32:2516–2524. doi: 10.1093/eurheartj/ehr329. [DOI] [PMC free article] [PubMed] [Google Scholar]