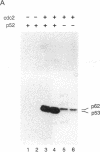
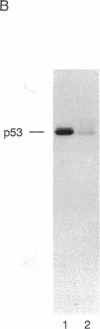
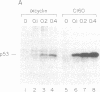
Abstract
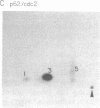
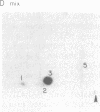
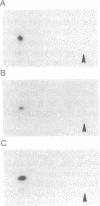
The human anti-oncoprotein p53 is shown to be a substrate of cdc2. The primary site of phosphorylation is serine-315. Serine-315 is phosphorylated by both p60-cdc2 and cyclin B-cdc2 enzymes. The phosphorylation of p53 is cell cycle-dependent. The abundance of p53 also oscillates during the cell cycle. The protein is largely absent from cells that have just completed division but accumulates in cells during G1 phase. Phosphorylation by cdc2 might regulate the antiproliferative activity of p53.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ajiro K., Borun T. W., Shulman S. D., McFadden G. M., Cohen L. H. Comparison of the structures of human histone 1A and 1B and their intramolecular phosphorylation sites during the HeLa S-3 cell cycle. Biochemistry. 1981 Mar 17;20(6):1454–1464. doi: 10.1021/bi00509a008. [DOI] [PubMed] [Google Scholar]
- Arion D., Meijer L., Brizuela L., Beach D. cdc2 is a component of the M phase-specific histone H1 kinase: evidence for identity with MPF. Cell. 1988 Oct 21;55(2):371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Banks L., Matlashewski G., Crawford L. Isolation of human-p53-specific monoclonal antibodies and their use in the studies of human p53 expression. Eur J Biochem. 1986 Sep 15;159(3):529–534. doi: 10.1111/j.1432-1033.1986.tb09919.x. [DOI] [PubMed] [Google Scholar]
- Beemon K., Hunter T. Characterization of Rous sarcoma virus src gene products synthesized in vitro. J Virol. 1978 Nov;28(2):551–566. doi: 10.1128/jvi.28.2.551-566.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R. N., Alfa C. E., Hyams J. S., Beach D. H. The fission yeast cdc2/cdc13/suc1 protein kinase: regulation of catalytic activity and nuclear localization. Cell. 1989 Aug 11;58(3):485–497. doi: 10.1016/0092-8674(89)90429-7. [DOI] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. Activation of human CDC2 protein as a histone H1 kinase is associated with complex formation with the p62 subunit. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4362–4366. doi: 10.1073/pnas.86.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkovich K., Duffy L. A., Harlow E. The retinoblastoma protein is phosphorylated during specific phases of the cell cycle. Cell. 1989 Sep 22;58(6):1097–1105. doi: 10.1016/0092-8674(89)90508-4. [DOI] [PubMed] [Google Scholar]
- Coulier F., Imbert J., Albert J., Jeunet E., Lawrence J. J., Crawford L., Birg F. Permanent expression of p53 in FR 3T3 rat cells but cell cycle-dependent association with large-T antigen in simian virus 40 transformants. EMBO J. 1985 Dec 16;4(13A):3413–3418. doi: 10.1002/j.1460-2075.1985.tb04098.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V., Lee W. M. Nuclear and nucleolar targeting sequences of c-erb-A, c-myb, N-myc, p53, HSP70, and HIV tat proteins. J Biol Chem. 1989 Oct 25;264(30):18019–18023. [PubMed] [Google Scholar]
- Draetta G., Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988 Jul 1;54(1):17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Draetta G., Brizuela L., Potashkin J., Beach D. Identification of p34 and p13, human homologs of the cell cycle regulators of fission yeast encoded by cdc2+ and suc1+. Cell. 1987 Jul 17;50(2):319–325. doi: 10.1016/0092-8674(87)90227-3. [DOI] [PubMed] [Google Scholar]
