ABSTRACT
Introduction:
Troponin release is common during critical illness. We hypothesized that there was an association between cardiac troponin T (cTnT) and biomarkers of systemic inflammation and ventricular dilatation.
Methods:
In an observational prospective cohort study, we enrolled consecutive adult patients admitted for noncardiac reasons to the intensive care unit (ICU) in two tertiary care centers. We measured cTnT, C-reactive protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT), and N-terminal pro brain natriuretic peptide (NT-proBNP) daily in the first week, and on alternate days in the second week. Using a peak cTnT cutoff ≥15 ng/L and concomitant changes on electrocardiogram, patients were categorized as “definite myocardial infarction (MI),” “possible MI,” “cTnT rise only,” or “no cTnT rise.” Within each group, associations between CRP, IL-6, PCT, NT-proBNP, and cTnT were investigated using mixed effect models.
Results:
One hundred seventy-two patients were included in the analysis of whom 84% had a cTnT rise ≥15 ng/L. Twenty-one patients (12%) had a definite MI, 51 (30%) had a possible MI, and 73 (42%) had a cTnT rise only. At the time of peak cTnT, 71% of patients were septic and 67% were on vasopressors.
Multivariable analysis showed a significant association between cTnT and IL-6 in all patients with a cTnT rise independent of age, gender, renal function, and cardiovascular risk factors. In patients without a definite MI, cTnT levels were significantly associated with PCT and NT-proBNP values. In patients without elevated cTnT levels, there was no associated NT-proBNP rise.
Conclusions:
In ICU patients admitted for non-cardiac reasons, serial cTnT levels were independently associated with markers of systemic inflammation and NT-proBNP.
Keywords: Critical illness, inflammation, inflammatory markers, sepsis, troponin, ventricular dilatation
INTRODUCTION
Troponin release is common during critical illness and associated with increased morbidity, mortality, and length of stay (1–15). We previously showed that 84% of patients admitted to the intensive care unit (ICU) for non-cardiac reasons had at least one elevated cardiac troponin T (cTnT) result (10). Only 14% of patients fulfilled criteria for a definite myocardial infarction (MI), and 27% had changes on electrocardiogram (ECG) suggestive of a possible MI.
Troponin T is a cardiac-specific molecule that is released into the systemic circulation following myocardial cell injury. Sepsis and inflammation are the leading non-cardiac causes of elevated troponin levels during critical illness (2, 14, 16–19). In sepsis, the heart undergoes physiologic and metabolic changes, including altered coronary blood flow, reduced oxygen extraction, regional and global wall hypokinesia, and ventricular dilatation (20–23). Coronary blood flow is often increased. In this situation, a potential explanation for troponin release is cellular ischemia due to microcirculatory changes within the myocardium (23–25). Circulating inflammatory cytokines appear to contribute through direct myocytotoxic effects, perturbations in microvascular flow and effects on cell permeability (20, 26). Regional and global wall hypokinesia resulting in increased cardiac filling pressures and ventricular dilatation may also play a role.
We hypothesized that there was a relationship between cTnT levels and biomarkers of systemic inflammation and ventricular dilatation during critical illness.
METHODS
Objectives
Our objectives were:
-
1.
to investigate the association between cTnT and C-reactive protein (CRP), interleukin-6 (IL-6), and procalcitonin (PCT) as markers of systemic inflammation;
-
2.
to investigate the association between cTnT and N-Terminal pro-brain natriuretic peptide (NT-proBNP) as a biomarker of ventricular dilatation.
Study protocol
The study protocol and methods have been described in detail elsewhere (10). We included a second centre (St George's University Hospital, London, UK) where the study was conducted during the same time period (June 2010 to April 2012) using the same protocol.
As previously described (10), we enrolled consecutive adult patients (≥18 years) admitted to the ICU for non-cardiac reasons. Patients with a high probability of cardiac injury or a primary cardiac diagnosis were excluded. In the first week, we measured cTnT, NT-proBNP, CRP, IL-6, and PCT and performed an ECG on a daily basis. During the second week, the blood tests and ECGs were taken on alternate days until discharge from ICU, death or day 14 after admission, whichever occurred first. Blood samples taken for research purposes were stored at −70°C and ECGs were kept in secure lockers until batch analysis at the end of the study. Clinicians were allowed to order ECGs, cTnT, and inflammatory markers separately as clinically indicated.
Laboratory analyses
All laboratory analyses were performed in the Department of Biochemistry at St George's University Hospital by two biochemists (ET and PC) who were blinded to the study and not involved in patient selection, data collection, and data analysis.
Cardiac Troponin T (cTnT) was measured using the Roche electrochemiluminescent high sensitivity sandwich immunoassay on the Elecsys 2010 (quoted analytical range: 3–10,000 ng/L; total coefficients of variation [CVs]: 1.5%–3.4% [measured between 24 and 2,665 ng/L] and reference range: <15 ng/L [99th percentile]). NT-proBNP was measured using the Diagnostic Products Corporation Immulite 2500 chemiluminescent sandwich immunoassay (analytical range: 20 and 35,000 pg/mL; quoted total CVs: 3.4%–5.6% [measured between 40.9 pg/mL and 32,096 pg/mL] and reference range: <125 pg/mL in patients <75 years and <450 pg/mL in those over 75). PCT was measured using a three-site sandwich immunoassay on the Advia Centaur (analytical range: 0.02–75 ng/mL and reference range: <0.1 ng/mL). CRP was measured using a latex enhanced immunoturbidimetric method on the Siemens Advia 2400 (analytical range: 4–336 mg/L; total CVs: 1.1%–1.4% (measured between 32 mg/L and 222 mg/L) and reference range: <10 mg/L]. IL-6 was measured using the Diagnostic Products Corporation Immulite 2500 chemiluminescent sandwich immunoassay (analytical range: 2–1,000 pg/mL; total CVs: 4.6%–7.2% (measured between 89 pg/mL and 724 pg/mL) and reference range: <5.9 pg/mL].
