Abstract
Life as we know it cannot exist without the nucleotide nicotinamide adenine dinucleotide (NAD). From the simplest organism, such as bacteria, to the most complex multicellular organisms, NAD is a key cellular component. NAD is extremely abundant in most living cells and has traditionally been described to be a cofactor in electron transfer during oxidation-reduction reactions. In addition to participating in these reactions, NAD has also been shown to play a key role in cell signaling, regulating several pathways from intracellular calcium transients to the epigenetic status of chromatin. Thus, NAD is a molecule that provides an important link between signaling and metabolism, and serves as a key molecule in cellular metabolic sensoring pathways. Importantly, it has now been clearly demonstrated that cellular NAD levels decline during chronological aging. This decline appears to play a crucial role in the development of metabolic dysfunction and age-related diseases. In this review we will discuss the molecular mechanisms responsible for the decrease in NAD levels during aging. Since other reviews on this subject have been recently published, we will concentrate on presenting a critical appraisal of the current status of the literature and will highlight some controversial topics in the field. In particular, we will discuss the potential role of the NADase CD38 as a driver of age-related NAD decline.
Keywords: CD38, PARP, SIRTUINS, NAD+, mitochondrial function, aging
INTRODUCTION
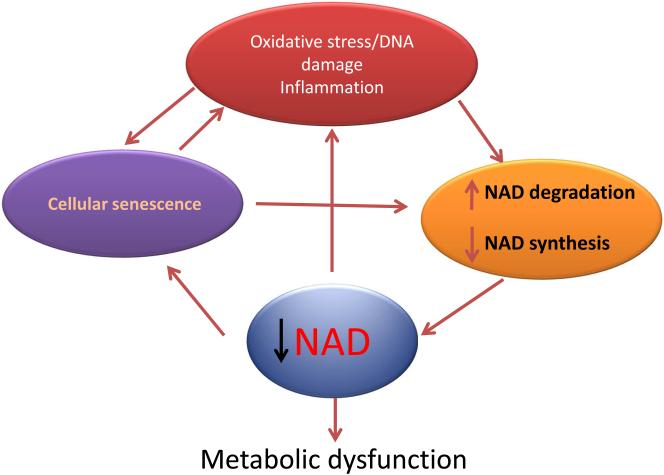
NAD was discovered over a hundred years ago (Harden and Young, 1906), and now that it has achieved its status as a super-centenarian molecule, its role in the biological process of aging is being recognized (Braidy et al., 2011; Gomes et al., 2013; Lin et al., 2000; Massudi et al., 2012; Scheibye-Knudsen et al., 2014; Zhu et al., 2015). It has been shown that NAD levels decline during chronological aging, and that this decline is both a consequence of the aging process and also a contributor to the development of age-related cellular dysfunction (Braidy et al., 2011; Gomes et al., 2013; Massudi et al., 2012; Scheibye-Knudsen et al., 2014; Verdin, 2015; Zhu et al., 2015). It is possible that a vicious cycle exists in which molecular mechanisms involved in the aging process, such as oxidative stress, DNA damage, senescence, and inflammation, lead to tissue NAD decline which subsequently exacerbates the processes that caused its decline in the first place (Figure 1). To potentially intervene in this vicious cycle it is crucial that we understand the mechanisms that lead to cellular NAD decrease during aging and, in particular, whether the decrease is mediated primarily by changes in its degradation, synthesis, or both. Furthermore, it is critical to understand how oxidative stress, DNA damage, inflammation, and senescence impact cellular NAD metabolism during the aging process. In the current review we will present a critical analysis of this subject, and will provide new mechanistic hypotheses to explain the age-related NAD decline.
Figure 1. A vicious cycle that amplifies tissue NAD decline, cellular senescence and damage during aging.
We propose that a vicious cycle exists in which molecular mechanisms involved in the aging process, such as oxidative stress, DNA damage, senescence, and inflammation, lead to tissue NAD decline which subsequently exacerbates the processes that caused its decline in the first place
THE DISCOVERY OF NAD AND ITS ROLE IN OXIDATION-REDUCTION REACTIONS
The study of NAD began around 1906 when Sir Arthur Harden and William John Young first identified a heat-stable low molecular weight fraction implicated in sugar fermentation in yeast (Harden and Young, 1906). These investigators observed that this fraction could support fermentation, and therefore postulated it to be a cofactor in this process (Harden and Young, 1906). Almost thirty years later, Hans von Euler-Chelpin identified this low molecular weight factor as being composed of two mononucleotides, adenosine monophosphate (AMP) and nicotinamide mononucleotide (NMN) (von Euler-Chelpin, 1930). Next, Otto Warburg isolated NAD and described its role in electron transfer in biological systems (Warburg and Christain, 1936). Since Warburg’s discovery, the electron-transferring properties of NAD have been widely recognized (Ziegler and Niere, 2004). The role of NAD in oxidation-reduction (oxidoreduction) reactions is due to the fact that its redox potential is at an intermediary value between biological donors and acceptors of electrons. NAD and its phosphorylated analogue NAD(P) are involved in both catabolic and anabolic reactions, serving as acceptors or donors of electrons. Some key cellular metabolic pathways where NAD(P)(H) plays a crucial role include, transfer of electrons during aerobic and anaerobic glycolysis, mitochondrial oxidative phosphorylation, biosynthetic lipid pathways, metabolism of several pharmacological and biological compounds, and cellular protection against oxidative stress (Ziegler and Niere, 2004). In these electron transfer reactions there is an inter-conversion between oxidized and reduced forms of NAD (NAD+, NADH), and these reactions are easily reversible. Therefore, the oxidoreduction reactions do not contribute directly to changes in total NAD levels in cells. This is not to say that electron transfer reactions do not modify the availability of NAD to other processes. In particular, the metabolic status of the organism can change the ratio between NAD+ and NADH (known as the NAD+/NADH ratio). Since NAD+ is the actual substrate of several NAD-dependent enzymes involved in protein modification and signal transduction, it is plausible that changes in NAD+/NADH ratios in cells could have a key role in the regulation of several cellular functions (Lin et al., 2004; Cantó et al., 2009).
