KEY TEACHING POINTS.
|
Introduction
Microreentrant tachycardias are well described and are thought to be responsible for a small proportion of atrial tachycardias post–atrial fibrillation (AF) ablation. However, because of the small size of these reentrant circuits and the poor spatial resolution of conventional mapping tools, they have not previously been mapped accurately in vivo in humans and have therefore been difficult to distinguish from nonreentrant focal tachycardias. The newly developed Rhythmia electroanatomic mapping system allows for the rapid creation of activation maps of ultra-high resolution. In this case report, we provide the first images of a microreentrant atrial tachycardia circuit in a post-AF setting, mapped with the high-resolution Rhythmia mapping system.
Case report
A 71-year-old woman with paroxysmal AF, who had an AF ablation procedure 8 years ago, attended for repeat ablation after a recurrence of AF in the past 12 months, which was not controlled despite flecainide. At her last ablation, she underwent pulmonary vein isolation with wide area circumferential ablation.
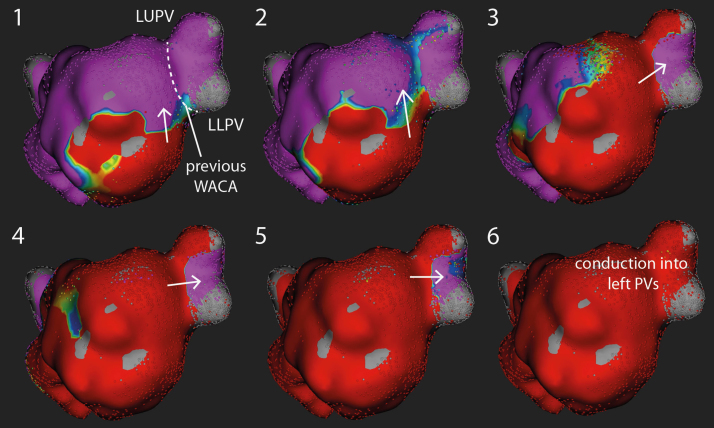
She was in sinus rhythm at the start of the procedure, and her procedure was performed under general anesthesia. After transseptal puncture, left atrial geometry was created using the Rhythmia mapping system (Boston Scientific, Marlborough, MA). Pacing from the coronary sinus demonstrated reconnection of the left pulmonary veins, with activation seen to cross the line of the previous wide area circumferential ablation into the left pulmonary veins (Figure 1).
Figure 1.
Electrical reconnection of left-sided pulmonary veins. Six sequential activation maps during pacing from the coronary sinus catheter demonstrating conduction into the left pulmonary veins. Conduction can be seen to cross the gap in the line of the previous wide area circumferential ablation (WACA) into the pulmonary veins (PVs). LLPV = left lower pulmonary vein; LUPV = left upper pulmonary vein.
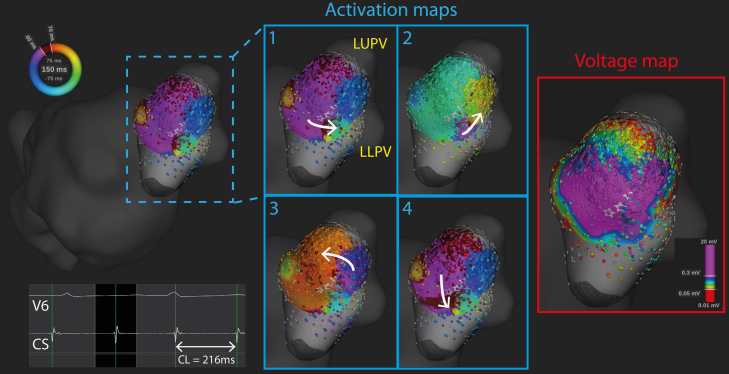
While mapping was performed, the patient developed an atrial tachycardia (cycle length 220 ms) with distal-to-proximal activation on the coronary sinus catheter. Further mapping anterior to the left pulmonary veins demonstrated a localized microreentrant circuit at the site of the gap on the previous ablation line (Figure 2 and Online Supplemental Video 1). The colors on the activation maps represent different timings throughout the tachycardia cycle length, and the wavefront can be tracked by following the regions where “early meets late,” that is, where red meets purple.1 The 4 sequential activation maps show activation progressing in an anticlockwise fashion, at this site anterior to the left-sided pulmonary veins. A corresponding bipolar voltage map during the tachycardia is also shown in Figure 2. In this case, the voltage map was not helpful in identifying the specific location of microreentrant circuit as the majority of bipolar electrograms in this region had amplitudes greater than 0.3 mV.
Figure 2.
