KEY TEACHING POINTS.
|
Introduction
Uhl’s disease is a rare congenital cardiac anomaly characterized by partial or complete absence of the right ventricular free wall myocardium, which is replaced by fibroelastic and adipose tissue. Complications that result can be divided into either right ventricular dysfunction with congestive heart failure, or arrhythmias. While ventricular arrhythmias predominate, there are case reports of atrial flutter, intra-atrial reentry tachycardia, atrioventricular (AV) block, and bilateral bundle branch block.1, 2, 3, 4 Sudden cardiac death has been reported in patients with Uhl’s anomaly.
Herein we report an adolescent patient with out-of-hospital cardiac arrest with 2 unusual arrhythmia mechanisms. What are the mechanisms for these 2 arrhythmias?
Case report
Our patient was a 12-year-old male subject with a known history of Uhl’s anomaly diagnosed at age 9 months, who had been well and was playing at home when he acutely became cyanotic and collapsed. Paramedics arrived quickly, continued cardiopulmonary resuscitation, delivered 2 DC shocks, and administered epinephrine for pulseless electrical activity. Eventually there was return of spontaneous circulation with sinus tachycardia. Prior to this event, the patient had been feeling well, without any significant limitations of physical activity or known palpitations.
Electrocardiogram, stress test, and Holter
The baseline electrocardiogram revealed large-amplitude P waves (Figure 1). The patient had undergone an exercise stress test approximately 1 year prior that revealed no exercise-induced arrhythmia or ischemic electrocardiographic changes and a VO2 max at the 71st percentile. A 24-hour Holter monitor done 18 months prior to his arrest revealed no arrhythmia.
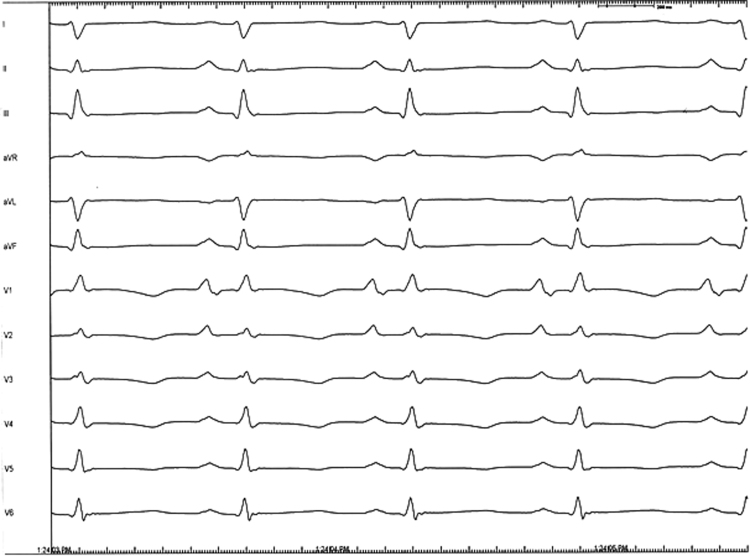
Figure 1.
Baseline 12-lead electrocardiogram prior to electrophysiology study. Large P waves were noted in lead V1 and V2.
Cardiac imaging
Cardiac magnetic resonance imaging performed 2 weeks after recovery from the cardiac arrest event revealed that the right ventricular parietal wall was severely thinned and noncontractile. Fat–water separation imaging demonstrated no fat present in the right ventricular parietal wall, making the diagnosis consistent with Uhl’s anomaly. Right ventricle size was severely enlarged, and right ventricular systolic function was severely depressed.
Electrophysiology study
The patient underwent a diagnostic electrophysiology study with ablation using 3 electrophysiologic catheters placed under nonfluoroscopic guidance with the Carto 3 electrophysiology mapping system (Biosense Webster, South Diamond Bar, CA). A 3-dimensional anatomic map of the right atrium, coronary sinus, and right ventricle was developed and used for catheter placement, electrophysiologic diagnosis, and catheter ablation. The baseline AH and HV intervals were normal, measuring 59 ms and 50 ms, respectively. Two sustained arrhythmias were induced, both resulting in extremely low blood pressure and hemodynamic instability (Figure 2A and B). The arrhythmia shown in Figure 2B required cardioversion despite attempts at pace termination with atrial and ventricular overdrive pacing. We concluded that either arrhythmia might have resulted in syncope and possible cardiac arrest.
