Abstract
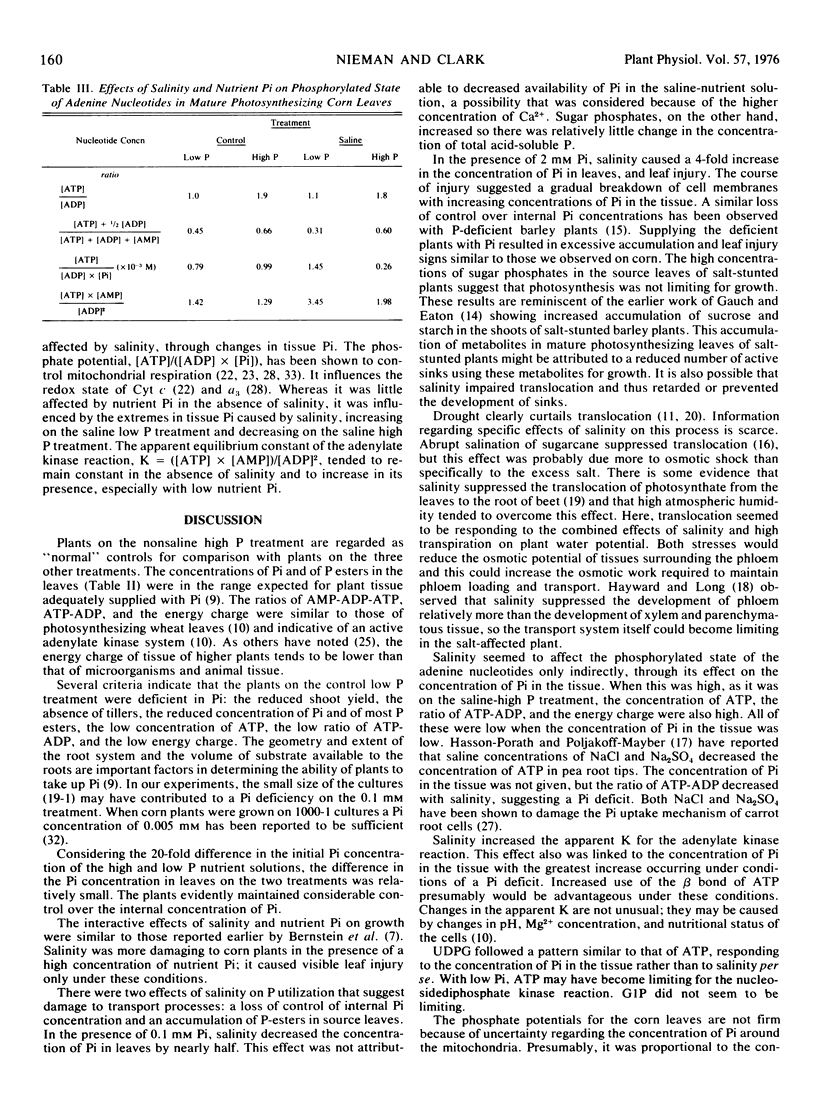
The effects of salinity on corn plants (Zea mays L.) are influenced by the concentration of nutrient orthophosphate. Salinity (−2 bars each of NaCl and CaCl2) was more injurious in combination with a high concentration of orthophosphate (2 mm) (that gave optimum yields in the absence of salinity) than it was with a lower concentration (0.1 mm). With 2 mm orthophosphate, salinity seemed to damage the plant mechanisms that normally regulate the internal concentration of orthophosphate resulting in excessive accumulation and P toxicity. On the other hand, with 0.1 mm orthophosphate, salinity decreased orthophosphate concentration in mature leaves. This effect was paralleled by decreases in the concentration of adenosine 5′-triphosphate and in the energy charge of the adenylate system, indicating an orthophosphate deficit. Even so, plants survived salinity better under these conditions than in the presence of 2 mm orthophosphate. The data indicated that salinity affected the phosphorylated state of the adenine nucleotides only indirectly through its effect on the concentration of orthophosphate in the cells.
