Abstract
Purpose
We compared HRQOL between adult survivors of childhood cancer and siblings by investigating the mediating role of emotional distress on HRQOL assessment, and examining the extent to which emotional distress affected the item responses of HRQOL measures given the same underlying HRQOL (i.e., measurement non-invariance).
Methods
7,103 cancer survivors and 390 siblings enrolled in Childhood Cancer Survivor Study who completed the SF-36 measuring HRQOL and the Brief Symptom Inventory-18 measuring anxiety, depression, and somatization were analyzed. Multiple Indicators & Multiple Causes modeling was performed to identify measurement non-invariance related to emotional distress on the responses to HRQOL items. Mediation analysis was performed to test the effects of cancer experience on HRQOL accounting for the mediating role of emotional distress.
Results
29%, 40%, and 34% of the SF-36 items were identified with measurement non-invariance related to anxiety, depression, and somatization, respectively. Survivors reported poorer HRQOL than siblings in all domains (p’s<0.05), except for pain. Other than physical functioning and general health perceptions, poorer HRQOL was explained by the mediating role of emotional distress (p’s<0.05).
Conclusions and implications of cancer survivors
Differences in HRQOL between survivors and siblings appear due, in part, to the mediating effect of emotional distress through which cancer experience influences the responses to HRQOL measures. Interventions to treat emotional distress may improve cancer survivors’ HRQOL.
Keywords: Cancer survivors, emotional distress, health-related quality of life, measurement non-invariance, mediation analysis
INTRODUCTION
With advances in treatment and follow-up care, the 5-year survival rate of childhood cancer has improved from <50% in the 1970s to >80% today [1]. However, childhood cancer survivors are vulnerable to significant late effects inclusive of peripheral neuropathy, cardiovascular, respiratory, metabolic, skeletal, and reproductive disorders resulting from anticancer therapies. The Childhood Cancer Survivor Study (CCSS) reported 62% of adult survivors of childhood cancer ≥1 chronic conditions [2], and by 50 years old, 53% of survivors had developed a severe, life –threatening or fatal condition [3]. Late effects can impact survivors’ health-related quality of life (HRQOL) [4] which is defined as perceived well-being and capability of performing daily functions [5].
Most studies comparing HRQOL in adult survivors of childhood cancer to siblings or general populations have revealed that survivors reported poorer HRQOL in physical, psychological, and social domains [4, 6, 7], though some studies demonstrated equivalent or even superior HRQOL, typically in the psychological domain [4, 7–10]. Differences in study designs, such as the inclusion of survivors of heterogeneous cancers [7] and the use of different HRQOL measures [11, 12], might contribute to the mixed findings. However, while the descriptive differences in HRQOL between survivors and controls have been investigated, evidence is sparse regarding the psychological mechanisms through which the cancer experience connects to different HRQOL.
Childhood cancer survivors experience more emotional distress, such as symptoms of anxiety and depression, compared to control groups [13, 14], and the presence of emotional distress significantly contributes to poor HRQOL [15]. The relationship between the cancer experience and HRQOL may be mediated through or explained by more emotional distress. Additionally, individuals with emotional distress tend to use different internal standards to interpret the meaning of life compared to those without distress, a process known as cognitive bias [16–18]. An individual with versus without cognitive bias may answer items of HRQOL differently even though both individuals possess the same underlying HRQOL. This bias issue can be tested using measurement non-invariance methodology [19]. In cancer survivors, psychosocial variables (emotional distress, coping skill, etc.) are more important than clinical/treatment variables to explain the variance of HRQOL [15, 20]. Therefore, if more survivors had emotional distress than siblings and emotional distress is associated with bias responding to HRQOL, the comparison of HRQOL between survivors and siblings will be misleading.
The main purpose of this study was to investigate the extent to which emotional distress affects the comparisons of HRQOL between adult survivors of childhood cancer and their siblings enrolled in the original CCSS. The first aim was to test measurement non-invariance related to emotional distress on the responses to HRQOL items. We hypothesize that individuals, regardless of survivors or siblings, who have emotional distress will rate the items of HRQOL measures differently than those who don’t have emotional distress. The second aim was to compare HRQOL between cancer survivors and siblings, and specifically include emotional distress as a mediating variable while addressing the influence of measurement non-invariance related to emotional distress on the HRQOL assessment. We hypothesize that differences in HRQOL between cancer survivors and siblings may be due, in part, to the mediating role of emotional distress through which cancer experience influences the responses to HRQOL measures.
METHODS
Sample and data collection
This study focuses on adult survivors of childhood cancer and their siblings who were enrolled in the original CCSS; the methodology for data collection form the original CCSS and characteristics of the population has been published previously [21]. Briefly, the original CCSS is a retrospective cohort study of children and adolescents who were treated for cancers at 26 medical centers in the US and Canada. Individuals who were under 21 years old of age; were diagnosed between 1970 and 1986 with leukemia, central nervous system malignancy, Hodgkin lymphoma, non-Hodgkin lymphoma, kidney cancer, neuroblastoma, soft tissue sarcoma, or malignant bone tumor; and were survived five or more years from diagnosis were recruited. Of 20,691 eligible survivors, 14,363 were enrolled in the CCSS cohort. Among a random sample of 5,857 siblings of survivors, 3,899 were enrolled. This current study utilizes data collected from a follow-up survey (2002–2005) in which 9,308 survivors and 500 siblings (out of 2,951) were eligible, and 7,103 survivors and 390 siblings completed the survey. The protocol was approved by Institutional Review Boards at the 26 participating institutions with participants providing informed consent.