SINCE THE 1960s IT HAS BEEN RECOGNIZED THAT NAD IS A SUBSTRATE FOR COVALENT PROTEIN MODIFICATIONS AND CELL SIGNALING
Interestingly, several years after the discovery of the role of NAD in electron transfer in biological systems, it was recognized that NAD is also involved in non-oxidoreduction pathways in cells (Chambon et.al, 1963). These “novel” roles of NAD include protein modification by ADP-ribosylation, generation of cellular second messengers, and modulation of the acetylation status of histones and other proteins (Chambon et al., 1963; Chini et. al., 1995; Clapper et al., 1987; Imai and Guarente, 2010; Lee, 2012). In these reactions the oxidized form of NAD (NAD+) serves as a substrate for a wide range of enzymes, such as poly-ADP-ribose polymerases (PARPs), CD38, and SIRTUINS that are involved in signal transduction and cell signaling. For example, NAD+ is used as a substrate for the generation of calcium-regulating second messengers, such as cyclic-ADP-ribose (cADPR) and likely nicotinic acid adenine dinucleotide phosphate (NAADP) (Chini et. al., 1995; Chini and Dousa, 1995; Lee, 2012). These signaling pathways have been shown to be very important in many physiological conditions, like egg fertilization, and pathological conditions such as cellular dysfunction induced by toxins and infectious agents (Chini, 2009; Wei et al., 2014). Due to the key role of NAD in multiple biological functions, it is crucial to characterize the mechanisms that control its metabolism. In recent years, we have learned much about the cellular mechanisms of NAD synthesis and degradation, and also about several of the in vivo NAD metabolites (Garten et al., 2015; Cantó et al., 2015)
NAD IS HIGHLY ABUNDANT IN CELLS AND CAN BE CONVERTED INTO SEVERAL MOLECULES OF BIOLOGICAL SIGNIFICANCE
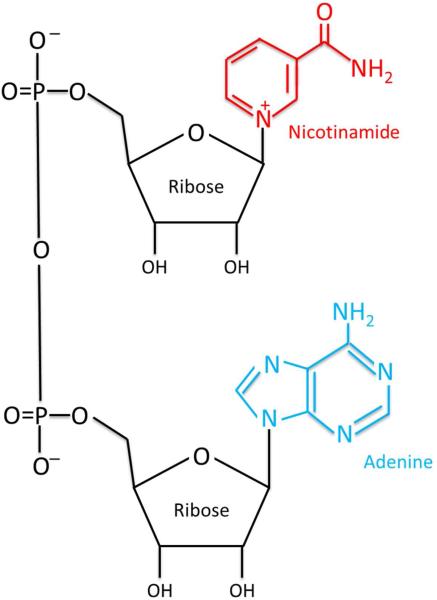
As discussed above, the molecular structure of NAD consists of two nucleotides: an adenine base and nicotinamide, which are joined by a phosphate group (Figure 2). The β diastereomer is the one that supports cellular biochemical reactions. Reported NAD concentrations in cells vary between studies and methodologies, but are generally in the range of 0.2-0.3 mM, making this a very abundant molecule in cells. The fact that NAD is a key molecular coin in energy metabolism and is abundant in cells raises the inevitable analogy with ATP (the main energy coin of living organisms). Several parallels between these two molecules are quite interesting and provide an important opportunity to understand the mechanisms that couple energy metabolism and cell signaling. Asides from being highly abundant in cells, both NAD and ATP are used to perform work coupling several catabolic and anabolic pathways in cells and also act as donors or acceptors for covalent protein modifications that further regulate metabolism and cell signaling. More than that, they are key components of metabolic sensing, and both NAD+/NADH and ATP/AMP ratios appear to be used by cells to integrate signaling and metabolism. Finally, both NAD and ATP are precursors of second messengers, such as cADPR and cAMP respectively, that integrate environmental signaling and cellular functions. These parallels are further supported by the fact that multiple biological compounds can be derived from both NAD and ATP. Thus, NAD and ATP appear to be at a similar hierarchical level, as far as the integration of cell metabolism, signaling, and function.
Figure 2. The structure of NAD+.
The molecular structure of NAD, including the two riboses, the adenine and the nicotinamide base.
As discussed above, NAD can be converted into several molecules that play a role in energy transduction and cell signaling such as NADP, NAADP, and cADPR. In addition, products of NAD degradation such as nicotinamide (NAM) and n-methyl-nicotinamide are emerging as key regulators of energy metabolism, epigenetics, aging, and longevity (Anderson et al., 2003; Kraus et al., 2014; Schmeisser et al., 2013). However, at this moment, it appears that we are far from understanding the complete picture of how all these NAD metabolites integrate during the course of the aging process. Below we will briefly discuss the metabolism of NAD in biological systems.
NAD METABOLISM
NAD Biosynthesis
NAD levels remain constant when used as a co-enzyme, but in non-redox reactions its levels are depleted from the cellular pool, requiring continuous synthesis of the dinucleotide (Nikiforov et al., 2015). The mechanisms of NAD synthesis have been extensively reviewed by others (Nikiforov et al., 2015; Yang and Sauve, 2016). Here we will only briefly describe some key aspects relevant to the aging field.