Microreentrant atrial tachycardia anterior to left-sided pulmonary veins. During mapping, the patient developed an atrial tachycardia (cycle length [CL] 220 ms). A high-density Rhythmia map was performed around the left pulmonary veins, which demonstrated a microreentrant circuit anterior to the left pulmonary veins, near the site of the gap in the wide area circumferential ablation line in Figure 1. The 4 panels are sequential activation maps demonstrating the microreentrant circuit. The corresponding bipolar voltage map during atrial tachycardia is also shown. LLPV = left lower pulmonary vein; LUPV = left upper pulmonary vein.
Interrogation of the bipolar electrograms in that region demonstrated significantly fractionated electrograms, with electrogram timings spanning the entire cycle length of the tachycardia within a localized region with an area of 0.32 cm2 (Figures 3A–3C). Entrainment mapping was performed close to the site of the microreentrant circuit using the Orion catheter, as shown in Figure 3D. Because of amplifier saturation, the postpacing interval was measured to the second tachycardia beat after entrainment (PPI(n+2)) and the difference between that value and twice the tachycardia cycle length was 50 ms. Mechanical termination of the tachycardia occurred during mapping at that location, and therefore no further activation mapping or entrainment mapping was performed. A cluster of ablation lesions were delivered at this location (Figure 3C), and no further atrial tachycardias were induced after ablation with burst pacing. The patient also proceeded to have reisolation of her pulmonary veins during that procedure.
Figure 3.
Fractionated electrograms spanning the entire tachycardia cycle length within the microreentrant circuit. A: Rhythmia isochronal activation map showing the location of the microreentrant circuit. B: Bipolar electrograms obtained with the Orion catheter at 5 locations within the circuit (1–5) shown in panel A. The black column denotes the window of interest (WOI) for activation mapping, and the Rhythmia auto-annotated activation times are shown by vertical yellow lines. Activation times are seen to span the entire tachycardia cycle length, with progressive activation denoted by blue arrows. The area bounded by the 5 points measured 0.32 cm2. The coronary sinus (CS) reference electrogram and surface electrocardiogram lead V6 are also shown. C: A cluster of radiofrequency lesions were delivered at the site of the microreentrant circuit, which rendered the arrhythmia subsequently noninducible. D: Intracardiac electrograms during entrainment near the site of the microreentrant circuit. Pacing was delivered from the Orion catheter (poles H4–5) at 200 ms, which was 20 ms shorter than the tachycardia cycle length of 220 ms. Because of amplifier saturation, the postpacing interval was measured to the second tachycardia beat postpacing (PPI(n+2) = 490 ms), which was 50 ms longer than twice the tachycardia cycle length (2 × TCL = 440 ms), suggesting that pacing was performed close to, but not exactly at, the site of the microreentrant circuit. LLPV = left lower pulmonary vein; LUPV = left upper pulmonary vein.
Discussion
In this case, we provide classical images of a microreentrant left atrial tachycardia localized to the site of ablation gaps from previous wide area circumferential ablation, mapped with the ultra-high-resolution Rhythmia mapping system. Atrial tachycardias occur in up to 30%–50% of patients with previous AF ablation, depending on the initial ablation strategy.2 The majority (75%) of post-AF ablation atrial tachycardias are macroreentrant in nature,3 the most common forms of which are the mitral isthmus–dependent and left atrial roof–dependent atrial tachycardias. The remaining 25% are composed of focal atrial tachycardias, resulting from triggered activity or enhanced automaticity, and microreentrant tachycardias, which have diameters of reentrant circuits <3 cm.2, 3
Focal or microreentrant atrial tachycardias post-AF ablation have been well described and are known to occur at sites of gaps of ablation, especially anterior to the left superior pulmonary vein.4 The differentiation between true focal and microreentrant tachycardias has utility during the targeting of ablation therapy, as the entrainment mapping can be used to localize the circuit if the tachycardia mechanism is known to be reentrant in nature. Furthermore, knowledge of the tachycardia mechanism can inform pharmacotherapy in the event of tachycardia recurrence, with focal tachycardias due to triggered activity more responsive to calcium channel blockers, while microreentrant tachycardias may be more suitably treated with antiarrhythmic drugs that modify refractoriness.