Measures
Emotional distress was measured using the Brief Symptom Inventory-18 (BSI-18) [22]. The BSI-18 captures 3 domains of emotional distress, depression (6 items), anxiety (6 items), and somatization (6 items), that the participants have experienced during the past 7 days. The raw domain score for each participant was calculated by summation of item scores ranging from 0 to 24, with higher scores indicating higher symptom levels. Raw domain scores were also converted to T-scores based on US population norms, and a cutoff of ≥63 was used to indicate clinically meaningful emotional distress for each domain. HRQOL was measured using the Medical Outcomes Study 36-Item Short-Form Health Survey (SF-36) [23]. The SF-36 captures 8 domains of HRQOL, including physical functioning, role limitations resulting from physical health problems, bodily pain, general health perceptions, vitality, social functioning, role limitations resulting from emotional problems, and mental health, that the participants have experienced during the past 4 weeks. The domain scores for each participant were calculated by a summation of observed item scores as well as a latent approach of structural equation modeling, with higher scores indicating better HRQOL.
Analytic framework
Chi-square tests were conducted to compare the differences in meaningfully elevated emotional distress (T-scores ≥ 63, %) [22] between cancer survivors and siblings. T-tests were conducted to compare the differences in HRQOL scores between cancer survivors and siblings. T-tests were also conducted to compare the differences in HRQOL scores between those with and without emotional distress.
Uniquely, this study used the framework “Multiple Indicators & Multiple Causes-Mediation Model (MIMIC-MM)” to address two fundamental measurement issues (measurement non-invariance [19] and mediation effects [24]) when comparing HRQOL between cancer survivors and siblings. Measurement non-invariance occurs when the individuals from subgroups (e.g., individuals with and without distress) rate a categorical item unequally given the same underlying HRQOL (e.g., physical functioning) the item intends to measure. Evidence of measurement non-invariance in HRQOL assessment suggests problematic construct validity of HRQOL measures, leading to misinterpretation of HRQOL comparisons between subgroups.
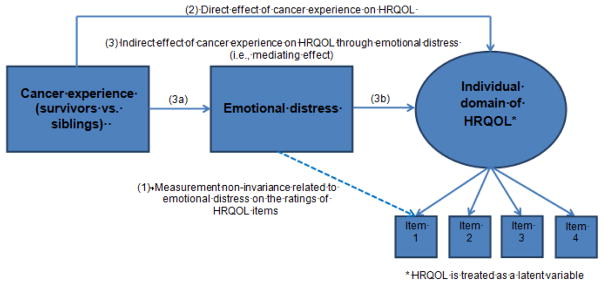
A MIMIC model extended from a general structural equation modeling was conducted to investigate measurement non-invariance related to emotional distress. Figure 1 displays a simplified MIMIC-MM framework that postulates the associations between items measuring the underlying HRQOL, and the linkage between the status of emotional distress and the responses to HRQOL items. Measurement non-invariance is evident if there is a significant association between emotional distress and the responses to individual HRQOL items given the same underlying HRQOL [19]. In contrast, measurement invariance is observed if there are no significant associations between emotional distress and individual HRQOL items. Measurement invariance implies any differences in emotional distress corresponding to HRQOL item responses operate directly through the underlying HRQOL, yet it does not suggest the underlying HRQOL is invariant between subgroups.
Figure 1.
Multiple Indicators & Multiple Causes Mediation Model for testing the effect of cancer experience on HRQOL through emotional distress
After measurement non-invariance was tested in a specific HRQOL domain, mediation analysis based on structural equation modeling was implemented to explicate the mechanism underling the relationship between the cancer experience and individual HRQOL domains via the inclusion of a mediating variable (i.e., emotional distress). A mediation model explicitly analyzes the indirect effect of the cancer experience on HRQOL through the process of emotional distress other than a direct relationship between the cancer experience and HRQOL [24]. The estimated direct and indirect effects of having had cancer on HRQOL could be biased if measurement non-invariance exists yet is not taken into consideration.
We developed a set of MIMIC-MM schemas, each focusing on individual domains of emotional distress and HRQOL using the following steps. In the first step, all paths between individual domain of emotional distress and each item from individual HRQOL domain were constrained to zero, and modification indices (MIs) were examined to suggest the improvement of model fit when specific paths were freely estimated. In the second step, starting with the largest MI, an individual pathway was added to the model in the first step one at a time until no MIs were >3.84 (df=1) (path 1 in Figure 1). In the third step, model fit improvement was tested using the indices of Root Mean Square Error of Approximation (RMSEA) <0.06 and Comparative Fit Index (CFI) >0.95 [25].
Within the MIMIC-MM framework (Figure 1), direct effect was estimated by the coefficient of the path from the cancer experience to HRQOL (path 2). Indirect effect was estimated by multiplying the coefficient of the path from cancer experience to emotional distress (path 3a) and the coefficient of the path from emotional distress to HRQOL (path 3b). The total effect of having experienced cancer on specific domain of HRQOL was estimated by a summation of direct and indirect effects. Implementing MIMIC-MM will improve the estimation of direct and indirect effects of cancer experience on HRQOL because the influence of measurement non-invariance related to emotional distress on HRQOL item responses is taken into consideration (i.e., the estimated associations in path 1). Potential confounding variables including age, sex, education, and household income were included.
MIMIC-MM analyses were conducted in Mplus 7.11 using the Mean and Variance-Adjusted Weighted Least Squares Extraction procedure which is a robust estimator for data with categorical response categories. The remaining analyses were conducted using STATA 13.1.
RESULTS
Sample characteristics (Table 1)
Table 1.