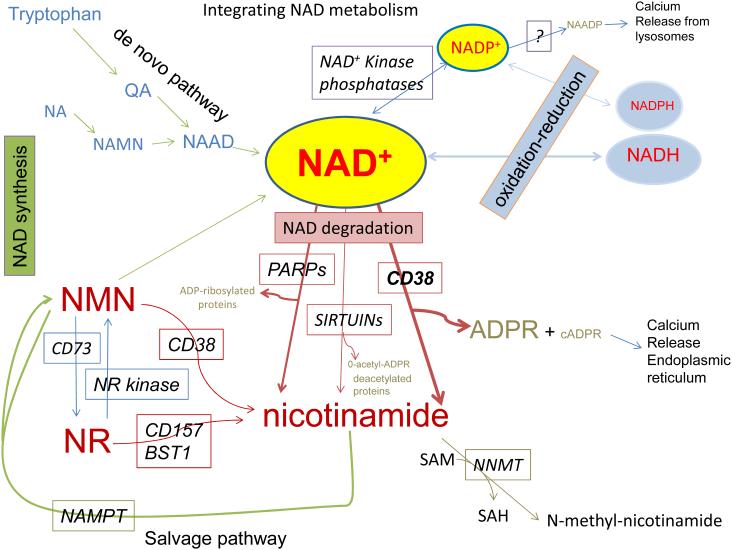
There are two main pathways for the synthesis of NAD, the so called de novo pathway that utilizes the essential amino acid L-tryptophan to generate quinolinic acid (QA) that is further metabolized into NAD (Figure 3) (Nikiforov et al., 2015), and the salvage pathway that utilizes nicotinamide (NAM), nicotinic acid (NA), and nicotinamide riboside (NR) (Figure 3) (Imai and Yoshino, 2013). The salvage pathways are the main source of NAD. Although NA and NAM are generically called niacin, these two distinct molecules serve as NAD precursors in two different reactions.
Figure 3. Pathways for synthesis and degradation of NAD and its metabolites.
On the upper left side of the figure is the simplified scheme of the de novo NAD synthetic pathway. On the left-lower part of the figure is the savage pathway and the interconversion of nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR). On the center and lower part of the figure are the mechanisms of degradation of NAD including CD38, PARPs, and SIRTUINs. On the upper right are the phosphorylation of NAD to NADP and the conversion of NADP to NAADP. Also on the upper right are the oxireduction reactions of NAD-NADH and NADP-NADH. On the lower portion of the figure is the metabolism of nicotinamide (NAM) via salvage pathway and (lower-right) via methylation. All of the abbreviations for metabolites and enzymes are found on the text.
In the Preiss-Handler pathway, NA is converted to nicotinic acid mononucleotide (NAMN) by the enzyme nicotinic acid phosphoribosyltransferase (NAPRT) (Figure 3). In mice, NAPRT seems to be expressed in tissues where NA is the preferential source for NAD biosynthesis, like liver, intestine, heart, and kidney (Jackson et al., 1995).
In mammalians, some studies indicate that NAM is the main niacin-derived NAD precursor (Collins and Chaykin, 1972; Terakata et al., 2013). The enzyme Nicotinamide phosphoribosyltransferase (NAMPT) generates NMN from NAM and 5′-phosphoribosyl-1-pyrophosphate (PRPP), thereby catalyzing the rate-limiting step in the mammalian NAD salvage pathway from NAM (Garten et al., 2015). In addition to being an NAD precursor, NAM is also responsible for the regulation of NAD-consuming enzymes through inhibition of NAD-binding sites (Zhao et al., 2013). Thus, understanding NAM metabolism is of great importance for modulation of NAD levels.
Another niacin-derived NAD precursor, NR, has been identified (Bieganowski and Brenner, 2004) and it is mainly obtained from the diet and from the partial digestion of NAD and NMN (Bogan and Brenner, 2008). NR is phosphorylated to NMN by the ATP-dependent nicotinamide riboside kinase (NRK) inside of cells (Bieganowski and Brenner, 2004). NR can also be generated via the enzyme CD73 (cluster of differentiation 73), an ecto-5'-nucleotidase. The CD73 catalytic site faces the outside of the cell and mediates the synthesis of NR from NAD and NMN, contributing to the availability of NR for intracellular synthesis of NAD (Figure 3) (Grozio et al., 2013).
The synthetic pathways described above converge in the formation of the dinucleotides NAMN and NMN (Figure 3). Both molecules are subsequently converted by the same enzyme, NMNAT (Houtkooper et al., 2010) which uses ATP as the adenyl donor, releasing pyrophosphate (Ruggieri et al., 2015). However, in the case of NAMN, it is first converted to NAAD, which is then amidated to NAD by glutamine-dependent NAD synthetase (NADS).
SYNTHESIS OF NAD-DERIVED CALCIUM SECOND MESSENGERS cADPR and NAADP
cADPR is a second messenger that induces calcium release from intracellular stores via activation of the ryanodine channel (Lee, 2012). It is synthesized from β-NAD+ by an ADP-ribosyl cyclase activity and then converted to ADPR in a cADPR hydrolase reaction (Fig. 3). It must be noted, however, that in many cell types studied so far, the precise nature of the enzyme(s) responsible for physiological ADP-ribosyl cyclase and cADPR hydrolase activities has not been well established. Nevertheless, ADP-ribosyl cyclase activity has been found across different species, suggesting that cADPR metabolism has been preserved in evolution (Kim et al., 1993; Malavasi et al., 2008; Rusinko and Lee, 1989). The first characterization of ADP-ribosyl cyclase was performed in Aplysia californica ovotestis and this soluble enzyme was found to have pure cyclase, but no hydrolase activity (Hellmich and Strumwasser, 1991; Lee and Aarhus, 1991).
The amino acid sequence of ADP-ribosyl cyclase from Aplysia has considerable homology with the human lymphocyte surface antigen CD38, which led to the discovery that CD38 has ADP-ribosyl cyclase activity (States et al., 1992). However, in contrast to the Aplysia cyclase, CD38 is a transmembrane protein and has both ADP-ribosyl cyclase and cADPR hydrolase activities. Surprisingly, CD38 bound to the plasma membrane has its catalytic site located on the extracellular domain of the cell (Chini, 2009; Quarona et al., 2013), which poses theoretical difficulties for understanding how CD38 generates cADPR in the cytoplasm, where it should be available to interact with calcium channels. Several mechanisms for generation of intracellular cADPR have been proposed, but a clear molecular model remains to be established (Wei et al., 2014). In cells from vertebrates, there are other enzymes with cyclase activity, although not as extensively characterized as CD38. The lymphocyte surface antigen named BST-1 (CD157) also has ADP-ribosyl cyclase activity and appears to be the product of a gene duplication of CD38 (Hirata et al., 1994).