Data collection
We collected demographics, admission diagnosis, cardiovascular risk factors (ischemic heart disease [IHD], diabetes, hypertension, any type of vascular disease), Acute Physiology and Chronic Health Evaluation (APACHE) II score, serum creatinine, vasopressor use, treatment with renal replacement therapy (RRT), presence of sepsis (as per previous consensus criteria (27)) and ICU and hospital outcome.
Interpretation of troponin levels
As described previously (10), the ECGs were analyzed independently by two senior cardiologists who were blinded to the cTnT results and clinical details. In case of discrepancy, adjudication was undertaken by a third senior cardiologist. The diagnosis of acute MI was based on the most recent consensus criteria (28). Accordingly, patients were classified into four groups depending on their peak cTnT level and ECG taken on the same day or the day before: “definite MI,” with cTnT ≥15 ng/L and ECG changes consistent with MI, “possible MI,” with cTnT ≥15 ng/L and ischemic changes on ECG but not fulfilling criteria for definite MI, “cTnT rise alone,” and “no cTnT rise” with peak cTnT <15 ng/L.
Statistics
We used means for continuous variables and proportions for categorical variables to describe baseline characteristics. The four groups were compared using the Kruskal–Wallis method for continuous variables where the distribution was not normal and Chi-squared test for categorical variables. The associations between cTnT and CRP, IL-6, PCT, and NT-proBNP were investigated using two-level mixed effect models to take into account the serial measurements over the duration of stay in ICU. The patient identifiers were modeled as a random effect and the repeated blood values were effectively averaged within patients.
The models were initially fitted separately for each predictor to evaluate the unifactorial relationship with the outcome cTnT. Multivariable mixed effects models were used to adjust for potential confounders including age, gender, and history of IHD, hypertension and diabetes, or any form of vascular disease. We adjusted for renal function by adding a binary covariate indicating “treatment with RRT or serum creatinine ≥140 μmol/L,” versus “no RRT and serum creatinine <140 μmol/L.” All variables included in the models were chosen a priori on clinical grounds and all chosen variables were included in the multivariable models.
Since cTnT was log-transformed to correct for positive skewness, the model results were back-transformed and presented as percentage change associated with a “one unit” change in the variable of interest. To make it easier to compare the results for different biomarkers, we presented a standardized measure of effect by calculating the percentage change in cTnT that would occur if the biomarker changed by one standard deviation (SD) of its distribution (29). It was necessary to log-transform IL-6. As a result, its results could not be expressed in a meaningful way in terms of a percentage associated with a unit change. Instead, we calculated the change in cTnT for a SD change in IL-6 using the SD of the log-transformed data. All results are presented as estimates with 95% confidence intervals (CI) wherever possible. To illustrate the variations of each biomarker over time in each of the four groups, the daily median values of CRP, IL-6, PCT, NT-proBNP, and cTnT in their original units were graphically displayed.
The four groups were analyzed as independent cohorts as we aimed to examine the differences between the characteristics of these, and the associations between the outcome cTnT and the different markers for each of the four groups. The model residuals were examined for normality using Quantile Quantile Plots. Stata version 14 was used for the analyses.
Ethics
The study was approved by a formal Research Ethics Committee (REC) and the Research and Development Department in our hospital. Written informed consent was obtained from the patients prior to enrolment. As previously described, if a patient did not have capacity to consent, the opinion of a personal consultee was sought in accordance with section 32 of the Mental Capacity Act 2005 (UK). In this case, the patients were asked to give informed consent after they had regained capacity. If consent was declined, all collected samples and ECGs were discarded. In case retrospective consent could not be obtained due to death or lack of capacity, the REC approved that these patients could be included in the analysis.
RESULTS
Patient population
We enrolled 179 patients of whom seven were excluded (five patients had an acute coronary syndrome on admission to ICU which was not recognized by the research team at enrolment and two patients were transferred to another hospital within 48 h of ICU admission). The baseline characteristics of the remaining 172 patients are shown in Table 1. The main reasons for admission to ICU were sepsis (37%), postemergency surgery (21%), respiratory failure (11%), neurological emergency, including drug overdose (10%), gastrointestinal bleed (7%), acute kidney injury (4%), and other noncardiac causes (10%).
Table 1.