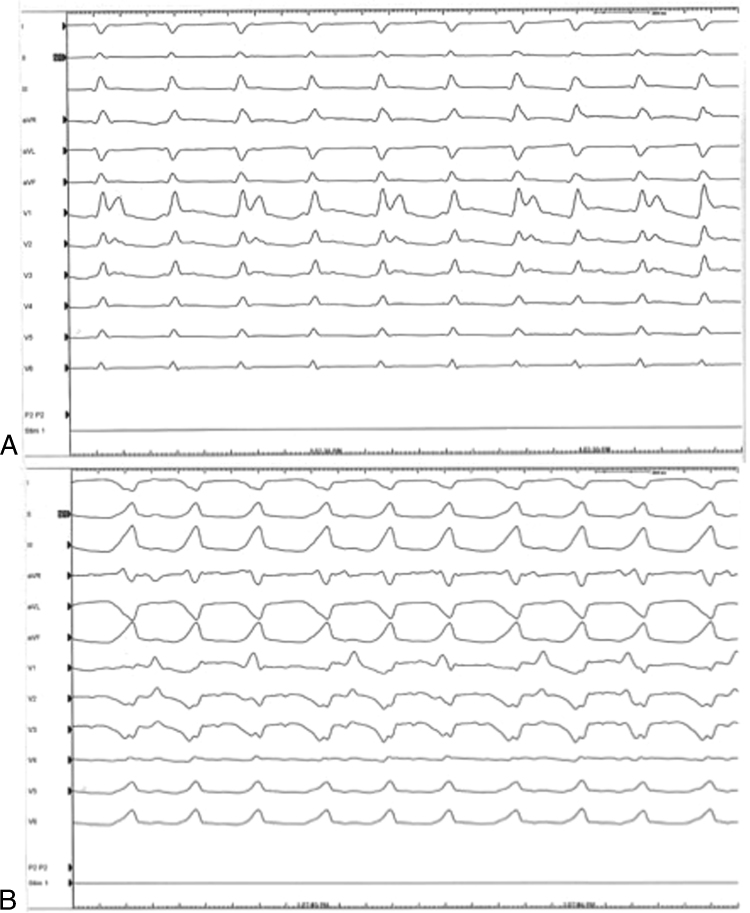
Figure 2.
Twelve-lead electrocardiograms during the 2 induced arrhythmias. A: Narrow QRS complex tachycardia (cycle length = 240 ms). During this tachycardia 2:1 ventricular-to-atrial (VA) conduction is evident. B: Wide complex tachycardia (left bundle branch block with right axis deviation, cycle length = 235 ms). During this tachycardia, VA dissociation was evident.
The narrow QRS complex tachycardia observed in Figure 2A was induced either during atrial extrastimulus testing or during rapid atrial pacing at cycle lengths less than 300 ms. During double atrial extrastimulus testing we observed an AH jump (105 ms) with a single AV nodal echo beat. During rapid atrial pacing, as the paced cycle length was decreased the PR and AH intervals gradually increased and were consistent with fast AV nodal pathway conduction (AH interval increased from 80 to 95 ms). However, at an atrial paced cycle length of 250 ms, the last paced atrial complex was conducted simultaneously over the antegrade fast and slow AV nodal pathways (1:2 atrial-to-ventricular conduction). Figure 3A suggests the presence of simultaneous dual antegrade AV conduction. A less likely possibility to explain this finding would be triggered junctional activity. The induced narrow QRS complex tachycardia (cycle length = 260 ms) was mostly associated with 2:1 VA conduction (Figure 3A), although at times 1:1 VA conduction was observed (Figure 3B). The pattern of VA conduction was concentric. Rapid atrial pacing at the Wenckebach cycle length was performed on multiple occasions to further understand the mechanism for the narrow QRS complex tachycardia seen in Figure 3A; however, the wide QRS complex tachycardia (WCT) (left bundle branch block [LBBB] and right axis deviation [RAD], cycle length = 235 ms) shown in Figure 2B was induced multiple times.