Demographic and clinical characteristics of the childhood cancer survivors and their siblings
| Cancer survivor (N=7,103) Mean (SD) |
Sibling (N=390) Mean (SD) |
p-value | |
|---|---|---|---|
|
| |||
| Age at interview (in years) | 31.8 (7.5) | 33.5 (8.2) | <0.001 |
| N (%) | N (%) | ||
| Time since diagnosis | N/A | ||
| 10–19 years | 1,916 (27.0%) | N/A┼ | |
| 20–29 years | 4,361 (61.4%) | N/A | |
| 30+ years | 826 (11.6%) | N/A | |
| Sex | 0.998 | ||
| Male | 3,388 (47.7%) | 186 (47.7%) | |
| Female | 3,715 (52.3) | 204 (52.3%) | |
| Race/ethnicity | 0.257 | ||
| White, non-Hispanic | 6,427 (91.0%) | 350 (93.8%) | |
| Black, non-Hispanic | 202 (2.9%) | 9 (2.4%) | |
| Hispanic | 272 (3.9%) | 9 (2.4%) | |
| Other | 165 (2.3%) | 5 (1.3%) | |
| Educational background | 0.713 | ||
| Below high school | 215 (3.1%) | 8 (2.1%) | |
| High school graduate | 899 (12.7%) | 50 (12.9%) | |
| Some college/training after high school | 2,589 (36.7%) | 133 (34.3%) | |
| College graduate | 2,384 (33.8%) | 137 (35.3%) | |
| Post graduate level | 967 (13.7%) | 60 (15.5%) | |
| Annual household incomes | <0.001 | ||
| <$19,999 | 737 (11.8%) | 25 (6.9%) | |
| $20,000 – $39,999 | 1,443 (23.2%) | 59 (16.4%) | |
| $40,000 – $59,999 | 1,266 (20.3%) | 79 (21.9%) | |
| $60,000 – $79,999 | 1,052 (16.9%) | 63 (17.5%) | |
| $80,000 – $99,999 | 695 (11.2%) | 47 (13.1%) | |
| ≥$100,000 | 1,032 (16.6%) | 87 (24.2%) | |
| Primary cancer diagnosis | N/A | ||
| Leukemia | 2,369 (33.4%) | ||
| Central nervous system tumors | 767 (10.8%) | N/A | |
| Hodgkin lymphoma | 999 (14.1%) | N/A | |
| Non-Hodgkin lymphoma | 541 (7.6%) | N/A | |
| Wilms tumor | 677 (9.5%) | N/A | |
| Neuroblastoma | 452 (6.4%) | N/A | |
| Soft tissue sarcoma | 646 (9.1%) | N/A | |
| Bone cancer | 652 (9.2%) | N/A | |
| Second cancer | N/A | ||
| Yes | 452 (6.4%) | N/A | |
| No | 6,651 (93.6%) | N/A | |
| Chemotherapy | N/A | ||
| Yes | 5,301 (80.0%) | N/A | |
| No | 1,353 (20.0%) | N/A | |
| Radiotherapy | N/A | ||
| Yes | 4,339 (65.8%) | N/A | |
| No | 2,288 (34.2%) | N/A | |
| Amputation | N/A | ||
| Yes | 434 (7.1%) | N/A | |
| No | 5,716 (92.9%) | N/A | |
N/A: Not applicable
Of 7,493 participants, 7,103 were survivors of childhood cancer and 390 were siblings. The mean age was 32 years in survivors and 34 years in siblings. Survivors and siblings had equal distribution by sex. The mean time since cancer diagnosis was 32 years, and the most common primary cancer diagnoses included leukemia (33%), lymphomas (22%), and central nervous system tumors (11%). The majority of survivors had received chemotherapy (80%), followed by radiotherapy (66%) and amputation (7%).
Associations between cancer experience, emotional distress, and HRQOL (Table 2, Appendixes 1–2)
Table 2.
| Anxiety | Depression | Somatization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Yes (N=6,765) Mean (SD) |
No (N=556) Mean (SD) |
p-value | Yes (N=6,466) Mean (SD) |
No (N=856) Mean (SD) |
p-value | Yes (N=6,320) Mean (SD) |
No (N=1,000) Mean (SD) |
p-value | |
| Physical functioning | 75.71 (26.03) | 88.63 (19.27) | <0.001 | 75.39 (27.15) | 89.27 (18.45) | <0.001 | 68.04 (27.94) | 90.76 (16.59) | <0.001 |
| Role limitations due to physical health problems | 59.35 (42.28) | 84.86 (31.12) | <0.001 | 60.62 (41.54) | 85.88 (30.26) | <0.001 | 48.79 (42.72) | 88.31 (27.30) | <0.001 |
| Bodily pain | 55.55 (26.25) | 76.99 (22.25) | <0.001 | 59.48 (27.28) | 77.47 (21.85) | <0.001 | 49.08 (24.04) | 79.51 (20.24) | <0.001 |
| General health perceptions | 48.31 (25.11) | 71.46 (21.66) | <0.001 | 50.16 (24.62) | 72.31 (21.20) | <0.001 | 43.79 (23.66) | 73.79 (19.74) | <0.001 |
| Vitality | 35.31 (20.75) | 58.37 (20.22) | <0.001 | 34.66 (18.90) | 59.54 (19.68) | <0.001 | 34.85 (19.94) | 60.06 (19.22) | <0.001 |
| Social functioning | 51.49 (27.49) | 86.43 (20.04) | <0.001 | 51.45 (25.97) | 88.06 (18.34) | <0.001 | 58.02 (28.03) | 87.87 (18.68) | <0.001 |
| Role limitations due to emotional problems | 37.64 (40.18) | 85.15 (30.37) | <0.001 | 38.63 (39.91) | 87.22 (28.23) | <0.001 | 54.84 (43.14) | 85.79 (29.77) | <0.001 |
| Mental health | 43.41 (17.84) | 76.17 (15.34) | <0.001 | 45.01 (16.54) | 77.49 (14.11) | <0.001 | 56.36 (20.95) | 76.43 (15.57) | <0.001 |
Based on t-tests
Means and standard deviations represent t-scores with a population mean = 50 and a standard deviation = 10. Higher t-scores reflect better HRQOL
Compared to siblings, more survivors have meaningfully elevated emotional distress (T-scores ≥ 63) on anxiety (8% vs. 4%; p<0.05), depression (12% vs. 8%; p<0.05), and somatization (14% vs. 7%; p<0.001) (Appendix 1). Before accounting for measurement non-invariance, survivors reported significantly worse HRQOL than siblings on the domains of physical functioning (87 vs. 94; p<0.001), role limitations due to physical health problems (83 vs. 87; p<0.01), general health perceptions (69 vs. 77; p<0.001), social functioning (84 vs. 88; p<0.001), and mental health (74 vs. 76; p<0.01) (Appendix 2). However, compared to siblings, survivors reported worse, but not statistical difference on the domains of vitality (56 vs. 59; p=0.05), role limitations due to emotional problems (81 vs. 85; p=0.05), and bodily pain (75 vs. 78; p=0.06).