Baseline characteristics of patients in the four groups
| Characteristics | All groups | Definite MI | Possible MI | Raised cTnT only | No cTn rise | P value* |
| Group size (n) | 172 | 21 | 51 | 73 | 27 | |
| Total number of cTnT results | 997 | 138 | 318 | 415 | 126 | |
| Median number of cTnT results per patient (min–max) | 6 (1–11) | 7 (1–11) | 6 (1–11) | 6 (1–11) | 3 (1–11) | |
| Age, mean (SD) | 63.0 (16.6) | 61.4 (14.4) | 68.6 (14.4) | 64.9 (15.2) | 48.4 (17.7) | <0.01 |
| Female sex, n (%) | 72 (42%) | 8 (38%) | 22 (43%) | 34 (47%) | 8 (30%) | 0.48 |
| White ethnicity, n (%) | 142 (83%) | 18 (86%) | 40 (80%) | 63 (90%) | 21 (84%) | 0.33 |
| APACHE II score on admission to ICU, mean (SD) | 18.2 (6.6) | 18.0 (6.22) | 18.9 (6.2) | 20.0 (6.5) | 12.5 (4.8) | <0.01 |
| Serum creatinine ≥140 μmol/L at any time during study period, n (%) | 76 (44.2%) | 10 (47.6%) | 26 (51%) | 39 (53.4%) | 1 (3.7%) | <0.001 |
| Treatment with RRT, n (%) | 54 (31.4%) | 7 (33.3%) | 16 (31.4%) | 30 (41.1%) | 1 (3.7%) | 0.005 |
| RRT or serum creatinine ≥140 μmol/L, n (%) | 87 (50.6%) | 10 (47.6%) | 31 (60.8%) | 45 (61.6%) | 1 (3.7%) | <0.001 |
| Comorbidities | ||||||
| Ischemic heart disease, n (%) | 33 (20%) | 6 (29%) | 12 (24%) | 13 (18%) | 2 (7%) | 0.23 |
| Hypertension, n (%) | 67 (39%) | 8 (38%) | 30 (59%) | 24 (33%) | 5 (19%) | <0.01 |
| Diabetes, n (%) | 51 (30%) | 8 (38%) | 20 (39%) | 20 (27%) | 3 (11%) | 0.06 |
| Any form of vascular disease, n (%) | 34 (20%) | 4 (19%) | 15 (29%) | 14 (19%) | 1 (3.7%) | 0.06 |
| Mortality | ||||||
| In hospital, n (%) | 42 (24%) | 6 (29%) | 16 (31%) | 19 (26%) | 1 (3.7%) | 0.03 |
| In ICU, n (%) | 32 (19%) | 5 (24%) | 11 (22%) | 15 (21%) | 1 (3.7%) | 0.44 |
*P values are for the test of heterogeneity among the four proportions or means as appropriate.
APACHE indicates acute physiology and chronic health; cTnT, cardiac troponin T; ICU, intensive care unit; RRT, renal replacement therapy; SD, standard deviation.
Prevalence of troponin rise
One hundred forty-five patients (84%) had at least one cTnT value ≥15 ng/L during their stay in ICU. Among them, 21 (12%) had contemporaneous ECG changes consistent with a definite MI of whom 14 were recognized by the clinical team and four underwent coronary angiography. Fifty-one patients (35%) had ECG changes suggestive of a possible MI and 73 (42%) had a cTnT rise without any ECG changes. Twenty-seven patients (16%) had no cTnT rise ≥15 ng/L. The mean, median, ranges (minimum – maximum), and interquartile ranges of the cTnT levels are shown in Table 2. Between 1 and 11 daily measurements were taken per patient, and estimates were based on repeated measures over the observation period. The highest cTnT values were observed in patients with a definite MI. Patients with a “raised cTnT only” had the highest APACHE II score on admission, the highest proportion of patients with sepsis and also the highest IL-6 results (Tables 1 and 2).
Table 2.
Biomarkers of inflammation and ventricular dilatation in the four groups
| Parameter | Definite MI (n = 21) | Possible MI (n = 51) | Raised cTnT only (n = 73) | No cTnT rise (n = 27) |
| cTnT (ng/L) | ||||
| Mean (SD) | 250.1 (477) | 238.5 (486) | 88.7 (143) | 3.9 (4.1) |
| Median | 80.5 | 61 | 46 | 1.7 |
| IQR | 171 | 150 | 82 | 4 |
| Range (min–max) | 9–3093 | 5–3335 | <0.3–1337 | <0.3–14 |
| IL-6 (pg/mL) | ||||
| Mean (SD) | 131.5 (323) | 386.7 (1,566) | 1919 (20,863) | 142.0 (434) |
| Median | 48.1 | 55.8 | 55.1 | 51.8 |
| IQR | 72 | 131.3 | 146.7 | 75.1 |
| Range (min–max) | <2–2,522 | <2–18,120 | <2 to >300,000 | 5.49–4,037 |
| CRP (mg/L) | ||||
| Mean (SD) | 113.1 (78) | 126.8 (86.5) | 132.6 (97) | 134.7 (84.3) |
| Median | 101.2 | 108.9 | 107.4 | 126.1 |
| IQR | 125.1 | 113.8 | 144.2 | 125.3 |
| Range (min–max) | <4–448.3 | <4–428.2 | <4–499.1 | <4–326.6 |
| PCT (ng/mL) | ||||
| Mean (SD) | 4.1 (10.3) | 6.3 (13.1) | 8.2 (18.1) | 2.3 (5.9) |
| Median | 0.7 | 1.5 | 2 | 0.4 |
| IQR | 2.4 | 5.1 | 6.7 | 0.9 |
| Range (min–max) | 0.1–75 | 0–75 | 0–199.5 | 0–41.3 |
| NT-pro-BNP (ng/mL) | ||||
| Mean (SD) | 15.8 (21.3) | 15.1 (19.5) | 7.27 (11.5) | 1.97 (3.6) |
| Median | 6.6 | 7.4 | 3.0 | 0.3 |
| IQR | 22.5 | 19.6 | 7.4 | 1.3 |
| Range (min–max) | 0.02–155 | 0.08–108 | 0.02–75.5 | 0.02–16.5 |
| Sepsis* | 15 (71%) | 38 (75%) | 57 (78%) | 13 (48%) |
| Any vasopressor use* | 14 (67%) | 37 (73%) | 49 (67%) | 10 (37%) |
| Noradrenaline use* | 13 (62%) | 32 (63%) | 44 (60%) | 9 (33%) |
Values preceded by < indicate minimum level detected. Estimates presented in this table are based on all observations for all patients.
*On the day of peak cTnT level.
CRP indicates C-reactive protein; cTnT, cardiac troponin T; IL-6, interleukin 6; IQR, interquartile range; MI, myocardial infarction; NT-pro-PNP, N-terminal pro brain natriuretic peptide; PCT, procalcitonin; SD, standard deviation.