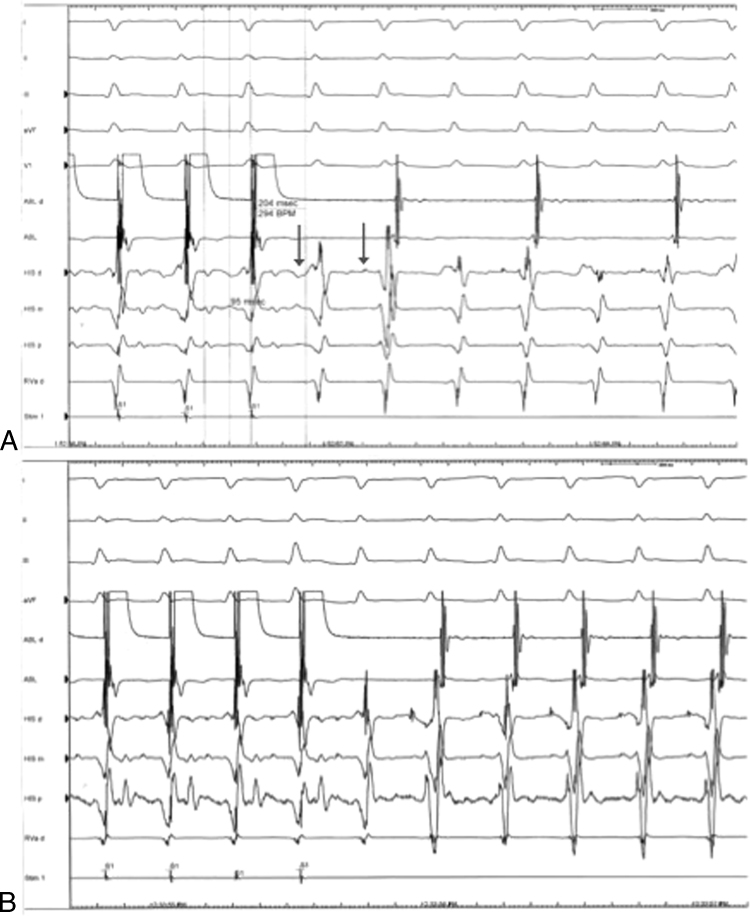
Figure 3.
Narrow QRS tachycardia. A: Induction of supraventricular tachycardia (SVT) with 1:2 atrioventricular (AV) response. Following termination of a train of rapid atrial pacing (atrial paced cycle length = 250 ms), a narrow QRS complex tachycardia is induced. The last paced atrial complex results in a double ventricular response, simultaneous AV conduction over the fast and slow AV nodal pathways (arrows). Conduction over the slow AV nodal pathway with a prolonged AH interval facilitates the occurrence of sustained AV nodal reentrant tachycardia (AVNRT) with 2:1 ventricular-to-atrial (VA) conduction. B: SVT with 1:1 VA conduction. Following a different attempt at induction of SVT during rapid atrial pacing, a double antegrade AV conduction response is again observed (1:2 AV conduction) with induction of AVNRT. During this episode of SVT, 1:1 VA conduction is evident. Format of electrocardiographic tracings: 5 surface electrocardiograms, and intracardiac electrograms recorded from the ablation catheter (high right atrium), His bundle catheter, and right ventricular catheter.
The WCT shown in Figure 2B was initially thought to represent AV nodal reentrant tachycardia (AVNRT) with aberration; however, on inspection of its induction mechanism and response to atrial and ventricular entrainment, a different mechanism became apparent. The WCT could be induced by 2 different stimulation paradigms. The easiest mechanism for induction of the WCT was rapid atrial pacing at short atrial paced cycle lengths, which produced HV block (Figure 4A). The atrial paced beat following the HV block initiated the WCT. The WCT could also be induced during ventricular extrastimulus testing (Figure 4B). The WCT started following 2 beats of slow–intermediate AV nodal reentry. Rapid atrial pacing during the WCT resulted in entrainment of the atrium and His bundle, with dissociation from the ventricle (Figure 4A). Ventricular pacing with the right ventricular pacing catheter located near the proximal right bundle branch resulted in concealed entrainment with a post-pacing interval of 15 ms (Figure 4C). After termination of the wide QRS tachycardia, a fascicular potential was noted at this site. Ventricular pacing at slower cycle lengths produced a QRS morphology closely matching the WCT–LBBB and right axis deviation (Figure 4D).
