Introduction
KEY TEACHING POINTS
|
Papillary muscles (PMs) of the left ventricle (LV) have been shown to be a potential origin of ventricular arrhythmias (VAs) in patients with or without structural heart disease. Recently, a “malignant” arrhythmia phenotype in patients with mitral valve prolapse (MVP) who experienced life-threatening VAs has been reported. This subset of patients with MVP was characterized by complex ventricular ectopy arising from one or both of the PMs, fascicular tissue, and outflow tracts,1, 2 and the arrhythmias have been shown to be successfully treated by catheter ablation (CA).3, 4, 5 Mitral valve dysfunction after radiofrequency (RF) CA of VAs of PM origin has been described in only 1 report,6 to our knowledge. Here we report a patient with a history of MVP and nonsustained ventricular tachycardia (NSVT) originating from the left ventricular posterior PM (PPM). Her ventricular tachycardia (VT) was successfully eliminated by repeated RF CA, but mitral valve regurgitation (MR) worsened from moderate to severe after CA. She underwent mitral valvuloplasty 8 months after the first CA session.
Case report
A 67-year-old woman with a 30-year history of MVP developed monomorphic NSVT at 170 beats/min. NSVT was refractory to medical treatment with verapamil and bisoprolol, and she had significant symptoms including palpitations. NSVT and ventricular premature complexes (VPCs) on the surface electrocardiogram exhibited a right bundle branch block with superior axis QRS morphology (Figure 1A), indicating a likely origin of the left posterior fascicle (LPF) or left ventricular PPM. Echocardiography showed a normal left ventricular ejection fraction of 63% and moderate MR due to anterior leaflet prolapse (Figure 2A). The first RF CA session was performed in a nonsedated state using a 3-dimensional electroanatomic mapping system with an intracardiac echocardiography (ICE) catheter (CartoSound software and SoundStar catheter, Biosense Webster Inc., Diamond Bar, CA) and a 3.5-mm open-irrigated-tip mapping and ablation catheter (NaviStar ThermoCool SmartTouch, Biosense Webster). At the beginning of the session, a SoundStar catheter was inserted via the femoral vein into the right atrium and right ventricle, and a SoundMap of the LV including both an anterior PM (APM) and a PPM was created. The VT was not sustained long enough to perform a precise electrophysiology study including entrainment pacing. Because of the insufficient inducibility and sustainability of the VT, the mechanism and origin of the VT were not clarified. We performed rough pace mapping in the entire LV and relatively better pace map was obtained at the LPF area, where we targeted first. The RF current was delivered by a retrograde transaortic approach targeting P1-like potential during NSVT in a mid-level of the LPF area on suspicion of verapamil-sensitive left posterior fascicular VT. However, VA was not eliminated. Extensive pace mapping was performed as a next step, and the best pace-map site was identified at the base of the PPM. The pace-map score7 was 19/24 and ventricular local electrocardiogram during VPC preceded the onset of the surface QRS complex by 16 ms, and Purkinje potential was not observed at that site. RF energy applications with 8 points and a total of 368 seconds guided by ICE with a power of 40 W and a contact force (CF) of 15–20 g were delivered on the base of the PPM (Figures 3A and 3B). Only 2 bonus applications with 45 W and 60 seconds each were delivered to opposite septal and lateral sides of the PPM. Despite repetitive ventricular response during RF applications and subsequent disappearance of VAs at the end of the session under isoproterenol infusion, NSVT relapsed a day after CA. We started alternative medical therapy; however, NSVT was refractory to sotalol and gradually became incessant.
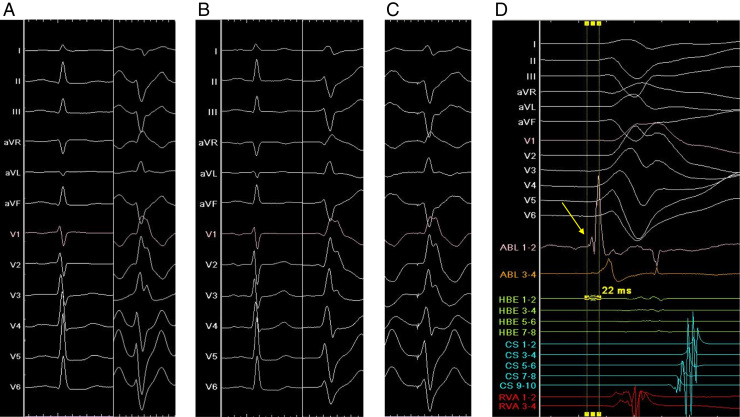
Figure 1.