We were the first to describe the synthesis of NAADP in several mammalian tissues including brain, liver, spleen, heart, and kidney (Chini and Dousa, 1995; Cheng et al., 2001). Synthesis of NAADP can be catalyzed in vitro by a NAD(P)ase such as the aplysia cyclase and by CD38 (Lee, 1999; Chini et al., 2002), in a reaction called the base-exchange reaction (Chini et al., 2002; Chini and Dousa, 1995). In vitro, these enzymes catalyze the exchange of NAM for NA on the molecule of NAD(P)+, generating NAADP (Chini et al., 2002). However, whether NAADP can be generated via the base-exchange reaction in vivo is still an open question (Kim et al., 2008; Palade, 2007; Soares et al., 2007). Using CD38 knockout mice, we and others have observed that NAADP could be produced by cells in the absence of CD38 (Kim et al., 2008; Soares et al., 2007). This indicates that the base-exchange reaction is unlikely to be the physiological route for the synthesis of NAADP.
Very little is known about the metabolism and role of these NAD-derived second messengers during the aging process. NAADP is an extremely potent calcium-releasing agent that promotes calcium release from lysosomal acid calcium stores (Soares et al., 2007; Morgan et al., 2015). NAADP appears to regulate a family of Ca2+/Na+ channels known as Two pore calcium channels (TPCN1 and 2) (Morgan et al., 2015). These messengers and channels are involved in the regulation of lysosomal function and the process of autophagy (Lu et al., 2013; Morgan et al., 2015). Furthermore, the TPCN channels are important regulators of lysosomal-anchored activation of the mTOR pathway (Cang et al., 2013). Interestingly, both autophagy and mTOR dysregulation have been implicated in the molecular mechanisms of aging. Thus, it is possible that both NAADP and its activated channels may play a role in the aging process by modulating autophagy and the mTOR pathway.
MECHANISMS OF NAD CATABOLISM
The reactions that consume NAD in cells and tissues are mediated by several enzymes that are involved in the non-oxidative roles of NAD (Chini, 2009). From all of the enzymes involved, CD38 and PARPs are the ones that appear to significantly contribute to the cellular NAD degradation. In contrast, SIRTUINS appear to have a minor role in NAD degradation, likely due to the fact that they are low turnover enzymes.
CD38 is the main NADase in mammalian tissues
Although CD38 clearly generates and degrades the second messenger cADPR, and plays key roles in the regulation of intracellular calcium transients, it is clear that CD38 also have other functions (Chini, 2009; Quarona et al., 2013). In fact, it is quite interesting that CD38 is a very inefficient second messenger-generating enzyme, as it will hydrolyze almost a hundred molecules of NAD to generate one molecule of the second messenger cADPR (Beers et al., 1995; Kim et al., 1993; Zielinska et al., 2004). In this regard, we have focused on the possible role of CD38, not as a second messenger enzyme, but as a NADase that can control cellular NAD levels (Aksoy et al., 2006a; Aksoy et al., 2006b; Barbosa et al., 2007; Chini, 2009; Escande et al., 2013; Hu et al., 2014). CD38 is clearly the main NADase in mammalian tissues and contributes to the degradation of NAD both in cultured cells and in tissues. CD38 will consume NAD during its normal catalysis, generating the products NAM and ADPR, and a minuscule amount of the second messenger cADPR (Beers et al., 1995; Chini, 2009) (Figure 3).
CD38 is mostly an ectoenzyme highly expressed in immune cells (Partida-Sanchez et al., 2001; Quarona et al., 2013). Although the great majority of the enzyme is on the cell surface with its catalytic site facing the extracellular space (Chini, 2009; Quarona et al., 2013), the enzyme has also been observed in intracellular membranes, including the nuclear membrane, mitochondria, and endoplasmic reticulum (Chini, 2009; Malavasi et al., 2008). Soluble intra and extracellular forms of CD38 have also been described (Chini, 2009; Malavasi et al., 2008). These data are quite intriguing, since the majority of NAD is intracellular. It is difficult to explain this topological paradox, where the extracellular catalytic enzymatic activity of CD38 can influence intracellular NAD levels. A possible mechanism to explain this paradox is if CD38 could degrade not only NAD, but also its extracellular precursors before they could enter the cell. Indeed, it has been shown that CD38 and its homolog BST-1/CD157 degrade both NMN and NR (Grozio et al., 2013; Preugschat et al., 2014). In particular, NMN is degraded by CD38 and NR can be hydrolyzed by BST-1 (Grozio et al., 2013; Preugschat et al., 2014). Thus, CD38 and BST-1 may have an important impact on the regulation of cellular metabolism and signaling, including regulation of the activity of SIRTUINS (Aksoy et al., 2006b; Barbosa et al., 2007). In fact, we and others have previously shown that CD38 regulates the organismal response to high caloric feeding and that genetic or pharmacological ablation of CD38 protects against high fat diet-induced metabolic dysfunction via modulation of SIRT1 activity (Barbosa et al., 2007; Chiang et al., 2015).
PARPs as NAD-degrading enzymes
Another group of enzymes that uses NAD as a substrate and can regulate cellular NAD levels are the PARP enzymes (Veith and Mangerich, 2015). PARPs were discovered in the 1960s, and are involved in the process of DNA damage repair and also regulate epigenetics and gene expression (Veith and Mangerich, 2015). It appears that PARPs are generally low turnover enzymes in the absence of DNA damage. During DNA damage, PARPs can be activated to a level that leads to significant consumption of cellular NAD, causing cellular NAD decline, metabolic collapse, and cell death (Bai et al., 2011; Veith and Mangerich, 2015). Genetic and pharmacological inhibition of PARP1 was shown to increase cellular NAD levels and regulate organismal metabolic function (Bai et al., 2011, Pirinen et al., 2014).