However, it had previously been difficult to differentiate true focal, that is, triggered activity or enhanced automaticity, from microreentrant tachycardias because existing mapping systems lacked adequate resolution to accurately define the paths of microreentrant circuits. The recently developed Rhythmia high-density, high-resolution electroanatomic mapping system, which uses a small basket array of 64 electrodes (Orion catheter), can rapidly create high-density activation maps with little or no manual annotation of activation.5 Using this system, we were able to characterize in detail a microreentrant circuit at the gap of previous wide area circumferential ablation. Importantly, using the Rhythmia algorithm, this was done rapidly without the need for manual annotation or verification of activation times. The automated mapping feature is a key advantage of the Rhythmia system with significantly reduced mapping times. In a recent study using the Rhythmia system, an average of 2753–3566 data points was collected with continuous mapping, taking an average of only 5.2–9.5 minutes.6 This compares favorably with other lower-density 3-dimensional mapping systems. For example, in a study using the NavX mapping system, even with the multipolar pentarray catheter, only 365 ± 108 points were collected during an average mapping time of 8 ± 3 minutes.7 High-resolution electroanatomic mapping systems such as the Rhythmia system enable more accurate and rapid mapping of localized reentrant atrial tachycardia circuits, which may improve success rates in the targeting of post-AF ablation atrial tachycardias.
Conclusion
We provide the first human in vivo evidence of a microreentrant circuit post-AF ablation, with an area of 0.32 cm2. High-resolution mapping systems such as the Rhythmia system allow for detailed characterization of small reentrant circuits and allow better targeting of ablation therapy for microreentrant tachycardias.
Footnotes
This study was supported by a National Institute of Health Research Clinical Lectureship (1716) and an Academy of Medical Sciences starter grant (grant no. AMS-SGCL8-Ng) to Dr Ng.
Dr Ng has received honoraria from Boston Scientific. Mr Guerrero is an employee of Boston Scientific. Dr Lim has received research grants from Medtronic and Boston Scientific and has received speaker’s fees from Biosense Webster, Boston Scientific, and St. Jude Medical.
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.hrcr.2017.01.008.
Appendix. Supplemantary data
Supplementary material
Video 1: Propagation map showing three cycles of a microreentrant tachycardia in the left atrium, anterior to the left sided pulmonary veins. The corresponding sequential activation maps are shown in Figure 2.
References
- 1.Del Carpio Munoz F., Buescher T., Asirvatham S.J. Teaching points with 3-dimensional mapping of cardiac arrhythmias: taking points: activation mapping. Circ Arrhythm Electrophysiol. 2011;4:e22–e25. doi: 10.1161/CIRCEP.110.960351. [DOI] [PubMed] [Google Scholar]
- 2.Morady F., Oral H., Chugh A. Diagnosis and ablation of atypical atrial tachycardia and flutter complicating atrial fibrillation ablation. Heart Rhythm. 2009;6:S29–S32. doi: 10.1016/j.hrthm.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Chae S., Oral H., Good E. Atrial tachycardia after circumferential pulmonary vein ablation of atrial fibrillation: mechanistic insights, results of catheter ablation, and risk factors for recurrence. J Am Coll Cardiol. 2007;50:1781–1787. doi: 10.1016/j.jacc.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 4.Mesas C.E., Pappone C., Lang C.C.E., Gugliotta F., Tomita T., Vicedomini G., Sala S., Paglino G., Gulletta S., Ferro A., Santinelli V. Left atrial tachycardia after circumferential pulmonary vein ablation for atrial fibrillation: electroanatomic characterization and treatment. J Am Coll Cardiol. 2004;44:1071–1079. doi: 10.1016/j.jacc.2004.05.072. [DOI] [PubMed] [Google Scholar]
- 5.Nakagawa H., Ikeda A., Sharma T., Lazzara R., Jackman W.M. Rapid high resolution electroanatomical mapping: evaluation of a new system in a canine atrial linear lesion model. Circ Arrhythm Electrophysiol. 2012;5:417–424. doi: 10.1161/CIRCEP.111.968602. [DOI] [PubMed] [Google Scholar]
- 6.Ptaszek L.M., Chalhoub F., Perna F., Beinart R., Barrett C.D., Danik S.B., Heist E.K., Ruskin J.N., Mansour M. Rapid acquisition of high-resolution electroanatomical maps using a novel multielectrode mapping system. J Interv Card Electrophysiol. 2013;36:233–242. doi: 10.1007/s10840-012-9733-y. [DOI] [PubMed] [Google Scholar]
- 7.Patel A.M., d’Avila A., Neuzil P., Kim S.J., Mela T., Singh J.P., Ruskin J.N., Reddy V.Y. Atrial tachycardia after ablation of persistent atrial fibrillation: identification of the critical isthmus with a combination of multielectrode activation mapping and targeted entrainment mapping. Circ Arrhythm Electrophysiol. 2008;1:14–22. doi: 10.1161/CIRCEP.107.748160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Video 1: Propagation map showing three cycles of a microreentrant tachycardia in the left atrium, anterior to the left sided pulmonary veins. The corresponding sequential activation maps are shown in Figure 2.