Surface electrocardiogram (50 mm/s) and intracardiac electrocardiogram (200 mm/s). A: Sinus rhythm and nonsustained ventricular tachycardia (NSVT) in the first session. B: Sinus rhythm and NSVT in the second session. C: An excellent pace map at a mid-level of the posterior papillary muscle in the second session. D: Intracardiac electrocardiogram at the successful ablation site in the second session. Prepotential (indicated by arrow) preceded the onset of the surface QRS complex by 22 ms during NSVT.
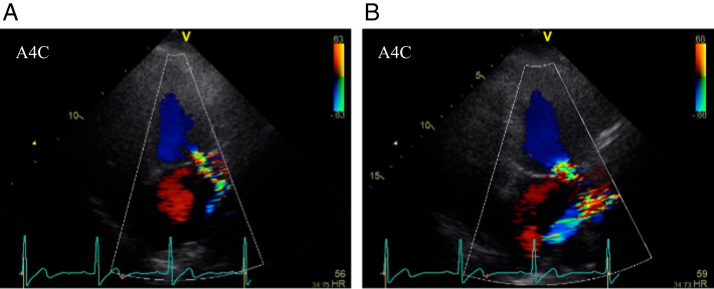
Figure 2.
A: Echocardiography before the first session showed moderate mitral valve regurgitation. B: Echocardiography before the second session showed severe mitral valve regurgitation. A4C = apical 4-chamber view.
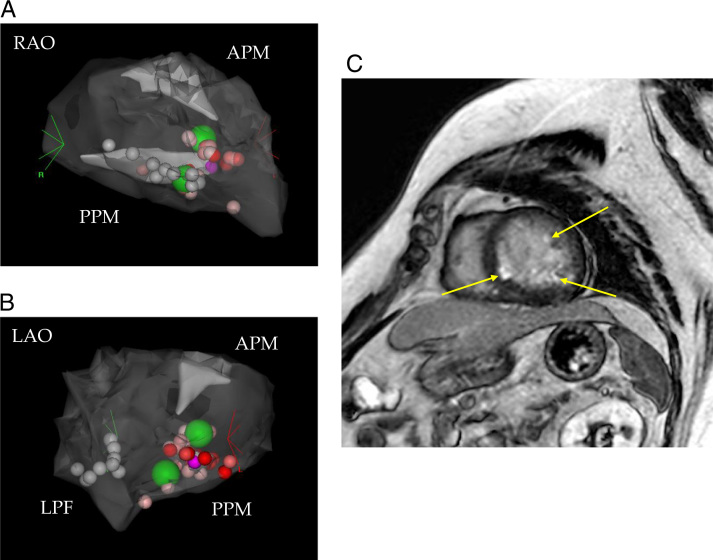
Figure 3.
Three-dimensional electroanatomic mapping and late gadolinium–enhanced cardiac magnetic resonance imaging. A: SoundMap of the left ventricle including anterior papillary muscle (APM) and posterior papillary muscle (PPM) in the first session in right anterior oblique (RAO) view. White tags indicate ablation sites in left posterior fascicular (LPF) area, purple tag indicates a best pace-map site, green tags indicate bonus application sites with 45 W, and other tags (VisiTag) indicate ablation sites with 40 W. B: SoundMap in left anterior oblique (LAO) view in the first session. C: Late gadolinium–enhanced cardiac magnetic resonance imaging before the second session revealed myocardial late gadolinium enhancements at the LPF area and both left ventricular papillary muscles (indicated by arrows).