At the time of peak cTnT levels, 71% patients had sepsis and 67% were on vasopressor treatment. Noradrenaline was the most frequently used vasopressor (89%).
Association between troponin levels and inflammatory markers
The medians for CRP, IL-6, PCT, NT-proBNP, and cTnT were displayed for the four groups separately (Fig. 1). The graph illustrates the large variations of the different biomarkers between the four groups and the patterns of change within each group.
Fig. 1.
Patterns of variations described by medians over time from Day 1 to Day 14 for the outcome cardiac troponin T and biomarkers of inflammation and ventricular strain in four groups.
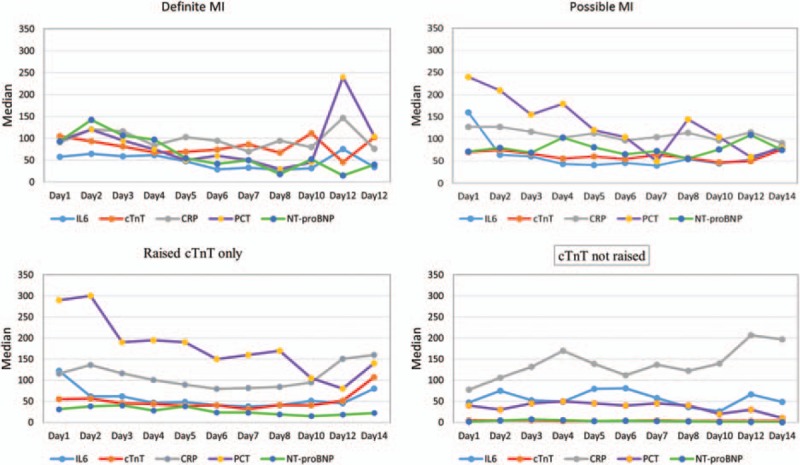
The daily median values of CRP, IL-6, PCT, NT-proBNP, and cTnT in their original units were graphically displayed to illustrate the variations of each biomarker over time in each of the four groups. NT-proBNP was scaled down for the graph by dividing it by 100 to allow the presentation on the same scale as other parameters. CRP indicates C-reactive protein; cTnT, cardiac troponin T; IL-6, interleukin-6; MI, myocardial infarction; NT-proBNP, N-terminal pro brain natriuretic peptide; PCT, procalcitonin.
Table 3 demonstrates the associations between cTnT and CRP, IL-6, and PCT and the presence of sepsis, both unadjusted and adjusted for potential confounders. Multivariable analysis showed an independent temporal association between cTnT and IL-6 in all patients with a cTnT rise. An increase in IL-6 by one SD was associated with a 16% to 23% rise in cTnT depending on individual cTnT group. In patients without a cTnT rise, there was no significant association between cTnT and IL-6.
Table 3.
Unadjusted and adjusted estimates of associations of cTnT with sepsis and CRP, IL-l, PCT, and NT-proBNP in different troponin groups
| Parameter | Unadjusted estimates: effect of factor on cTnT as a percentage per unit cTnT, except sepsis* (95% CI) | Adjusted estimates: effect of factor on cTnT as a percentage per unit cTnT, except sepsis* (95% CI) | Standardized effect sizes for adjusted analysis equivalent to a SD change in the relevant marker | ||||||||
| Effect size (%) | LCI | UCI | P value | Effect size (%) | LCI | UCI | P value | Effect size (%) | LCI | UCI | |
| Patients with definite MI (n = 21) | |||||||||||
| Sepsis | 33 | 6.0 | 68 | 0.01 | 28.85 | 1.99 | 62.79 | 0.03 | |||
| CRP | 0.13 | −0.07 | 0.33 | 0.20 | 0.12 | −0.08 | 0.32 | 0.25 | 9.70 | −6.16 | 28.23 |
| IL-6 (log)† | N/A | 0.006 | N/A | 0.02 | 23.37 | 3.68 | 46.80 | ||||
| PCT | 1.62 | −0.06 | 3.33 | 0.06 | 1.30 | −0.42 | 3.04 | 0.14 | 14.20 | −4.21 | 36.15 |
| NT-proBNP | 0.00 | −0.0003 | 0.0012 | 0.23 | 0.0004 | −0.0004 | 0.0012 | 0.31 | 0.01 | −0.01 | 0.02 |
| Patients with a possible MI (n = 51) | |||||||||||
| Sepsis | 10.01 | −5.48 | 28.03 | 0.22 | 5.98 | −8.81 | 23.18 | 0.45 | |||
| CRP | 0.13 | 0.03 | 0.23 | 0.01 | 0.11 | 0.01 | 0.21 | 0.03 | 10.08 | 0.85 | 20.16 |
| IL-6 (log)† | N/A | <0.001 | N/A | <0.001 | 17.45 | 8.14 | 27.56 | ||||
| PCT | 1.32 | 0.36 | 2.29 | 0.007 | 1.20 | 0.25 | 2.15 | 0.01 | 16.86 | 3.31 | 32.17 |
| NT-proBNP | 0.00 | 0.0006 | 0.0016 | <0.001 | 0.0010 | 0.0005 | 0.0015 | <0.001 | 0.02 | 0.01 | 0.03 |
| Patients with raised cTnT only (n = 73) | |||||||||||
| Sepsis | 22.12 | 6.56 | 39.95 | 0.004 | 23.17 | 4.99 | 37.80 | 0.01 | |||
| CRP | 0.09 | 0.00 | 0.17 | 0.045 | 0.08 | 0.00 | 0.16 | 0.07 | 7.87 | −0.47 | 16.92 |
| IL-6 (log)† | N/A | 0.001 | N/A | <0.001 | 15.68 | 6.58 | 25.56 | ||||
| PCT | 0.68 | 0.30 | 1.07 | <0.001 | 0.70 | 0.28 | 1.03 | 0.001 | 12.58 | 5.23 | 20.45 |
| NT-proBNP | 0.00 | 0.0010 | 0.0028 | <0.001 | 0.0018 | 0.0006 | 0.0024 | 0.001 | 0.02 | 0.01 | 0.03 |
| Patients without raised cTnT (n = 27) | |||||||||||
| Sepsis | −1.58 | −15.80 | 15.03 | 0.84 | −8.72 | −21.03 | 5.51 | 0.22 | |||
| CRP | 0.06 | −0.03 | 0.15 | 0.16 | 0.00 | −0.08 | 0.09 | 0.92 | 0.36 | −6.35 | 7.55 |
| IL-6 (log)† | N/A | 0.31 | N/A | 0.31 | −3.32 | −9.45 | 3.24 | ||||
| PCT | 2.86 | 1.35 | 4.38 | <0.001 | 2.21 | 0.89 | 3.54 | 0.001 | 13.74 | 5.35 | 22.79 |
| NT-proBNP | 0.01 | 0.0036 | 0.0089 | <0.001 | 0.0049 | 0.0025 | 0.0073 | <0.001 | 0.02 | 0.01 | 0.03 |
Adjusted mixed model gives average cTnT (outcome) after allowing for gender, age, ischemic heart disease, hypertension, RRT use, or creatinine ≥140 μmol/L, diabetes, and any form of vascular disease.