Participants with more symptoms of anxiety, depression, and somatization reported significantly poorer HRQOL on all domains of the SF-36 than those who reported less emotional distress (all p’s<0.001) (Table 2).
Items of the SF-36 with measurement non-invariance related to emotional distress (Table 3)
Table 3.
| Anxiety | Depression | Somatization | |
|---|---|---|---|
|
| |||
| Physical functioning | |||
| PF01: Vigorous activities | - | 0.042* | −0.118*** |
| PF02: Moderate activities | - | - | −0.062** |
| PF03: Lifting or carrying groceries | - | - | −0.099*** |
| PF04: Climbing several flights of stairs | - | - | −0.111*** |
| PF09: Walking one block | - | - | 0.089*** |
| PF10: Bathing or dressing | −0.079* | −0.103** | - |
| Role limitations due to physical health problems | |||
| RP1: Limited in the kind of work or other activities | - | 0.079*** | - |
| General health perceptions | |||
| RGH1: My health is excellent, very good, good, fair, poor | −0.052* | −0.109*** | −0.164*** |
| GH2: My health is excellent | - | - | −0.215*** |
| RGH3: I am as healthy as anybody I know | 0.061* | 0.048** | - |
| GH4: I seem get sick a little earlier than other people | - | −0.091*** | - |
| RGH5: I expect my health to get worse | 0.066* | - | - |
| Vitality | |||
| RVT1: Feel full of pep | 0.243*** | 0.115** | 0.226*** |
| RVT2: Have a lot of energy | 0.324*** | 0.201*** | 0.256*** |
| VT3: Feel worn out | - | - | −0.088*** |
| VT4: Feel tired | 0.118*** | 0.089*** | - |
| Role limitations due to emotional problems | |||
| RE2: Accomplished less than would like | - | - | 0.083*** |
| RE3: Didn’t do work or other activities as carefully as usual | - | −0.100** | - |
| Mental health | |||
| MH1: Been a very nervous person | −0.575*** | - | 0.306*** |
| MH2: Felt so down in the dumps nothing could cheer up | - | −0.590** | - |
| RMH3: Felt calm and peaceful | 0.388*** | −0.307*** | - |
| MH4: Felt downhearted and blue | - | −0.616*** | - |
| RMH5: Been a happy person | 1.230*** | −0.583*** | - |
| # of items with measurement non-invariance, % | 10 (28.6%) | 14 (40.0%) | 12 (34.3%) |
No items were identified with measurement non-invariance in domains of bodily pain and social functioning
Standardized estimates reflect strength of the association between the specified HRQOL item and individual distress domains. Since higher scores represent more emotional distress and better HRQOL, a negative sign indicates that the participants with more emotional distress likely to rate lower item scores on HRQOL domains (i.e., poorer HRQOL) than those with more emotional distress given the same underlying HRQOL.
p<0.05
p<0.01
p<0.001
Overall, 10 (29%), 14 (40%), and 12 (34%) of the SF-36 items were identified with measurement non-invariance associated with anxiety, depression, and somatization, respectively. Interestingly, over 50% the measurement non-invariance items related to symptoms of depression (57%) and somatization (58%) were presented in a negative direction. For example, the magnitude of measurement non-invariance for “PF10: Bathing or dressing” (physical functioning) associated with depression was −0.103, meaning that participants with more depression symptoms tend to report more problems in performing bathing or dressing than those with less depression symptoms given the same underlying physical functioning. In contrast, over 50% the measurement non-invariance items related to anxiety (70%) were in a positive direction. For example, the magnitude of measurement non-invariance for “GRH3: I am healthy as anybody as I know” (general health perception) associated with anxiety was 0.061, meaning that participants with more anxiety symptoms tend to perceive healthier than those with less anxiety symptoms given the same underlying general health perceptions. The positive direction of measurement non-invariance items related to symptoms of anxiety, depression, and somatization was commonly observed on vitality domain (item labels: RVT1, RVT2, and VT4).
Effect of the cancer experience on HRQOL through emotional distress using MIMIC-MM (Table 4)
Table 4.