Three months after the first CA session, she was eventually taken by ambulance to our hospital because of intolerable repeated palpitations. Although the morphology of incessant NSVT on surface electrocardiography was almost the same as the morphology before the first CA session, the R/S ratio in lead I had slightly changed from <1 to >1 (Figure 1B). Apart from this clinical NSVT, another type of VPC was observed. It was isolated and less frequent and exhibited a left bundle branch block with inferior axis QRS morphology indicative of an outflow tract origin. Several signs of MR worsening were also noted at the time of the second admission. Echocardiography showed worsening of MR from moderate to severe (Figure 2B) and progressive dilatation of the left atrium from 55 to 86 mL/m2, and serum brain natriuretic peptide level had increased from 59 to 209 pg/mL. Cardiac magnetic resonance (CMR) imaging revealed myocardial late gadolinium enhancements at the LPF area and at both left ventricular PMs, although RF energy application was not delivered to the APM in the first CA session (Figure 3C). Redo CA was performed via the transseptal approach with a long steerable sheath (Agilis, St. Jude Medical, St. Paul, MN) and using the same system and catheters as those used in the first CA session. At a mid-level of the PPM, an excellent pace map was obtained and the earliest ventricular activation with slightly dull prepotential was identified during NSVT. The pace-map score7 was 20/24, and the prepotential preceded the onset of the surface QRS complex by 22 ms (Figures 1C and 1D). The little difference between the pace map and VAs (Figures 1B and 1C) could be explained by deep location of the VA origin in the PM.3 Purkinje potential around the PPM was clearly sharper than the prepotential, and it was not observed at application sites. VAs were eliminated by irrigated RF ablation with a power of 40 W and a CF of 5–10 g under the guidance of ICE, and several bonus applications with a maximum power of 50 W were delivered close to the origin of VA at the mid-level of the PPM. VA was no longer inducible by right ventricular programmed stimulation during isoproterenol infusion, and she was free of recurrence thereafter. In contrast, MR remained severe and brain natriuretic peptide level increased further after the second CA session, even though NSVT was completely abolished and diuretics were administered. Transesophageal echocardiography showed broad anterior leaflet prolapse ranging from segment A1 (anterolateral) to segment A3 (anteromedial) and thickening of the anterior leaflet without ruptured chordae tendineae or posterior leaflet prolapse. Since shortness of breath on exertion was worsened, she underwent mitral valvuloplasty 8 months after the first CA session. A diffusely redundant and thickened anterior leaflet and elongated chordae were clearly visible, and thickening and hardening of the PPM was detected during the surgery.
Discussion
There is limited information on adverse effects of RF CA of a PM on the mitral valve function. Yamada et al3 reported that there was no evidence of significant MR after RF CA of PMs in 19 patients during a median follow-up period of 21 months (APM, n = 7; PPM, n = 12). Similarly, Rivera et al5 reported that there was no increase in the incidence of MR or an increase in MR severity in 21 patients, including 5 patients with MVP, after CA of PMs using either cryoenergy (n = 12) or RF energy (n = 9). However, a case of bileaflet MVP and VT who developed severe MR after CA targeting PM-derived VA was reported by DeSimone et al.6 In the present case, MR worsened during an 8-month follow-up period. Possible explanations for the aggravation of MR are attenuated contraction of the PPM and its discoordination with the APM, leading to worsening of anterior mitral leaflet prolapse. Since each PM distributes chords to the ipsilateral half of both leaflets,8 ablation of the PPM could have worsened anterior leaflet prolapse.
Differences in the system and protocols used for CA might explain why worsening of MR was observed after CA of the PM in the present case but not in cases described in previous reports.3, 4, 5 It is difficult to achieve stable catheter contact with a PM because of the vigorous and constant motion associated with normal PM contraction, the variability in PM anatomy, and the difficulty in recognizing the precise location of the PM only by x-ray fluoroscopy. A CF-sensing catheter in combination with the CartoSound system contributes to powerful and stable tissue contact, and this technology was not used in CA cases in previous studies. However, the optimal setting of energy power and CF for CA of VA originating from a PM has not been established. We performed RF ablation with a power of 40 W and a CF of 15–20 g in the first session of CA and with a power of 40 W and a CF of 5–10 g in the second session. Although elimination of VA was achieved by the second session, multiple irrigated RF applications to the PM in the first session might have been excessive, predisposing the PM to dysfunction after additional CA.
In addition to the effect of CA on PM function, progression of MVP itself is a possible explanation for worsening of MR after the second session of CA. However, it is not possible to critically differentiate the 2 possibilities. CMR imaging is useful for identifying structural abnormality contributing to arrhythmogenesis. Three-dimensional high-resolution late gadolinium–enhanced CMR imaging enables to visualize myocardial fibrosis involving the PMs in patients with MVP,9 and Basso et al2 reported that fibrosis of the PMs and inferobasal left ventricular wall was the structural hallmark of VA origin in patients with MVP. Unfortunately, however, assessment of PM function and ventricular fibrosis by CMR imaging were not performed before and after the 2 sessions of CA in the present case.