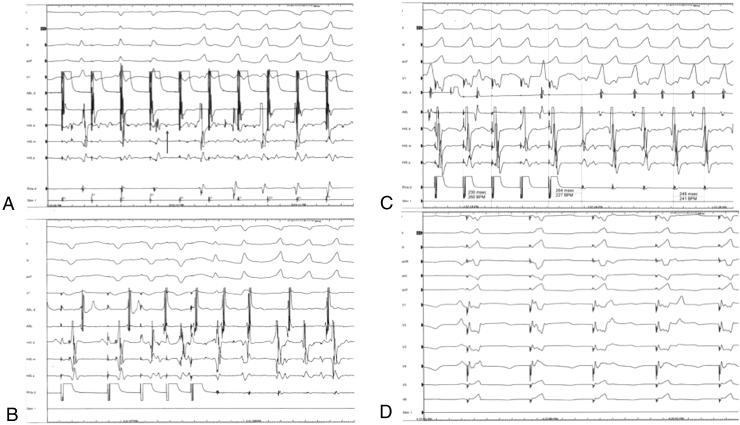
Figure 4.
Wide QRS complex tachycardia. A: Induction of wide complex tachycardia (WCT) with rapid atrial pacing. Following the fourth atrial paced event from the left, His-to-ventricular conduction time (HV) block is noted. Gradual HV prolongation is evident on the previous 2 atrial paced beats. The subsequent fifth atrial paced complex is associated with a shortened HV interval and possible pre-excitation. Given the dissociation of atrial and His electrograms from the ventricle on ensuing atrial paced events, it is probable that the tachycardia started on the fourth QRS complex, following the HV block. B: Induction of the WCT during delivery of triple ventricular extrastimuli. The third ventricular paced complex was delivered in the ventricular refractory and does not capture the ventricle. Following the second ventricular extrastimuli 2 beats of atrioventricular node reentry occur, which facilitates the occurrence of the WCT. C: Ventricular pacing at a cycle length = 230 ms in the region of the proximal right bundle branch resulted in concealed entrainment with a post-pacing interval of 15 ms (recovery interval = 264 ms). D: QRS morphology during pacing at the fascicular potential site elicits QRS morphology similar to the WCT (compare with Figure 2B). Format of electrocardiographic tracings is similar to Figure 3.
Radiofrequency ablation of the slow AV nodal pathway rendered both tachycardias noninducible.
Discussion
Electrophysiologic study in our patient revealed 2 different arrhythmia mechanisms that resulted in hemodynamic compromise and may have been responsible for syncope and sudden cardiac arrest. One arrhythmia mechanism involved an unusual form of AVNRT (narrow QRS tachycardia), and the other a fascicular–ventricular reentrant circuit (WCT).
1:2 AV conduction is a rare form of antegrade simultaneous conduction through 2 AV nodal pathways (fast and slow AV nodal pathways), and has been reported to result in both AVNRT and a nonreentrant form of tachycardia.5, 6 Previous reports of double ventricular response have only included adult subjects. Further atypical for our patient’s narrow QRS complex tachycardia was the presence of 2:1 ventricular-to-atrial conduction during AVNRT.
What was the mechanism of the wide QRS tachycardia? Our electrophysiologic data provide evidence that this arrhythmia was an unusual form of fascicular–ventricular reentry.
The WCT was characterized by the following observations:
-
1)
induction during rapid atrial pacing associated with HV prolongation and block, as well as during programmed ventricular stimulation associated with AV nodal reentry
-
2)
a very prolonged HV interval, more likely secondary to distal to proximal retrograde His activation
-
3)
variable ventricular-to-atrial conduction during tachycardia
-
4)
ability to dissociate the atrium and His bundle from the ventricle with atrial pacing during tachycardia
-
5)
concealed entrainment with a short post-pacing interval from the right ventricular septum near the right bundle branch block (RBBB)
-
6)
recording of a fascicular potential at this site in sinus rhythm
-
7)
development of a paced QRS morphology similar to the WCT QRS morphology during ventricular pacing at this site
-
8)
a QRS morphology during the WCT of LBBB and RAD.