Use of standard effect sizes to estimate equivalent changes in cTnT:
Examples:
Relationship between cTnT and NT-proBNP: the standardised effect size for NT-proBNP in patients with a definite MI is 0.01. To obtain the equivalent change in cTnT for a change in NT-proBNP, calculate the following:
(value + 1)a where value is the estimate as a proportion, and a is the change in NT-proBNP.
For instance, the value of NT-proBNP is 0.01% (= 0.0001); to compute a 10-unit change, the formula translates into: (0.0001 + 1)10 = 1.001. This means, for a 10-unit change in NT-proBNP, the associated change of cTnT is 0.001 (i.e., 0.1%).
Impact of sepsis: the adjusted effect size describes the difference in cTnT between those with and without sepsis, e.g., if the value is 28.85%, the mean cTnT is raised by 28.85% in patients with sepsis compared with those without sepsis.
*Results are presented as the percentage change in cTnT as the inflammatory marker increases by one unit.
†Since Il-6 was log transformed as well as cTnT, the “one-unit change” estimates are not very meaningful and have been omitted.
CI indicates confidence interval; CRP, C-reactive protein; cTnT, cardiac troponin T; IL-6, interleukin 6; LCI, lower confidence interval; MI, myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCT, procalcitonin; RRT, renal replacement therapy; UCI, upper confidence interval.
Cardiac troponin T was also independently associated with PCT levels in all patients except those with a definite MI. An increase in PCT by one SD was associated with a 13% to 17% rise in cTnT.
There was an independent association between sepsis and cTnT results in patients with a definite MI and those with raised cTnT levels only. In cTnT-positive patients without contemporaneous ECG changes, the mean cTnT levels were raised by 23% in patients with sepsis compared with those without sepsis (Table 3). The adjusted estimates of percentage effect size and 95% CI were displayed using a high low graph for the four groups (Fig. 2).
Fig. 2.
Adjusted percentage effect size and 95% confidence intervals for different biomarkers in four groups.
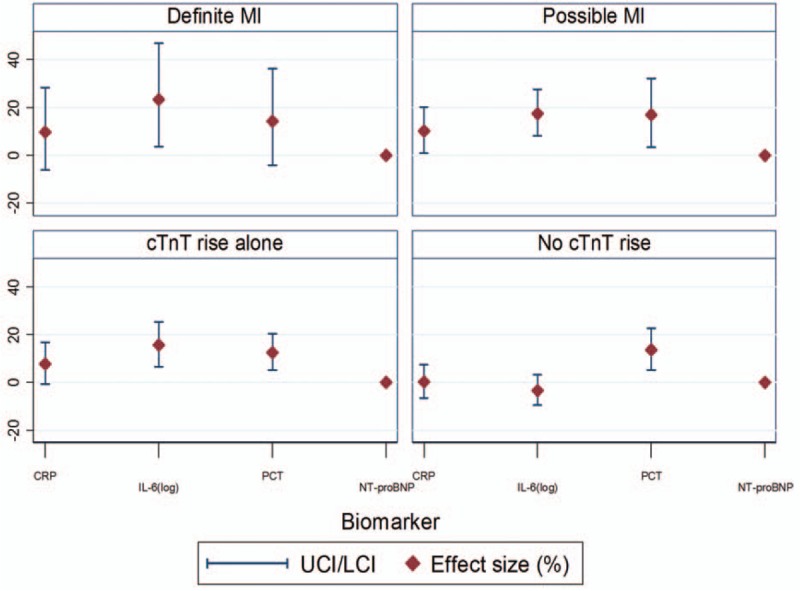
CRP indicates C-reactive protein; cTnT, cardiac troponin T; IL-6, Interleukin 6; LCI, lower confidence interval; MI, myocardial infarction; NT-proBNP, N-terminal pro brain natriuretic peptide; PCT, procalcitonin; UCI, upper confidence interval.
Association between troponin levels and NT-proBNP
There was a strong independent association between cTnT and NT-proBNP levels in all patient groups except those with a definite MI (Table 3). An increase in NT-proBNP by one SD was associated with a 0.02% increase in cTnT. In patients without elevated cTnT levels, there was no associated NT-proBNP rise.