The clinical benefit of CA of VAs in patients with MVP is supported by earlier studies.5, 10 Syed et al10 showed that CA of clinically dominant ventricular ectopy improved symptoms and reduced appropriate implantable cardioverter-defibrillator shocks in patients with MVP. In addition, a “malignant” subset of patients with MVP who experienced life-threatening VAs was reported.1, 2 This subset of patients was characterized by bileaflet prolapse, a higher percentage of women, presence of biphasic or inverted T waves in inferior leads, and VPCs originating from the outflow tract alternating with the PM. In the present case, mitral prolapse was observed only in the anterior leaflet while other characteristics were similar to those in the malignant subsets of patients with MVP, and indication of CA was significant symptoms of NSVT. However, the safety of CA of PM-derived VAs has not been established. Ikeda et al11 reported that lesion depths and diameters were 5.9 ± 1.2 and 8.0 ± 1.9 mm by application of 40 W and mild CF (median of 8 g) and 8.8 ± 1.6 and 12.5 ± 1.7 mm by application of 40 W and moderate CF (median of 21 g), respectively. The depth of the lesion induced by CA is greater than the diameter of PMs, 0.7 ± 0.2 cm, that was determined by echocardiography.12 We usually attempt to create a transmural lesion by CA; however, such an attempt might not be appropriate in CA of PM-derived VAs.
Conclusion
The present case indicates that PM ablation has a potential risk of MR aggravation in patients with MVP and that patients require careful follow-up.
References
- 1.Sriram C.S., Syed F.F., Ferguson M.E. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222–230. doi: 10.1016/j.jacc.2013.02.060. [DOI] [PubMed] [Google Scholar]
- 2.Basso C., Perazzolo Marra M., Rizzo S. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 2015;132:556–566. doi: 10.1161/CIRCULATIONAHA.115.016291. [DOI] [PubMed] [Google Scholar]
- 3.Yamada T., Doppalapudi H., McElderry H.T. Electrocardiographic and electrophysiological characteristics in idiopathic ventricular arrhythmias originating from the papillary muscles in the left ventricle: relevance for catheter ablation. Circ Arrhythm Electrophysiol. 2010;3:324–331. doi: 10.1161/CIRCEP.109.922310. [DOI] [PubMed] [Google Scholar]
- 4.Yokokawa M., Good E., Desjardins B. Predictors of successful catheter ablation of ventricular arrhythmias arising from the papillary muscles. Heart Rhythm. 2010;7:1654–1659. doi: 10.1016/j.hrthm.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rivera S., Ricapito Mde L., Tomas L. Results of cryoenergy and radiofrequency-based catheter ablation for treating ventricular arrhythmias arising from the papillary muscles of the left ventricle, guided by intracardiac echocardiography and image integration. Circ Arrhythm Electrophysiol. 2016;9:e003874. doi: 10.1161/CIRCEP.115.003874. [DOI] [PubMed] [Google Scholar]
- 6.Desimone C.V., Hu T., Ebrille E. Catheter ablation related mitral valve injury: the importance of early recognition and rescue mitral valve repair. J Cardiovasc Electrophysiol. 2014;25:971–975. doi: 10.1111/jce.12439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coggins D.L., Lee R.J., Sweeney J. Radiofrequency catheter ablation as a cure for idiopathic tachycardia of both left and right ventricular origin. J Am Coll Cardiol. 1994;23:1333–1341. doi: 10.1016/0735-1097(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 8.Silbiger J.J., Bazaz R. Contemporary insights into the functional anatomy of the mitral valve. Am Heart J. 2009;158:887–895. doi: 10.1016/j.ahj.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Han Y., Peters D.C., Salton C.J. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294–303. doi: 10.1016/j.jcmg.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Syed F.F., Ackerman M.J., McLeod C.J. Sites of successful ventricular fibrillation ablation in bileaflet mitral valve prolapse syndrome. Circ Arrhythm Electrophysiol. 2016;9 doi: 10.1161/CIRCEP.116.004005. http://dx.doi.org/10.1161/CIRCEP.116.004005. [DOI] [PubMed] [Google Scholar]
- 11.Ikeda A., Nakagawa H., Lambert H. Relationship between catheter CF and radiofrequency lesion size and incidence of steam pop in the beating canine heart: electrogram amplitude, impedance, and electrode temperature are poor predictors of electrode-tissue CF and lesion size. Circ Arrhythm Electrophysiol. 2014;7:1174–1180. doi: 10.1161/CIRCEP.113.001094. [DOI] [PubMed] [Google Scholar]
- 12.Kobashi A., Suwa M., Ito T. Solitary papillary muscle hypertrophy as a possible form of hypertrophic cardiomyopathy. Jpn Circ J. 1998;62:811–816. doi: 10.1253/jcj.62.811. [DOI] [PubMed] [Google Scholar]