SARM1 and NAD depletion
Another emerging factor that appears to be involved in NAD metabolism is the sterile alpha and TIR motif-containing 1 (SARM1) protein. It has been reported that SARM1 is involved in the mechanisms that link NAD depletion and neuronal Wallerian degeneration (Gerdts et al., 2015). In particular, formation of the SARM1-TIR dimer triggered rapid breakdown of cellular NAD+, whereas SARM1-induced axon destruction could be counteracted by increased NAD+ synthesis. However, it is not known if SARM1 has NADase activity, and how it correlates with the other mechanisms of NAD degradation (Gerdts et al., 2015). It would be interesting to understand the potential role of SARM1 in the mechanisms of age-related NAD decline.
THE EMERGING ROLE OF NAD METABOLISM IN AGING AND LONGEVITY
Cellular NAD levels decline during the aging process
NAD levels change during many physiological processes. Mounting evidence indicates that intracellular NAD levels are significantly affected by nutritional and environmental stimuli. These changes in NAD content are reflected into NAD-dependent enzymatic activities, which in turn lead to changes in cellular metabolism, gene expression, and protein function. Therefore, maintenance of a proper intracellular NAD concentration appears critical to maintain tissue homeostasis (Cantó et al., 2012; Imai and Guarente, 2014; Ruggieri et al., 2015).
Recently, it has been described that cellular NAD levels decline during chronological aging and in progeroid states. This decline has been shown to be about twofold in old worms and in multiple mice tissues, including liver and skeletal muscle, leading to mitochondrial dysfunction and metabolic abnormalities (Braidy et al., 2011; Gomes et al., 2013; Massudi et al., 2012; Mouchiroud et al., 2013; Scheibye-Knudsen et al., 2014; Zhu et al., 2015). NAD decline was also observed in animals submitted to high fat diet and during senescence (Borradaile and Pickering, 2009; van der Veer et al., 2007; Yoshino et al., 2011). In contrast, NAD increases in cells and tissues after interventions associated with metabolic and age-related benefits, such as exercise and caloric restriction (CR) (Cantó et al., 2010; Fulco et al., 2008). Moreover, NAD also oscillates in a circadian fashion (Nakahata et al., 2009; Ramsey et al., 2009), confirming the relationship between NAD levels and the nutritional state of the organism.
Recent studies show that NAD levels are regulated independently in different cell compartments, but the different NAD pools are still interconnected (Gomes et al., 2013; Kato and Lin, 2014; Stein and Imai, 2014; Yang et al., 2007). In fact, the study of Gomes et al. indicate that mitochondria are regulated by nuclear NAD and that the impairment in OXPHOS function during aging may be precipitated by depletion of the nuclear NAD pool (Gomes et al., 2013). Recently, the development of a genetically encoded biosensor allowed the direct measurement of the NAD concentration within specific subcellular compartments (Cambronne et al, 2016). This new study proposes that NAD in the mitochondria fluctuates distinctly from that in the nucleocytoplasm. They also report that there appears to be at least two mechanisms for maintaining mitochondrial NAD in various cell types, the conversion of NMN by NMNAT3 and transport of cytoplasmic NAD+ (Cambronne et al, 2016). However, it is not clear in which cellular compartment(s) is NAD decline relevant to aging.
These studies highlight the need to understand the mechanisms that lead to age-related NAD decline. There are several key questions in aging research such as: (a) What causes the NAD decline observed during the aging process? (b) What are the consequences of this NAD decline to the cellular dysfunction during aging? (c) Finally, which interventions can be used to prevent NAD decline during aging?
WHAT CAUSES THE NAD DECLINE OBSERVED DURING THE AGING PROCESS?
The decline in NAD levels during aging appears to be a potential hallmark of the aging process and has been demonstrated by several independent investigators using multiple techniques and biological models (Braidy et al., 2011; Gomes et al., 2013; Massudi et al., 2012; Mouchiroud et al., 2013; Scheibye-Knudsen et al., 2014; Zhu et al., 2015). Thus, it is crucial to understand the mechanisms that lead to the decline of this molecule during the aging process. While decline in NAD+ levels is a potential mediator of aging phenotypes, it remains unclear whether this deficit is the result of a single underlying mechanism (Wiley and Campisi, 2014). Three possible scenarios could explain this decline: (A) NAD synthesis could be decreased during the aging process, or (B) NAD degradation could be increased or (C) any combination of these two processes.
Decrease in NAD synthesis as a cause of age-related NAD decline: Role of NAMPT
One explanation for the loss in NAD during aging is that one or more of the NAD-biosynthetic pathways, such as the NAMPT pathway, decline with aging. NAMPT is an important regulator of the intracellular NAD pool. Through its NAD-biosynthetic activity, NAMPT influences the activity of NAD-dependent enzymes, such as SIRTUINS and PARPs, and thereby regulates cellular metabolism, mitochondrial biogenesis, and responses to inflammatory, oxidative, proteotoxic, and genotoxic stress (Garten et al., 2015; Imai and Yoshino, 2013) There is some evidence indicating that levels of NAMPT decline during replicative senescence of human smooth muscle cells, and in peripheral tissue of old mice, such as WAT and skeletal muscle (van der Veer et al., 2007; Yoshino et al., 2011), whereas exercise training has the opposite effect, at least in skeletal muscle (Costford et al., 2010). In addition, lifelong muscle-specific Nampt transgene expression preserved muscle NAD levels and exercise capacity in aged mice (Frederick et al., 2016).