DISCUSSION
Our study confirmed an association between cTnT levels and markers of systemic inflammation and NT-proBNP in critically ill patients admitted for non-cardiac reasons. In cTnT-positive patients, there was a significant association between cTnT release and IL-6 levels independent of age, gender, renal function, and presence of cardiovascular risk factors. An increase in IL-6 by one SD was associated with a 16% to 23% rise in cTnT. In addition, there was an independent association between cTnT levels and NT-proBNP in all patients except in those with a definite MI.
These results support the current theories for troponin release in the absence of myocardial necrosis. A popular theory is that troponin release may be caused by cellular ischemia without irreversible cell necrosis due to altered microcirculation (30, 31). Using isolated cultured myocardial cells, Schwartz et al. showed that during limited periods of ischemia, cultured cardiac myocytes remained viable but developed blebs containing cytoplasmic material (31). If ischemia was corrected relatively quickly, the blebs were either reabsorbed or shed into the circulation without myocyte damage. In patients with septic shock, post-mortem examinations have also shown more pronounced histological abnormalities in troponin-positive patients compared with patients without a troponin rise (32).
Ventricular dilatation and wall stress are other possible explanations for troponin release during sepsis. Studies in humans and animals suggest that cytokines, in particular IL-1β, IL-6, and tumor necrosis factor alpha (TNF-α), may play a role (33). TNF-α is known to increase the permeability of endothelial cells. Its effect on myocardial cells appears to be similar, thus leading to leakage of cTn (34). However, in healthy volunteers who underwent intravenous injection of endotoxin and subsequently developed significant elevations in serum TNF-α, IL-6, and IL-8 levels, there was no significant increase in systemic cTn levels which suggests that TNF-α alone may not be sufficient to induce significant changes (35).
In vitro studies have shown that serum obtained from patients with septic shock was able to depress rat cardiomyocytes’ contractility and induce ventricular dilatation (36, 37). Fernandes et al. (38) described a correlation between raised troponin levels and left ventricular systolic dysfunction in critically ill patients with sepsis. Similarly, ver Elst et al. showed that in patients with septic shock, elevated cTn levels were strongly associated with left ventricular dysfunction, as assessed by transoesophageal echocardiography (30). In a separate study of 37 patients with septic shock, those with raised troponin levels had a higher incidence of regional wall motion abnormalities on echocardiography (56% vs. 6%, P = 0.002), lower ejection fraction (46% vs. 62%, P = 0.04), and higher mortality (56% vs. 24%, P = 0.04) compared with patients with normal troponin levels (39). Finally, Landesberg et al. (40) investigated general ICU patients with severe sepsis/septic shock and demonstrated that left ventricular diastolic dysfunction and right ventricular dilatation were the echocardiographic signs that correlated best with troponin elevation.
Atrial and ventricular filling pressures in the left and right chambers are the main determinants of release of natriuretic peptides. NT-proBNP is a marker of ventricular dilatation and strain. In a subanalysis of the Albumin Italian Outcome Sepsis trial including 995 patients with severe sepsis or septic shock, Masson et al. showed that 97% of patients had NT-proBNP levels above the upper limit of normal and 85% of patients had cTnT levels greater than normal (41). However, cardiac function was not directly assessed.
Our findings support the hypothesis that troponin release during critical illness may be representative of both systemic inflammation and ventricular strain. With serial troponin levels of 172 patients from two different centers for up to 14 days, our study is one of the largest in the literature. The findings are strengthened by the fact that we only included ICU patients admitted for non-cardiac reasons, analyzed patients in four different groups depending on whether they had a definite, possible, or no MI during their stay in ICU, corrected for important confounding factors, had all ECGs interpreted independently by senior cardiologists who were blinded to the troponin results, and tested hypotheses that had been formed a priori.
It is still important to acknowledge some limitations. The observational nature of the study prevents us from defining causality between inflammatory markers and cTnT elevations. Second, we analyzed patients with and without sepsis which may have impacted the strength of the associations. Third, we did not measure IL-1β or TNF-α, two key inflammatory cytokines. Similarly, we did not perform routine echocardiography in all patients to correlate the NT-proBNP results with ventricular dilatation and acknowledge that other mechanisms beyond ventricular stretch stimulate BNP release (42). Finally, we defined sepsis according to the previous consensus criteria as our study was conducted before the new sepsis criteria were published (27).
Despite these limitations, we believe that our findings advance the understanding of troponin release during critical illness. Obviously, the results do not allow any recommendations regarding optimal management of troponin-positive patients after an MI has been excluded. We note the findings of a nationwide survey of 310 intensivists in the United States regarding the management of ICU patients with elevated troponin levels without typical features of acute coronary syndrome (43). Seventy-six percent of respondents stated that they would start either aspirin or clopidogrel, and 47% would commence heparin, 49% recommended high-dose statins, 69% would start beta-blockers, and 38% would use an angiotensin-converting-enzyme inhibitor. In addition, 73% of the intensivists would request a cardiology consultation and 52% would make a referral for coronary angiography. Given the wide variety of opinions, a better understanding and more guidance regarding the management of troponin-positive patients is urgently required.
In conclusion, our results show that cTnT is associated with NT-pro-BNP and markers of systemic inflammation, especially in patients without a definite MI.
Footnotes
Professor David Bennett made significant contributions to the study protocol. He sadly died on February 21, 2012 and is still deeply missed.
MO and DT designed the protocol and led the research project. KL, JS, BS, and CM recruited patients and collected the necessary specimens and ECGs. PC and ET performed the laboratory analyses. SA, JL, and JP performed the statistical analyses. MO wrote the first draft. All authors revised the manuscript and contributed to the data interpretation. All authors approved the final manuscript.