Effects of childhood cancer on HRQOL with or without adjusting for measurement non-invariance related to emotional distress┼‡
| Anxiety | Depression | Somatization | |
|---|---|---|---|
|
| |||
| Physical functioning (PF) | |||
| Direct effect: cancer to PF | −0.106*** | −0.108*** | −0.078*** |
| Indirect effect: cancer to PF through emotional distress | −0.021* | −0.019* | −0.048*** |
| Cancer to emotional distress | 0.062* | 0.048* | 0.091*** |
| Emotional distress to PF | −0.339*** | −0.391*** | −0.524*** |
| Total effect: direct plus indirect effects | −0.127*** | −0.127*** | −0.126*** |
| RMSEA/CFI& | 0.042/0.996 | 0.042/0.996 | 0.038/0.997 |
| Role limitations due to physical health problems (RP) | |||
| Direct effect: cancer to RP | −0.019 | −0.024 | 0.013 |
| Indirect effect: cancer to RP through emotional distress | −0.026* | −0.022* | −0.058*** |
| Cancer to emotional distress | 0.062* | 0.048* | 0.091*** |
| Emotional distress to RP | −0.418*** | −0.464*** | −0.633*** |
| Total effect: direct plus indirect effects | −0.045** | −0.046*** | −0.045** |
| RMSEA/CFI& | 0.032/0.998 | 0.024/0.999 | 0.032/0.998 |
| Bodily pain (BP) | |||
| Direct effect: cancer to BP | 0.009 | 0.001 | 0.043* |
| Indirect effect: cancer to BP through emotional distress | −0.028* | −0.020* | −0.062*** |
| Cancer to emotional distress | 0.062* | 0.049* | 0.092*** |
| Emotional distress to BP | −0.448*** | −0.399*** | −0.674*** |
| Total effect: direct plus indirect effects | −0.019 | −0.019 | −0.019 |
| RMSEA/CFI& | 0.029/0.999 | 0.029/0.999 | 0.029/0.999 |
| General health perceptions (GH) | |||
| Direct effect: cancer to GH | −0.041* | −0.051** | 0.008 |
| Indirect effect: cancer to GH through emotional distress | −0.034** | −0.024* | −0.075*** |
| Cancer to emotional distress | 0.067** | 0.047* | 0.115*** |
| Emotional distress to GH | −0.509*** | −0.506*** | −0.651*** |
| Total effect: direct plus indirect effects | −0.075*** | −0.074*** | −0.066*** |
| RMSEA/CFI& | 0.059/0.991 | 0.060/0.990 | 0.057/0.991 |
| Vitality (VT) | |||
| Direct effect: cancer to VT | 0.011 | 0.010 | 0.033* |
| Indirect effect: cancer to VT through emotional distress | −0.046** | −0.038* | −0.067*** |
| Cancer to emotional distress | 0.065** | 0.051* | 0.091*** |
|
| |||
| Emotional distress to VT | −0.697*** | −0.731*** | −0.737*** |
| Total effect: direct plus indirect effects | −0.034* | −0.028* | −0.034* |
| RMSEA/CFI& | 0.037/0.998 | 0.041/0.998 | 0.037/0.998 |
| Social functioning (SF) | |||
| Direct effect: cancer to SF | −0.007 | −0.012 | 0.012 |
| Indirect effect: cancer to SF through emotional distress | −0.043* | −0.038* | −0.061*** |
| Cancer to emotional distress | 0.062* | 0.049* | 0.092*** |
| Emotional distress to SF | −0.691*** | −0.776*** | −0.667*** |
| Total effect: direct plus indirect effects | −0.050** | −0.050** | −0.050** |
| RMSEA/CFI& | 0.028/0.999 | 0.028/0.999 | 0.028/0.999 |
| Role limitations due to emotional problems (RE) | |||
| Direct effect: cancer to RE | 0.010 | 0.004 | 0.016 |
| Indirect effect: cancer to RE through emotional distress | −0.041* | −0.035* | −0.050*** |
| Cancer to emotional distress | 0.062* | 0.049* | 0.092*** |
| Emotional distress to RE | −0.664*** | −0.721*** | −0.546*** |
| Total effect: direct plus indirect effects | −0.032* | −0.031* | −0.034* |
| RMSEA/CFI& | 0.022/0.999 | 0.024/0.999 | 0.024/0.999 |
| Mental health (MH) | |||
| Direct effect: cancer to MH | 0.011 | −0.004 | 0.006 |
| Indirect effect: cancer to MH through emotional distress | −0.056*** | −0.026** | −0.051*** |
| Cancer to emotional distress | 0.061*** | 0.043** | 0.079*** |
| Emotional distress to MH | −0.911*** | −0.604*** | −0.648*** |
| Total effect: direct plus indirect effects | −0.045*** | −0.030* | −0.045*** |
| RMSEA/CFI& | 0.040/0.994 | 0.045/0.994 | 0.057/0.987 |
p<0.05
p<0.01
p<0.001
Adjust for age, gender, education, and household income
Since higher scores represent more emotional distress and better HRQOL, a negative sign would ideally indicate that cancer experience was related to more emotional distress, more emotional distress was related to poorer HRQOL, and cancer experience was related to poorer HRQOL.
Acceptable fit index: Root Mean Square Error of Approximation (RMSEA) <0.05 and Comparative Fit Index (CFI) >0.95
Bold: Without adjusting for the influence of measurement non-invariance; otherwise, with adjusting for the influence of measurement non-invariance
Using structural equation modeling, the estimated total effects suggest that cancer survivors experienced significantly poorer HRQOL in 7 domains (i.e., physical functioning, role limitations due to physical health problems, general health perceptions, vitality, social functioning, role limitations due to emotional problems, and mental health; all p’s<0.05) than siblings except for bodily pain (p=0.06). However, the total effect of cancer on HRQOL was greatly explained by the mechanisms (i.e., mediating/indirect effects) of emotional distress rather than by the direct contribution of having been diagnosed and treated for cancer. The magnitude of the indirect effects of cancer experience on all domains of HRQOL through 3 emotional distress domains were all significant (p<0.05), whereas the direct effects of the cancer experience were only significant on physical functioning and general health perceptions (p<0.05). For example, compared to siblings, cancer survivors reported more role limitations due to physical health problems (total effect: −0.046; p<0.001) which was directly contributed by the cancer experience (direct effect: −0.024; p>0.05) and the mechanism of depressive symptoms (indirect effect: −0.022; p<0.05). This mechanism was further evident by the fact that having cancer was associated with greater depression (0.048; p<0.05), and greater depression was associated with greater role limitations due to physical health problems (−0.464; p<0.001). Adjustment for measurement non-invariance did not significantly change the magnitude and direction in the associations of the cancer experience with HRQOL versus non-adjustment. However, adjusting for measurement non-invariance improved the indices of model fit to a satisfactory level (RMSEA<0.06, CFI>0.95).
DISCUSSION
We recently have reported that 96% of childhood cancer survivors develop at least one chronic health conditions by age 45 years [26]. Preserving suboptimal functional status and HRQOL in this high risk population is an important clinical concern. Using the same dataset from the original CCSS (a follow-up survey 2002–2005), both previous [7] and present studies found that childhood cancer survivors experienced significantly elevated emotional distress and poorer HRQOL than their siblings except for bodily pain. Using the MIMIC-MM methodology, the present study further demonstrate stronger indirect effects of cancer experience through the mechanisms of emotional distress symptoms (anxiety, depression and somatization) on all HRQOL domains (except for physical functioning and general health perceptions) relative to the direct effects. The evidence of indirect effects suggests that poorer HRQOL in cancer survivors than siblings was governed by the presence of different emotional distress.