Interestingly, NAMPT has been implicated as a critical regulator of neuronal stem and /or progenitor cell (NSPC) proliferation, self-renewal, and differentiation. It was described that hippocampal NAD levels and NAMPT expression decline with age, and in vivo ablation of Nampt impaired NSPC proliferation and self-renewal. In addition, acute ablation of NAMPT in hippocampal neurospheres significantly reduced NAD levels in NSPCs and stalled them in G1 of the cell cycle, while chronic ablation of NAMPT in hippocampal neurospheres abrogated oligodendrogenesis. These results together reveal that Nampt deficiency in adult NSPCs recapitulates their functional defects observed during the aging process (Stein and Imai, 2014).
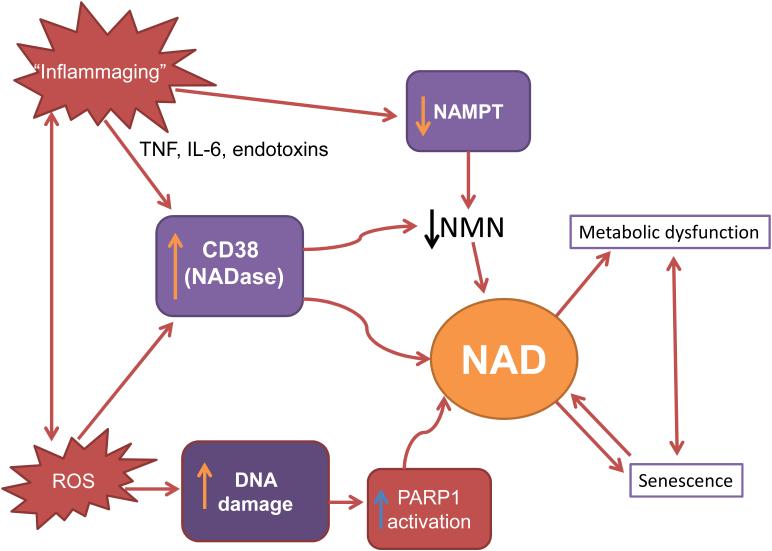
If NAMPT is indeed an important regulator of NAD decline during aging, an essential question is how NAMPT is down regulated during aging. Inflammatory cytokines and oxidative stress appear to be major contributors to the development of chronic inflammation during aging (Franceschi and Campisi, 2014; Kim et al., 2016). One possibility is that chronic inflammation during aging could decrease NAMPT expression and cause a decline in NAD synthesis and subsequent cellular NAD decline (Imai and Yoshino, 2013) (Figure 4). However, neither the role of cytokines on NAMPT expression, nor the role of NAMPT in age-related NAD decline, has been clearly established. In fact, interestingly, inflammatory cytokines can cause either a reduction or increase in NAMPT expression (Iqbal and Zaidi, 2006; Yoshino et al., 2011), implying a complex connection between chronic inflammation and NAMPT-mediated NAD biosynthesis.
Figure 4. Hypothetical interactions between molecular mechanisms of aging and cellular NAD decline.
Molecular mechanisms of aging such as oxidative stress via reactive oxygen species (ROS), DNA damage, and chronic inflammation of aging (Inflammaging) may promote dysregulation of NAD metabolism via activation of CD38 and PARPs, or inhibition of NAMPT. NAD decline can amplify the aging process via acceleration of cellular metabolic dysfunction and senescence.
NAD degradation as a cause of age-related NAD decline
PARP1
A role for PARP1 in the regulation of NAD levels was proposed by studies of PARP1 knockout mice. In one study, it was shown that PARP1 knockout mice had a systemic elevation in NAD+ levels, SIRT1 activity, and metabolic benefits (Bai et al., 2011). Interestingly, in this study, PARP1 knockout mice phenocopied many aspects of SIRT1 activation, such as a higher mitochondrial content, increased energy expenditure, and protection against metabolic disease. Also, pharmacologic inhibition of PARP in cells increased NAD content, SIRT1 activity, and enhanced oxidative metabolism (Bai et al., 2011). In sharp contrast, others have reported a decrease in metabolic flexibility in PARP1 knockout mice, showing that these animals present glucose intolerance and metabolic decline (Devalaraja-Narashimha and Padanilam, 2010). These discrepant results could be due to different animal backgrounds used in each of the studies. Furthermore, the role of PARP1 in aging and longevity is also controversial. For example, one of the current hypotheses to explain the age-related NAD decline is that this phenomenon could be mediated by accumulation of DNA damage and activation of PARP1 during aging (Figure 4) (Imai and Guarente, 2014). However, depending on the study, it has been observed that levels and activity of PARPs may either decrease or increase during chronological aging or accelerated aging disorders (Bakondi et al., 2011; Braidy et al., 2011; Noren Hooten et al., 2012; Scheibye-Knudsen et al., 2014; Zhang et al., 2014). For example, it was reported that in Cockayne syndrome (CS), an accelerated aging disorder characterized by progressive neurodegeneration, there is aberrant PARP activation leading to decreased SIRT1 activity and mitochondrial dysfunction (Scheibye-Knudsen et al., 2014). PARP inhibition, or NAD supplementation, could activate SIRT1 and rescue the CS-associated phenotypes (Scheibye-Knudsen et al., 2014). Also, PARP activity in nuclei from the liver, heart, kidney, and lung of aging rats was found to be upregulated and there was an increase in formation of poly(ADP-ribosylated proteins in all these tissues (Braidy et al., 2011). In contrast, a different study showed that there was a positive correlation between life span and PARP expression across several species (Grube and Bürkle, 1992). In addition, senescent fibroblasts showed a decline in the expression and activity of PARP1, and the protein expression level of PARP1 was significantly lower in the skin of aged donors compared to that of young ones (Bakondi et al., 2011). Thus, the precise role of PARP1 in aging and lifespan need to be further explored. In particular, at the same time that PARPs appear to have a beneficial effect on the control of DNA integrity and may positively contribute to an increase in organismal longevity, its over-activation during the aging process may contribute to cellular NAD decline and metabolic dysfunction (Figure 4). Interestingly, this appears to be the case, since it was proposed that over-activation of PARP1 in skeletal muscle of old mice during exercise can lead to cellular NAD decline that may contribute to exercise-induced fatigue and reduced muscle performance in response to exercise during aging (Mohamed et al., 2014).