Professor JP and Dr SA are supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas’ NHS Foundation Trust and King's College London. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, or the Department of Health.
The authors report no conflicts of interest.
REFERENCES
- 1.Lim W, Cook DJ, Griffith LE, Crowther MA, Devereux PJ. Elevated cardiac troponin levels in critically ill patients: prevalence, incidence, and outcomes. Am J Crit Care 2006; 15 3:280–288. [PubMed] [Google Scholar]
- 2.Bessiere F, Khenifer S, Dubourg J, Durieu I, Lega JC. Prognostic value of troponin in sepsis: a meta-analysis. Intensive Care Med 2013; 39:1181–1189. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton MA, Toner A, Cecconi M. Troponin in critically ill patients. Minerva Anestesiol 2012; 78 9:1039–1045. [PubMed] [Google Scholar]
- 4.Klein Gunnewiek JM, van de Leur JJ. Elevated troponin T concentrations in critically ill patients. Intensive Care Med 2003; 29 12:2317–2322. [DOI] [PubMed] [Google Scholar]
- 5.Guest TM, Ramanathan AV, Tuteur PG, Schechtmann KB, Ladenson JH, Jaffe AS. Myocardial injury in critically ill patients. A frequently unrecognized complication. JAMA 1995; 273 24:1945–1949. [PubMed] [Google Scholar]
- 6.Ammann P, Maggiorini M, Bertel O, Haenseler E, Joller-Jemelka HI, Oechslin E, Minder EI, Rickli H, Fehr T. Troponin as a risk factor for mortality in critically ill patients without acute coronary syndromes. J Am Coll Cardiol 2003; 41 11:2004–2009. [DOI] [PubMed] [Google Scholar]
- 7.Stein R, Gupta B, Agarwal S, Golub J, Bhutani D, Rosman A, Eng C. Prognostic implications of normal (<0.10 ng/ml) and borderline (0.10 to 1.49 ng/ml) troponin elevation levels in critically ill patients without acute coronary syndrome. Am J Cardiol 2008; 102 5:509–512. [DOI] [PubMed] [Google Scholar]
- 8.Wu TT, Yuan A, Chen CY, Chen WJ, Luh KT, Kuo SH, Lin FY, Yang PC. Cardiac troponin I levels are a risk factor for mortality and multiple organ failure in noncardiac critically ill patients and have an additive effect to the APACHE II score in outcome prediction. Shock 2004; 22 2:95–101. [DOI] [PubMed] [Google Scholar]
- 9.Relos RP, Hasinoff IK, Beilman GJ. Moderately elevated serum troponin concentrations are associated with increased morbidity and mortality rates in surgical intensive care unit patients. Crit Care Med 2003; 31 11:2598–2603. [DOI] [PubMed] [Google Scholar]
- 10.Ostermann M, Lo J, Toolan M, Tuddenham E, Sanderson B, Lei K, Smith J, Griffiths A, Webb I, Coutts J, et al. A prospective study of the impact of serial troponin measurements on the diagnosis of myocardial infarction and hospital and 6-month mortality in patients admitted to ICU with non-cardiac diagnoses. Crit Care 2014; 18 2:R62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed AN, Blonde K, Hackam D, Iansavichene A, Mrkobrada M. Prognostic significance of elevated troponin in non-cardiac hospitalized patients: a systematic review and meta-analysis. Ann Med 2014; 46:653–663. [DOI] [PubMed] [Google Scholar]
- 12.Audimooolam VK, McPhail MJ, Sherwood R, Willars C, Bernal W, Wendon JA. Elevated troponin I and its prognostic significance in acute liver failure. Crit Care 2012; 16 6:R228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim W, Whitlock R, Khera V, Devereaux PJ, Tkaczyk A, Heels-Ansdell D, Jacka M, Cook D. Etiology of troponin elevation in critically ill patients. J Crit Care 2010; 25 2:322–328. [DOI] [PubMed] [Google Scholar]
- 14.Sheyin O, Davies O, Duan W, Perez X. The prognostic significance of troponin elevation in patients with sepsis: a meta-analysis. Heart & Lung 2015; 44:75–81. [DOI] [PubMed] [Google Scholar]
- 15.Vasile VC, Babuin L, Rio Perez JA, Alegria JR, Song LM, Chai HS, Afessa B, Jaffe AS. Long-term prognostic significance of elevated cardiac troponin levels in critically ill patients with acute gastrointestinal bleeding. Crit Care Med 2009; 37 1:140–147. [DOI] [PubMed] [Google Scholar]
- 16.Maeder M, Fehr T, Rickli H, Amman P. Sepsis-associated myocardial dysfunction: diagnostic and prognostic impact of cardiac troponins and natriuretic peptides. Chest 2006; 129:1349–1366. [DOI] [PubMed] [Google Scholar]
- 17.Spies C, Haude V, Fitzner R, Schröder K, Overbeck M, Runkel N, Schaffartzik W. Serum cardiac troponin T as a prognostic marker in early sepsis. Chest 1998; 113 4:1055–1063. [DOI] [PubMed] [Google Scholar]
- 18.Baillard C, Boussarsar M, Fosse JP, Girou E, Le Toumelin P, Cracco C, Jaber S, Cohen Y, Brochard L. Cardiac troponin I in patients with severe exacerbation of chronic obstructive pulmonary disease. Intensive Care Med 2003; 29:584–589. [DOI] [PubMed] [Google Scholar]
- 19.Røsjø H, Varpula M, Hagve TA, Karlsson S, Ruokonen E, Pettilä V, Kurola J, Omland T. FINNRESUSCI Laboratory Study Group:. Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med 2011; 37:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parker MM, Shelhamer JH, Bacharach SL, Green MV, Natanson C, Frederick TM, Damske BA, Parrillo JE. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med 1984; 100:483–490. [DOI] [PubMed] [Google Scholar]