Emotional distress is a critical problem in cancer survivors who face prolonged uncertainty about prognosis such as cancer recurrence and late effects. Dalton and colleagues reported a 1.2 to 3.1-fold risk of developing depression in Danish adult cancer survivors one year after cancer diagnosis compared to those without cancer, and the risk fell slowly over 10-years of follow-up [27]. Mitchell and colleagues found that cancer survivors had a 1.1- to 1.5-fold risk of developing anxiety compared to healthy controls, and the risk was higher for >10 years after diagnosis than <2 years [28]. Psychiatric disorder or emotional symptoms can negatively impact HRQOL [29]. We previously reported that emotional symptoms were most significantly associated with poorer HRQOL compared to other symptoms such as cardiac and pulmonary symptoms [15]. Nevertheless, these previous studies regarded emotional distress and HRQOL as two distinct outcome variables or considered emotional distress as a confounding variable on the association of cancer experience with HRQOL. This line of earlier research neither explains why some cancer survivors reported better or worse HRQOL than controls, nor does it inform the design of interventions to improve HRQOL.
A uniqueness of this study is the evaluation of measurement non-invariance in responding to HRQOL items. The direction of measurement non-invariance related to emotional distress on the HRQOL item responses was mixed. We found that more than 50% of HRQOL items with measurement non-invariance related to depression and somatization, respectively, were in a negative direction, meaning the participants with higher level of distress likely to rate lower item scores on HRQOL measures than those with lower distress given the same underlying HRQOL. Psychological research in information processing suggests that depression-prone individuals tend to hold negative schemata; when the life is affected by stressful events (e.g., cancer), negative schemata produce cognitive bias in attention, interpretation, and memory for self-relevant information [16]. Specifically, depressed individuals may possess selective attention for negative information [30], interpret emotionally subjective, ambiguous information in a negative manner [31], and/or have difficulties in inhibiting and shifting negative information in working memory [32]. Cognitive bias may explain a negative direction in measurement non-invariance.
More than 50% of HRQOL items with measurement non-invariance related to anxiety were in a positive direction. A mixed psychological state has been observed in cancer survivors who have an imminent worry at a time of apparent happiness and celebration. After cancer therapies, survivors often develop greater resilience as an adaptive process to manage the prospect of future life [33]. Recent evidence also reveals that individuals with moderate anxiety and depression commonly live with a high level of resilience and benefit-finding [34], which may explain a positive direction of measurement non-invariance related to emotional distress.
Specifically, we found that the positive direction of measurement non-invariance related to symptoms of anxiety, depression, and somatization was captured by vitality items. This finding can be interpreted by the notion “focusing illusion” (one form of cognitive bias), suggesting that individuals with and without health problems may interpret the meaning of HRQOL items differently depending upon the content or description of the items [35]. Since vitality is a vague HRQOL concept, individuals without emotional distress may interpret vitality as a broader daily activity impact related to physical and emotional health and various symptoms. Instead, individuals with emotional distress may envision vitality as an impact related to emotional health. As a result, focusing illusion between individuals with and without emotional distress causes a positive direction of measurement non-invariance. Future studies should apply cognitive debriefing methods to elucidate this finding.
In this study, we examine the mediating effects of respective emotional distress (anxiety, depression, and somatization) on the association of cancer experience with HRQOL. It is possible that the mediating effects will differ by comorbid emotional distress. Using latent class analysis, a recent CCSS study identified four clusters of emotional distress measured by the BSI-18: asymptomatic, elevated somatization, elevated anxiety and depression, and elevated anxiety, depression, and somatization clusters [36]. Further studies are encouraged to apply the methodology created in this study and the four emotional distress clusters identified by a recent CCSS study to examine the mediating effects of different emotional distress clusters on the association of cancer experience with HRQOL. In addition, to generalize our findings, it is important to test whether the same results would be held in individuals with other chronic conditions. A recent study found that cancer survivors without chronic conditions have better HRQOL across all domains of the SF-36 than those with one non-cancer chronic condition. However, individuals with multiple chronic conditions have the worst HRQOL than that of cancer survivors [37]. Another study also found that emotional distress in individuals with severe chronic conditions such as back pain and chronic obstructive pulmonary disease was significantly higher than that of cancer populations [38]. Future studies should test the mediating effects of emotional distress on the association of chronic conditions with HRQOL.
Establishing a link between cancer and HRQOL through emotional distress provides useful insight for designing distress-targeted interventions to improve HRQOL. Several non-pharmacological (e.g., cognitive-behavioral, psycho-educational, and exercise) and pharmacological interventions show effectiveness in improving depression, which in turn improves HRQOL [39–41]. Apart from research examining cognitive content as a vulnerability factor (i.e., negative thoughts), a promising line of research highlights the role of cognitive biases in the development, maintenance, and release of emotional distress. Cognitive bias modification, an experimental paradigm that uses training to induce adaptive cognitive biases, has demonstrated promising results for managing anxiety and depression, especially with cognitive biases that are stronger for interpretation than for attention biases [42–45].
Several limitations of this study are worth noting. First, we only used self-report measures to investigate health outcomes. Future studies might use clinical interviews to evaluate emotional distress to improve the precision of our estimates. Second, we used cross-sectional data to test the direct and indirect effects of cancer experience on HRQOL. Our findings do not infer a causal effect although the literature hypothesizes that “disease experience-distress-HRQOL” is a reasonable pathway [46]. Investigating a unique association between emotional distress and HRQOL over time may rule out the concern that HRQOL changes emotional distress. Finally, the current study merely tested measurement non-invariance related to distress symptoms on the responses to HRQOL items. It is possible that measurement non-invariance related to the cancer experience (survivors versus siblings) may occur when responding to HRQOL items. Survivors may change their internal standard in perceiving or interpreting the meanings of HRQOL that is different from the general population. Data collection for the change of internal standard relies on a longitudinal design, and future studies are needed to test these complex pathways.