CD38
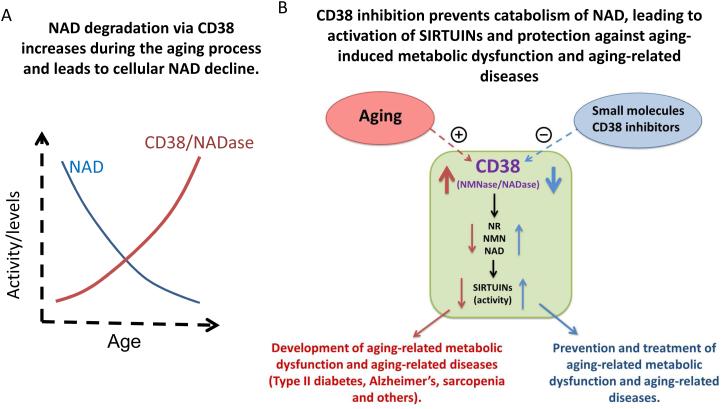
CD38 regulates numerous processes, including cell activation and proliferation, muscle contraction, hormone release, and cell immunity (Chini, 2009; Malavasi et al., 2008). Impaired CD38 function is associated with effects on immunity, metabolic dysfunction, and behavioral deficits in mice (Barbosa et al., 2007; Lopatina et al., 2012; Partida-Sanchez et al., 2001). Tissue NAD levels were found to be tenfold to 20-fold higher in CD38-deficient mice compared to wild-type animals. In addition, NADase activity was almost absent in CD38 knockout mice (Aksoy et al., 2006a; Young et al., 2006), suggesting that CD38 is the main NADase in mammalian tissues. We and others have previously shown that CD38 knockout mice have higher NAD levels and are protected against obesity and metabolic syndrome (Barbosa et al., 2007; Chiang et al., 2015). In addition, treatment of obese mice with CD38 inhibitors increased intracellular NAD levels and improved several aspects of glucose and lipid homeostasis (Escande et al., 2013; Haffner et al., 2015). Importantly, we recently demonstrated that the expression and activity of CD38 increase with aging and that CD38 is required for the age-related NAD decline and mitochondrial dysfunction via a pathway mediated, at least in part ,by regulation of SIRT3 activity (Camacho-Pereira et al., 2016) (Figure 5). We also identified that CD38 is the main enzyme involved in the degradation of the NAD precursor nicotinamide mononucleotide (NMN) in vivo (Camacho-Pereira et al., 2016), indicating that CD38 has a key role in age-related NAD decline and as a modulator of NAD-replacement therapy for aging and metabolic diseases.
Figure 5. CD38 plays a key role in NAD decline in aging.
NAD degradation via CD38 increases during the aging process. In A, we have recently observed that CD38 expression and activity increases during the aging process and that CD38 knock out is sufficient to inhibit the age-related NAD decline (Camacho-Pereira et al., 2016). In B, we present a schematic representation of the role of CD38 in age-related NAD and NAD precursor degradation, and its impact in age-related metabolic decline and age-related diseases.
An interesting question that remain to be addressed is how is CD38 expression regulated during aging. CD38 expression and activity are induced by cytokines and bacterial endotoxins such as lipopolysaccharides (LPS) (Lee et al., 2012; Musso et al., 2001; Sun et al., 2006, Yamamoto-Katayoma et al., 2002). Thus, the chronic inflammation observed during aging could lead to an increase in expression of CD38 and subsequently cause NAD decline (Figure 4 and 5). This hypothesis provides a link between the “inflammaging” theory of aging and the age-related cellular NAD decline (Figure 4).
The consequences of the age-related NAD decline: Role of SIRTUINS
Decreased activity of SIRTUIN family members during aging, especially SIRT1, SIRT3, and SIRT6, has been strongly associated with the susceptibility of organs to aging and age-related diseases (Brown et al., 2013; Choudhury et al., 2011; Kanfi et al., 2012; Yuan et al., 2016). A major cause of the decline in sirtuin activity is a decrease in NAD levels with age, a decline that is accelerated by obesity and counteracted by caloric restriction (CR) and physical activity (Chang and Guarente, 2014; Choudhury et al., 2011; Kincaid and Bossy-Wetzel, 2013; Koltai et al., 2010; Rappou et al., 2016; Yoshino et al., 2011).
SIRT1 has the capability to extend life span, delay aging, and prevent aging-related diseases, mainly by catalyzing the deacetylation of histones, and regulation of transcription factors, or coactivators, such as p53, forkhead box O (FOXO), nuclear factor-κB (NF-κB), PGC-1α, and Ku70 (Ramis et al., 2015). Although there is no decline in SIRT1 protein with age, SIRT1 activity might be compromised in old mice due to the systemic decline in NAD. Indeed, several studies have reported that the activity of SIRTUINS decays with aging (Braidy et al., 2011; Cho et al., 2015; Quintas et al., 2012).
Pharmacological interventions directed to increase SIRT1 activity have been found to slow the onset of aging and delay age-associated diseases. Studies show that overexpression of SIRT1 in mice and use of small molecule activators of SIRT1, such as resveratrol and SRT1720, enhance insulin sensitivity and protect against diet-induced impairments in mitochondrial capacity and oxidative metabolism in animals (Banks et al., 2008; Feige et al., 2008; Milne et al., 2007; Pfluger et al., 2008). SIRT1 activators have also been shown to improve health and extend lifespan of mice maintained on a standard diet and also on a high-fat diet (Baur et al., 2006; Minor et al., 2011). Also, the effects of CR on spontaneous activity, cell survival, and lifespan extension require SIRT1 (Boily et al., 2008; Chen et al., 2005)
Like SIRT1, SIRT6 expression is correlated with longevity and its expression decreased with age in human dermal fibroblasts (Sharma et al., 2013). SIRT6 is also induced by CR in rats (Kanfi et al., 2008). Accumulating evidence also suggest a role for the mitochondrial SIRTUINS like SIRT3 in age-related pathologies (Choudhury et al., 2011; McDonnell et al., 2015; Osborne et al., 2016). For example, SIRT3 plays a central role in mitochondrial function and it has been implicated in the effects of caloric restriction (Someya et al., 2010; Osborne et al., 2016). As SIRT3 knockout mice age, they show accelerated signs of aging and dysfunction in the heart, including cardiac hypertrophy and fibrosis (Hafner et al., 2010; McDonnell et al., 2015).