- 21.Bouhemad B, Nicolas-Robin A, Arbelot C, Arthaud M, Féger F, Rouby JJ. Acute left ventricular dilatation and shock-induced myocardial dysfunction. Crit Care Med 2009; 37:441–447. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu SD. Cytokine-induced modulation of cardiac function. Circ Res 2004; 95:1140–1153. [DOI] [PubMed] [Google Scholar]
- 23.Dhainaut JF, Huyghebaert MF, Monsallier JF, Jefevre G, Dall’Ava-Santucci J, Brunet F, Villemant D, Carli A, Raichvarg D. Coronary hemodynamics and myocardial metabolism of lactate, free fatty acids, glucose, and ketones in patients with septic shock. Circulation 1987; 75:533–541. [DOI] [PubMed] [Google Scholar]
- 24.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. J Exp Med 1996; 183:949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Altmann DR, Korte W, Maeder MT, Fehr T, Haager P, Rickli H, Kleger GR, Rodriguez R, Ammann P. Elevated cardiac troponin I in sepsis and septic shock: no evidence for thrombus associated myocardial necrosis. PLoS One 2010; 5:e9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunnion RE, Schaer GL, Parker MM, Natanson C, Parrillo JE. The coronary circulation in human septic shock. Circulation 1986; 73:637–644. [DOI] [PubMed] [Google Scholar]
- 27.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992; 101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Lindahl B, Morrow DA, Clemmensen PM, et al. Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial InfarctionAT Third universal definition of myocardial infarction. Circulation 2012; 126 16:2020–2035. [DOI] [PubMed] [Google Scholar]
- 29.Peacock J, Kerry S. Presenting Medical Statistics form Proposal to Publication. 2007; Oxford: Oxford University Press, page 102. [Google Scholar]
- 30.Hickman PE, Potter JM, Aroney C, Koerbin G, Southcott E, Wu AH, Roberts MS. Cardiac troponin may be released by ischemia alone, without necrosis. Clin Chim Acta 2010; 411:318–323. [DOI] [PubMed] [Google Scholar]
- 31.Schwartz P, Piper HM, Spahr R, Spieckermann PG. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am J Pathol 1984; 115:349–361. [PMC free article] [PubMed] [Google Scholar]
- 32.Ver Elst KM, Spapen HD, Nguyen DN, Garbar C, Huyghens LP, Gorus FK. Cardiac troponins I and T are biological markers of left ventricular dysfunction in septic shock. Clin Chem 2000; 46:650–657. [PubMed] [Google Scholar]
- 33.Favory R, Neviere R. Bench-to-bedside review: significance and interpretation of elevated troponin in septic patients. Crit Care 2006; 10 4:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piper HM, Schwartz P, Spahr R, Hütter JF, Spieckermann PG. Early enzyme release from myocardial cells is not due to irreversible cell damage. J Mol Cell Cardiol 1984; 16:385–388. [DOI] [PubMed] [Google Scholar]
- 35.van Bockel EA, Tulleken JE, Muller Kobold AC, Ligtenberg JJ, van der Werf TS, Spanjersberg R, Zijlstra JG. Cardiac troponin I release and cytokine response during experimental human endotoxaemia. Intensive Care Med 2003; 29 9:1598–1600. [DOI] [PubMed] [Google Scholar]
- 36.Parrillo JE, Burch C, Shelhamer JH, Parker MM, Natanson C, Schuette W. A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. J Clin Invest 1985; 76:1539–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoffmann JN, Werdan K, Hartl WH, Jochum M, Faist E, Inthorn D. Hemofiltrate from patients with severe sepsis and depressed left ventricular contractility contains cardiotoxic compounds. Shock 1999; 12:174–180. [DOI] [PubMed] [Google Scholar]
- 38.Fernandes CJ, Jr, Akamine N, Knobel E. Cardiac troponin: a new serum marker of myocardial injury in sepsis. Intensive Care Med 1999; 25:1165–1168. [DOI] [PubMed] [Google Scholar]
- 39.Mehta NJ, Khan IA, Gupta V, Jani K, Gowda RM, Smith PR. Cardiac troponin I predicts myocardial dysfunction and adverse outcome in septic shock. Int J Cardiol 2004; 95:13–17. [DOI] [PubMed] [Google Scholar]
- 40.Landesberg G, Jaffe AS, Gilon D, Levin PD, Goodman S, Abu-Baih A, Beeri R, Weissman C, Sprung CL, Landesberg A. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med 2014; 42 4:790–800. [DOI] [PubMed] [Google Scholar]
- 41.Zakynthinos E, Kiropoulos T, Gourgoulianis K, Filippatos G. Diagnostic and prognostic impact of brain natriuretic peptide in cardiac and noncardiac diseases. Heart Lung 2008; 37 4:275–285. [DOI] [PubMed] [Google Scholar]
- 42.Masson S, Caironi P, Fanizza C, Carrer S, Caricato A, Fassini P, Vago T, Romero M, Tognoni G, Gattinoni L, et al. Albumin Italian Outcome Sepsis Study Investigators. Sequential N-Terminal Pro-B-type natriuretic peptide and high-sensitivity cardiac troponin measurements during albumin replacement in patients with severe sepsis or septic shock. Crit Care Med 2016; 44:707–716. [DOI] [PubMed] [Google Scholar]
- 43.Gundre P, Kleyn M, Kulbak G, Kupfer Y, Tessler S. Elevated troponin Cs in intensive care units—a nationwide survey of critical care physicians. Chest 2011; 140:1013A. [Google Scholar]