CONCLUSIONS
The effects of the cancer experience on HRQOL are not straightforward. We observed that childhood cancer survivors reported poorer HRQOL than their siblings through the mechanism of emotional distress. Additionally, emotional distress was associated with measurement non-invariance on item responses to HRQOL measures, suggesting that simply comparing HRQOL between survivors and siblings without considering the role of emotional distress might be misleading. Our framework leverages new insights for future research to affirm these mechanisms and develop interventions to improve HRQOL for childhood cancer survivors.
Acknowledgments
Funding sources
This work was supported by the National Cancer Institute grant U24 CA55727 (ICH, TMB, GTA [PI], WL, LLR and KRK), grant R21 CA202210 (ICH [PI], KRK), the Cancer Center Support grant P30 CA21765 (ICH, TMB, GTA, LLR, and KRK), and the ALSAC (ICH, TMB, GTA, LLR, and KRK).
Footnotes
COMPLIANCE WITH ETHICAL STANDARDS
Conflict of Interest:
All co-authors have declares that they have no conflict of interest.
Ethical Approval:
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Specifically, the study protocol was approved by Institutional Review Boards at the 26 participating institutions with participants providing informed consent.
Informed Consent:
Informed consent was obtained from all individual participants included in the study.
References
- 1.American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. The New England Journal of Medicine. 2006;355(15):1572–82. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, et al. Aging and risk of severe, disabling, life-threatening, and fatal events in the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2014;32(12):1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. Psychological status in childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Journal of Clinical Oncology. 2009;27(14):2396–404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: A quality-of-life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute. 1993;85(5):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 6.Zeltzer LK, Chen E, Weiss R, Guo MD, Robison LL, Meadows AT, et al. Comparison of psychologic outcome in adult survivors of childhood acute lymphoblastic leukemia versus sibling controls: A cooperative Children’s Cancer Group and National Institutes of Health study. Journal of Clinical Oncology. 1997;15(2):547–56. doi: 10.1200/JCO.1997.15.2.547. [DOI] [PubMed] [Google Scholar]
- 7.Zeltzer LK, Lu Q, Leisenring W, Tsao JC, Recklitis C, Armstrong G, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: A report from the Childhood Cancer Survivor Study. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(2):435–46. doi: 10.1158/1055-9965.EPI-07-2541. [DOI] [PubMed] [Google Scholar]
- 8.Elkin TD, Phipps S, Mulhern RK, Fairclough D. Psychological functioning of adolescent and young adult survivors of pediatric malignancy. Medical and Pediatric Oncology. 1997;29(6):582–8. doi: 10.1002/(sici)1096-911x(199712)29:6<582::aid-mpo13>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 9.Maunsell E, Pogany L, Barrera M, Shaw AK, Speechley KN. Quality of life among long-term adolescent and adult survivors of childhood cancer. Journal of Clinical Oncology. 2006;24(16):2527–35. doi: 10.1200/JCO.2005.03.9297. [DOI] [PubMed] [Google Scholar]
- 10.Stam H, Grootenhuis MA, Caron HN, Last BF. Quality of life and current coping in young adult survivors of childhood cancer: Positive expectations about the further course of the disease were correlated with better quality of life. Psycho-oncology. 2006;15(1):31–43. doi: 10.1002/pon.920. [DOI] [PubMed] [Google Scholar]
- 11.Huang IC, Quinn GP, Krull K, Eddleton KZ, Murphy DC, Shenkman EA, et al. Head-to-head comparisons of quality of life instruments for young adult survivors of childhood cancer. Supportive Care in Cancer. 2012;20(9):2061–71. doi: 10.1007/s00520-011-1315-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang IC, Quinn GP, Wen PS, Shenkman EA, Revicki DA, Krull K, et al. Using three legacy measures to develop a health-related quality of life tool for young adult survivors of childhood cancer. Quality of Life Research. 2012;21(8):1437–50. doi: 10.1007/s11136-011-0055-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zebrack BJ, Zeltzer LK, Whitton J, Mertens AC, Odom L, Berkow R, et al. Psychological outcomes in long-term survivors of childhood leukemia, Hodgkin’s disease, and non-Hodgkin’s lymphoma: A report from the Childhood Cancer Survivor Study. Pediatrics. 2002;110(1 Pt 1):42–52. doi: 10.1542/peds.110.1.42. [DOI] [PubMed] [Google Scholar]
- 14.Zebrack BJ, Zevon MA, Turk N, Nagarajan R, Whitton J, Robison LL, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: A report from the childhood cancer survivor study. Pediatric Blood & Cancer. 2007;49(1):47–51. doi: 10.1002/pbc.20914. [DOI] [PubMed] [Google Scholar]
- 15.Huang IC, Brinkman TM, Kenzik K, Gurney JG, Ness KK, Lanctot J, et al. Association between the prevalence of symptoms and health-related quality of life in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort study. Journal of Clinical Oncology. 2013;31(33):4242–51. doi: 10.1200/JCO.2012.47.8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beck AT. The evolution of the cognitive model of depression and its neurobiological correlates. The American Journal of Psychiatry. 2008;165(8):969–77. doi: 10.1176/appi.ajp.2008.08050721. [DOI] [PubMed] [Google Scholar]
- 17.Ingram RE. Toward an information-processing analysis of depression. Cognitive Therapy and Research. 1984;8(5):443–78. [Google Scholar]
- 18.Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22(6):539–60. [Google Scholar]
- 19.Meredith W, Teresi JA. An essay on measurement and factorial invariance. Medical Care. 2006;44(11 Suppl 3):S69–77. doi: 10.1097/01.mlr.0000245438.73837.89. [DOI] [PubMed] [Google Scholar]