Potential arrhythmia mechanisms excluded:
-
1)
AVNRT with aberration, excluded by observations 4 and 5
-
2)
manifest nodoventricular accessory pathway, excluded by observation 4, since the nodoventricular accessory pathway is intimately connected to the lower AV node
-
3)
bundle branch reentry tachycardia, excluded by observations 1 and 4, and lack of a consistent HV interval during tachycardia
-
4)
left ventricular interfascicular reentrant tachycardia, excluded by the QRS morphology during the WCT (observation 8)
-
5)
ventricular tachycardia (VT) (observation 8, and vide infra).
Induction of VT during atrial stimulation has been reported on rare occasions (observation 1). The most common “VT” induced during atrial stimulation is left ventricular fascicular tachycardia, which exhibits a QRS morphology of RBBB with either left axis deviation (LAD) or RAD.7 Other forms of VT induced during atrial stimulation have been studied and reported. Simons et al8 and Iesaka et al9 each reported a single case of bundle branch reentry VT (LBBB with superior axis induced with rapid atrial pacing, and RBBB with LAD induced during programmed atrial stimulation, respectively). Belhassen et al10 reported 9 patients in whom VT was induced by supraventricular beats. The VT had RBBB morphology in 5, LBBB morphology in 2, and both RBBB and LBBB in 2 patients. Wellens et al11 reported 7 patients in whom sustained VT could be induced with a single atrial or single ventricular premature beat. All induced VTs had RBBB morphology. While LBBB tachycardia can occur, most atrial induced “VTs” exhibit RBBB.
Bharati et al3 have studied the conduction system in an individual with Uhl’s anomaly and recurrent WCT who died. Examination of the conduction system revealed a septated bundle of His. The authors speculated that the WCT (LBBB with normal or LAD) might have originated in the septated AV bundle or one of its major divisions. The same group12 reported on 2 patients with congenital abnormalities of the conduction system and recurrent tachyarrhythmias. One of these 2 patients with recurrent LBBB WCT demonstrated on histologic examination of the conduction system septation of the bundle of His and division of the right bundle branch into 3 parts. At one point in its course, the penetrating bundle of His joined the ventricular septum, allowing for a fascicular–ventricular connection, which the authors termed a Mahaim fiber. These anatomic observations support our hypothesis as to the proposed mechanism we put forth for the WCT observed in our patient (Figure 5).
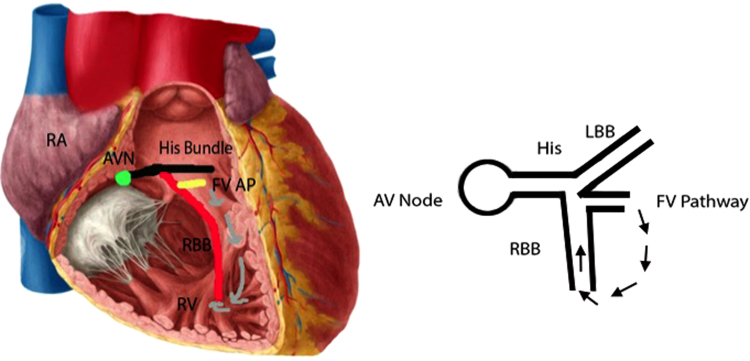
Figure 5.
Proposed arrhythmia circuit. The proposed arrhythmia circuit suggests antegrade conduction from the right bundle branch (RBB) to the right ventricle (RV) mid septum via a fascicular–ventricular pathway (yellow line). The wide complex tachycardia morphology suggests an origin from the medial right ventricular septum (R wave transition – V4/V5, and right axis deviation). After reaching the moderate band, the wave front reengages the distal RBB and conducts antidromically through ventricular myocardium and back up the proximal right bundle. AVN = atrioventricular node; FV AP = fascicular–ventricular accessory pathway; RA = right atrium. Permission for using the left hand figure granted by @ Kenhub (www.kenhub.com<http://www.kenhub.com>) / Illustration by Y. Koh.
Conclusion
We report a patient with Uhl’s anomaly and cardiac arrest in whom 2 unusual arrhythmias (narrow and wide QRS complex tachycardia) were induced during an electrophysiology study, both associated with severe hemodynamic compromise. The narrow QRS tachycardia was initiated with 1:2 AV conduction and AVNRT. The WCT was secondary to a fascicular–ventricular reentrant mechanism from the distal bundle of His or right bundle branch.