- Draetta G., Piwnica-Worms H., Morrison D., Druker B., Roberts T., Beach D. Human cdc2 protein kinase is a major cell-cycle regulated tyrosine kinase substrate. Nature. 1988 Dec 22;336(6201):738–744. doi: 10.1038/336738a0. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Giordano A., Whyte P., Harlow E., Franza B. R., Jr, Beach D., Draetta G. A 60 kd cdc2-associated polypeptide complexes with the E1A proteins in adenovirus-infected cells. Cell. 1989 Sep 8;58(5):981–990. doi: 10.1016/0092-8674(89)90949-5. [DOI] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Williamson N. M., Ralston R., Helfman D. M., Adams T. E. Molecular cloning and in vitro expression of a cDNA clone for human cellular tumor antigen p53. Mol Cell Biol. 1985 Jul;5(7):1601–1610. doi: 10.1128/mcb.5.7.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Lane D. P., Crawford L. V. T antigen is bound to a host protein in SV40-transformed cells. Nature. 1979 Mar 15;278(5701):261–263. doi: 10.1038/278261a0. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Miller L. K. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J Virol. 1978 Sep;27(3):754–767. doi: 10.1128/jvi.27.3.754-767.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey D., Brizuela L., Mohr I., Marshak D. R., Gluzman Y., Beach D. Phosphorylation of large tumour antigen by cdc2 stimulates SV40 DNA replication. Nature. 1989 Oct 12;341(6242):503–507. doi: 10.1038/341503a0. [DOI] [PubMed] [Google Scholar]
- Meek D. W., Eckhart W. Phosphorylation of p53 in normal and simian virus 40-transformed NIH 3T3 cells. Mol Cell Biol. 1988 Jan;8(1):461–465. doi: 10.1128/mcb.8.1.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P., Bissett Y. Gene required in G1 for commitment to cell cycle and in G2 for control of mitosis in fission yeast. Nature. 1981 Aug 6;292(5823):558–560. doi: 10.1038/292558a0. [DOI] [PubMed] [Google Scholar]
- O'Reilly D. R., Miller L. K. Expression and complex formation of simian virus 40 large T antigen and mouse p53 in insect cells. J Virol. 1988 Sep;62(9):3109–3119. doi: 10.1128/jvi.62.9.3109-3119.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Reed S. I., Hadwiger J. A., Lörincz A. T. Protein kinase activity associated with the product of the yeast cell division cycle gene CDC28. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4055–4059. doi: 10.1073/pnas.82.12.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Oren M., Levine A. J. Two distinct mechanisms regulate the levels of a cellular tumor antigen, p53. Mol Cell Biol. 1983 Dec;3(12):2143–2150. doi: 10.1128/mcb.3.12.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Rihs H. P., Peters R. Nuclear transport kinetics depend on phosphorylation-site-containing sequences flanking the karyophilic signal of the Simian virus 40 T-antigen. EMBO J. 1989 May;8(5):1479–1484. doi: 10.1002/j.1460-2075.1989.tb03531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad A., Anderson C. W., Carroll R. B. Mapping of phosphomonoester and apparent phosphodiester bonds of the oncogene product p53 from simian virus 40-transformed 3T3 cells. Proc Natl Acad Sci U S A. 1986 Feb;83(4):897–901. doi: 10.1073/pnas.83.4.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Simanis V., Nurse P. The cell cycle control gene cdc2+ of fission yeast encodes a protein kinase potentially regulated by phosphorylation. Cell. 1986 Apr 25;45(2):261–268. doi: 10.1016/0092-8674(86)90390-9. [DOI] [PubMed] [Google Scholar]
- Taya Y., Yasuda H., Kamijo M., Nakaya K., Nakamura Y., Ohba Y., Nishimura S. In vitro phosphorylation of the tumor suppressor gene RB protein by mitosis-specific histone H1 kinase. Biochem Biophys Res Commun. 1989 Oct 16;164(1):580–586. doi: 10.1016/0006-291x(89)91759-2. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Wang E. H., Friedman P. N., Prives C. The murine p53 protein blocks replication of SV40 DNA in vitro by inhibiting the initiation functions of SV40 large T antigen. Cell. 1989 May 5;57(3):379–392. doi: 10.1016/0092-8674(89)90913-6. [DOI] [PubMed] [Google Scholar]
- Whyte P., Buchkovich K. J., Horowitz J. M., Friend S. H., Raybuck M., Weinberg R. A., Harlow E. Association between an oncogene and an anti-oncogene: the adenovirus E1A proteins bind to the retinoblastoma gene product. Nature. 1988 Jul 14;334(6178):124–129. doi: 10.1038/334124a0. [DOI] [PubMed] [Google Scholar]