- 20.Kenzik K, Huang IC, Rizzo JD, Shenkman E, Wingard J. Relationships among symptoms, psychosocial factors, and health-related quality of life in hematopoietic stem cell transplant survivors. Supportive Care in Cancer. 2015;23(3):797–807. doi: 10.1007/s00520-014-2420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: A National Cancer Institute-supported resource for outcome and intervention research. Journal of Clinical Oncology. 2009;27(14):2308–18. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derogatis L. Brief Symptom Inventory 18: Administration, scoring, and procedures manual. Minneapolis, MN: National Computer Systems; 2000. [Google Scholar]
- 23.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 24.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annual Review of Psychology. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- 26.Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA. 2013;309(22):2371–81. doi: 10.1001/jama.2013.6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalton SO, Laursen TM, Ross L, Mortensen PB, Johansen C. Risk for hospitalization with depression after a cancer diagnosis: A nationwide, population-based study of cancer patients in Denmark from 1973 to 2003. Journal of Clinical Oncology. 2009;27(9):1440–5. doi: 10.1200/JCO.2008.20.5526. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell AJ, Ferguson DW, Gill J, Paul J, Symonds P. Depression and anxiety in long-term cancer survivors compared with spouses and healthy controls: A systematic review and meta-analysis. The Lancet Oncology. 2013;14(8):721–32. doi: 10.1016/S1470-2045(13)70244-4. [DOI] [PubMed] [Google Scholar]
- 29.Hays RD, Wells KB, Sherbourne CD, Rogers W, Spritzer K. Functioning and well-being outcomes of patients with depression compared with chronic general medical illnesses. Archives of General Psychiatry. 1995;52(1):11–9. doi: 10.1001/archpsyc.1995.03950130011002. [DOI] [PubMed] [Google Scholar]
- 30.Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27(12):1135–42. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- 31.Wisco BE, Nolen-Hoeksema S. Interpretation bias and depressive symptoms: The role of self-relevance. Behaviour Research and Therapy. 2010;48(11):1113–22. doi: 10.1016/j.brat.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 32.Joormann J, Siemer M. Memory accessibility, mood regulation, and dysphoria: difficulties in repairing sad mood with happy memories? Journal of Abnormal Psychology. 2004;113(2):179–88. doi: 10.1037/0021-843X.113.2.179. [DOI] [PubMed] [Google Scholar]
- 33.Antoni MH, Lehman JM, Kilbourn KM, Boyers AE, Culver JL, Alferi SM, et al. Cognitive-behavioral stress management intervention decreases the prevalence of depression and enhances benefit finding among women under treatment for early-stage breast cancer. Health Psychology. 2001;20(1):20–32. doi: 10.1037//0278-6133.20.1.20. [DOI] [PubMed] [Google Scholar]
- 34.Tomich PL, Helgeson VS. Posttraumatic growth following cancer: Links to quality of life. Journal of Traumatic Stress. 2012;25(5):567–73. doi: 10.1002/jts.21738. [DOI] [PubMed] [Google Scholar]
- 35.Ubel PA, Loewenstein G, Jepson C. Whose quality of life? A commentary exploring discrepancies between health state evaluations of patients and the general public. Quality of Life Research. 2003;12(6):599–607. doi: 10.1023/a:1025119931010. [DOI] [PubMed] [Google Scholar]
- 36.D’Agostino NM, Edelstein K, Zhang N, Recklitis CJ, Brinkman TM, Srivastava D, et al. Comorbid symptoms of emotional distress in adult survivors of childhood cancer. Cancer. 2016;122(20):3215–24. doi: 10.1002/cncr.30171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heins MJ, Korevaar JC, Hopman PE, Donker GA, Schellevis FG, Rijken MP. Health-related quality of life and health care use in cancer survivors compared with patients with chronic diseases. Cancer. 2016;122(6):962–70. doi: 10.1002/cncr.29853. [DOI] [PubMed] [Google Scholar]
- 38.Schalet BD, Pilkonis PA, Yu L, Dodds N, Johnston KL, Yount S, et al. Clinical validity of PROMIS Depression, Anxiety, and Anger across diverse clinical samples. Journal of Clinical Epidemiology. 2016;73:119–27. doi: 10.1016/j.jclinepi.2015.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jacobsen PB, Jim HS. Psychosocial interventions for anxiety and depression in adult cancer patients: Achievements and challenges. CA: A Cancer Journal for Clinicians. 2008;58(4):214–30. doi: 10.3322/CA.2008.0003. [DOI] [PubMed] [Google Scholar]
- 40.Osborn RL, Demoncada AC, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: Meta-analyses. International Journal of Psychiatry in Medicine. 2006;36(1):13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. [DOI] [PubMed] [Google Scholar]
- 41.Uitterhoeve RJ, Vernooy M, Litjens M, Potting K, Bensing J, De Mulder P, et al. Psychosocial interventions for patients with advanced cancer - a systematic review of the literature. British Journal of Cancer. 2004;91(6):1050–62. doi: 10.1038/sj.bjc.6602103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940–58. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- 43.Holmes EA, Lang TJ, Shah DM. Developing interpretation bias modification as a “cognitive vaccine” for depressed mood: Imagining positive events makes you feel better than thinking about them verbally. Journal of Abnormal Psychology. 2009;118(1):76–88. doi: 10.1037/a0012590. [DOI] [PubMed] [Google Scholar]
- 44.MacLeod C, Mathews A. Cognitive bias modification approaches to anxiety. Annual Review of Clinical Psychology. 2012;8:189–217. doi: 10.1146/annurev-clinpsy-032511-143052. [DOI] [PubMed] [Google Scholar]
- 45.Wells TT, Beevers CG. Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cognition & Emotion. 2010;24(5):719–28. [Google Scholar]
- 46.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life. A conceptual model of patient outcomes. JAMA. 1995;273(1):59–65. [PubMed] [Google Scholar]