References
- 1.Tanoue Y., Kado H., Shiokawa Y. Uhl׳s anomaly complicated with critical ventricular arrhythmia in a 2-month-old infant. Eur J Cardiothorac Surg. 2003;24:1040–1042. doi: 10.1016/j.ejcts.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Deal B.J., Mavroudis C., Backer C.L. Beyond Fontan conversion: surgical therapy of arrhythmias including patients with associated complex congenital heart disease. Ann Thorac Surg. 2003;76:542–553. doi: 10.1016/s0003-4975(03)00469-7. [DOI] [PubMed] [Google Scholar]
- 3.Bharati S., Feld A.W., Bauernfeind R., Kattus A.A., Lev M. Hypoplasia of the right ventricular myocardium with ventricular tachycardia. Arch Pathol Lab Med. 1983;1075:249–253. [PubMed] [Google Scholar]
- 4.Bharati S., Ciraulo D.A., Bilitch M., Rosen K.M., Lev M. Inexcitable right ventricle and bilateral bundle branch block in Uhl׳s disease. Circulation. 1978;57:636–644. doi: 10.1161/01.cir.57.3.636. [DOI] [PubMed] [Google Scholar]
- 5.Sakurada H., Sakamoto M., Hiyoshi Y., Tejima T., Motomiya T., Sugiura M., Hiraoka M. Double ventricular responses to a single atrial depolarization in a patient with dual AV nodal pathways. Pacing Clin Electrophysiol. 1992;15:28–33. doi: 10.1111/j.1540-8159.1992.tb02898.x. [DOI] [PubMed] [Google Scholar]
- 6.Dixit S., Callans D.J., Gerstenfeld E.P., Marchlinski F.E. Reentrant and nonreentrant forms of atrio-ventricular nodal tachycardia mimicking atrial fibrillation. J Cardiovasc Electrophysiol. 2006;17:312–316. doi: 10.1111/j.1540-8167.2006.00410.x. [DOI] [PubMed] [Google Scholar]
- 7.Gopi A., Nair S.G., Shelke A., Saggu D.K., Yalagudri S., Reddy P., Narasimhan C. A stepwise approach to the induction of idiopathic fascicular ventricular tachycardia. J Interv Card Electrophysiol. 2015;44:17–22. doi: 10.1007/s10840-015-0022-4. [DOI] [PubMed] [Google Scholar]
- 8.Simons G.R., Sorrentino R.A., Zimerman L.I., Wharton J.M., Natale A. Bundle branch reentry tachycardia and possible sustained interfascicular reentry tachycardia with a shared unusual induction pattern. J Cardiovasc Electrophysiol. 1996;7:44–50. doi: 10.1111/j.1540-8167.1996.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 9.Iesaka Y., Lister J.W., Blankstein R.L., Gosselin A.J., Rozanski J.J. Macroreentry as a mechanism of atrial-induced ventricular ectopic beats: a laboratory curiosity? Pacing Clin Electrophysiol. 1983;6:746–750. doi: 10.1111/j.1540-8159.1983.tb05335.x. [DOI] [PubMed] [Google Scholar]
- 10.Belhassen B., Shapira I., Kauli N., Keren A., Laniado S. Initiation of ventricular tachycardia by supraventricular beats. Cardiology. 1982;69:203–213. doi: 10.1159/000173505. [DOI] [PubMed] [Google Scholar]
- 11.Wellens H.J., Bär F.W., Farré J., Ross D.L., Wiener I., Vanagt E.J. Initiation and termination of ventricular tachycardia by supraventricular stimuli. Incidence and electrophysiologic determinants as observed during programmed stimulation of the heart. Am J Cardiol. 1980;46:576–582. doi: 10.1016/0002-9149(80)90506-8. [DOI] [PubMed] [Google Scholar]
- 12.Bharati S., Bauernfiend R., Scheinman M., Massie B., Cheitlin M., Denes P., Wu D., Lev M., Rosen K.M. Congenital abnormalities of the conduction system in two patients with tachyarrhythmias. Circulation. 1979;59:593–606. doi: 10.1161/01.cir.59.3.593. [DOI] [PubMed] [Google Scholar]