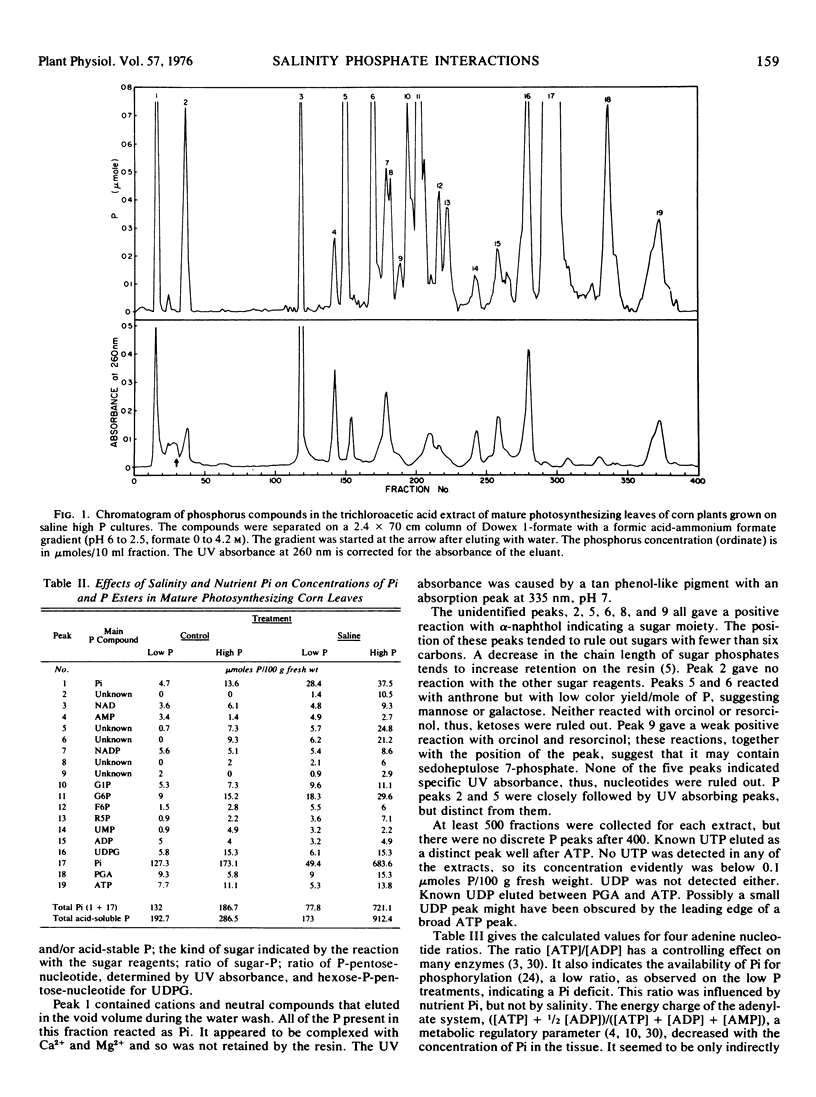
Salinity, especially in the presence of 2 mm orthophosphate, resulted in an increase in the concentrations of sugar phosphates in mature photosynthesizing leaves, suggesting that translocation rather than photosynthesis was a limiting process. Decreased translocation could be a secondary effect of decreased growth. However, a decreased translocation rate could cause decreased growth by limiting the supply of essential metabolites reaching growing tissues.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson D. E. The energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968 Nov;7(11):4030–4034. doi: 10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Methods for the isolation of glycolytic intermediated by column chromatography with ion exchange resins. J Biol Chem. 1959 Mar;234(3):459–465. [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- Bomsel J. L., Pradet A. Study of adenosine 5'-mono-,di- and triphosphates in plant tissues. IV. Regulation of the level of nucleotides, in vivo, by adenylate kinase: theoretical and experimental study. Biochim Biophys Acta. 1968 Aug 20;162(2):230–242. doi: 10.1016/0005-2728(68)90105-9. [DOI] [PubMed] [Google Scholar]
- Gauch H. G., Eaton F. M. EFFECT OF SALINE SUBSTRATE ON HOURLY LEVELS OF CARBOHYDRATES AND INORGANIC CONSTITUENTS OF BARLEY PLANTS. Plant Physiol. 1942 Jul;17(3):347–365. doi: 10.1104/pp.17.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartt C. E. Effect of Moisture Supply upon Translocation and Storage of C in Sugarcane. Plant Physiol. 1967 Mar;42(3):338–346. doi: 10.1104/pp.42.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasson-Porath E., Poljakoff-Mayber A. Content of adenosine phosphate compounds in pea roots grown in saline media. Plant Physiol. 1971 Jan;47(1):109–113. doi: 10.1104/pp.47.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isherwood F. A., Barrett F. C. Analysis of phosphate esters in plant material. Extraction and purification. Biochem J. 1967 Sep;104(3):922–933. doi: 10.1042/bj1040922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLINGENBERG M. [On the reversibility of oxidative phosphorylation. IV. Relation between the redox state of cytochrome c and the phosphorylation potential of adenosine triphosphate]. Biochem Z. 1961;335:263–272. [PubMed] [Google Scholar]
- Koobs D. H. Phosphate mediation of the Crabtree and Pasteur effects. Science. 1972 Oct 13;178(4057):127–133. doi: 10.1126/science.178.4057.127. [DOI] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman R. H., Willis C. Correlation between the Suppression of Glucose and Phosphate Uptake and the Release of Protein from Viable Carrot Root Cells Treated with Monovalent Cations. Plant Physiol. 1971 Sep;48(3):287–293. doi: 10.1104/pp.48.3.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen C. S., Wilson D. F. Control of respiration by the mitochondrial phosphorylation state. Arch Biochem Biophys. 1974 Apr 2;161(2):581–591. doi: 10.1016/0003-9861(74)90341-5. [DOI] [PubMed] [Google Scholar]
- Wilson D. F., Owen C., Mela L., Weiner L. Control of mitochondrial respiration by the phosphate potential. Biochem Biophys Res Commun. 1973 Jul 2;53(1):326–333. doi: 10.1016/0006-291x(73)91437-x. [DOI] [PubMed] [Google Scholar]