The cytoplasmic SIRT2 protein remains one of the least understood of the SURTUINS, but it was shown that, along with NAD+, it is upregulated in some tissues during CR (Wang et al., 2007). Recently, it was shown that SIRT2 overexpression extends both mean and maximum lifespan of BubR1-deficient mice, a model that lives shorter and show signs of accelerated aging (North et al., 2014). However, although it appears that this process is mediated by SIRT2, other sirtuins could also be involved (North et al., 2014).
Mitochondria as a target of NAD decline during aging
One of the hallmarks of aging is a decline in mitochondrial function. During aging there are distinct mitochondrial morphological changes, such as abnormal rounded mitochondria, reduction of mitochondrial DNA with increase in mutation rate, and impaired mitochondrial biogenesis (Yuan et al., 2016). A decrease in mitochondrial biogenesis may reduce the turnover of mitochondrial components, resulting in the accumulation of oxidized lipids, proteins, and DNA (Ungvari et al., 2008). Thus, it is believed that maintaining mitochondrial biogenesis capacity during aging is vital to prevent the progression of aging-related diseases (Ungvari et al., 2008; Yuan et al., 2016).
It is now clear that a decrease in SIRT1 activity during aging has deleterious effects on mitochondria (Imai and Guarente, 2014). A reduction in SIRT1 activity down-regulates mitochondrial biogenesis, oxidative metabolism, and antioxidant defense pathways, leading to damage to complex I of the electron transport chain and a decline in mitochondrial function (Imai and Guarente, 2014). In addition, a defect in SIRT1 activity led to a decrease in expression of mitochondria-encoded proteins and metabolic decline in the skeletal muscle of 24-month-old mice (Gomes et al., 2013). These changes were reversed by supplementation with NMN, indicating that the NAD shortage appears to be the primary trigger. Consistent with these studies, some of the health benefits of SIRT1 have been linked to improved mitochondrial function (Baur et al., 2006; Gerhart-Hines et al., 2007; Mouchiroud et al., 2013).
Among the three SITUINS present in the mitochondria (SIRT3, SIRT4 and SIRT5), SIRT3 is the major mitochondrial deacetylase (Lombard et al., 2007). In mitochondria, SIRT3 serves as an important regulator of energy metabolism (Liu et al., 2014) and is highly expressed in metabolically active tissues such as brown adipose tissue, muscle, liver, kidney, heart, and brain. Particularly in the skeletal muscle, SIRT3 expression is sensitive to the diet (Palacios et al., 2009). SIRT3 deacetylates several proteins of the electron transport chain and oxidative phosphorylation, being important for mitochondrial energy production (Ahn et al., 2008). Importantly, SIRT3 expression and activity are suppressed with aging, and SIRT3 up-regulation in aged hematopoietic stem cells improves their regenerative capacity (Brown et al., 2013).
PARP activation also appears to be involved in mitochondrial dysfunction. PARP1 inhibition in cellular models has been shown to increase mitochondrial metabolism through SIRT1 activation (Bai et al., 2011). Also, in the Sco2 knockout/knockin mouse, a mitochondrial disease model characterized by impaired cytochrome c oxidase biogenesis, PARP inhibition led to a reduction of NAD consumption, and a marked improvement of the respiratory chain defect and exercise intolerance (Cerutti et al., 2014). However, it is still not clear whether PARP activation regulates the mitochondria dysfunction that happens during aging. In addition, in our recent study, we observed that the increased CD38 activity during the aging process appears to contribute to the development of mitochondrial dysfunction by decreasing cellular NAD availability (Camacho-Pereira et al., 2016).
Nicotinamide metabolism in aging and longevity
One of the main products of the degradation of NAD is NAM. Recent data clearly indicate that NAM and its derived cellular metabolites have a role in the regulation of aging, energy metabolism, and epigenetics (Bitterman et al., 2002; Anderson et al., 2003; Kraus et al., 2014). For example, in yeast it has been shown that accumulation of cellular NAM has a negative effect on longevity (Anderson et al., 2003). This effect is likely due to the fact that NAM serves as an inhibitor of the Sir2 enzyme, the yeast homolog of mammalian SIRT1 (Anderson et al., 2003). Thus, yeast has a mechanism to clear cellular NAM via a nicotinamidase (PNC1) that promotes the deamidation of this molecule (Anderson et al., 2003). PNC1 has been implicated as a longevity gene in yeast and is both necessary and sufficient for lifespan extension by calorie restriction and low-intensity stress in this organism (Anderson et al., 2003).
An analogous role of NAM in mammalians appears likely. In fact, NAM is an inhibitor of SIRTUINS (Bitterman et al., 2002). It is possible that during NAD metabolism, cellular NAM may accumulate and need to be metabolized (Bitterman et al., 2002; Anderson et al., 2003; Kraus et al., 2014). Indeed, it has been shown that NAM metabolism appears to play a key role in the regulation of energy metabolism, obesity, and liver steatosis (Kraus et al., 2014). The enzyme Nicotinamide N-methyltransferase (NNMT) was shown to be involved in the regulation of cellular metabolism and obesity (Kraus et al., 2014). NNMT is an enzyme that methylates nicotinamide using S-adenosylmethionine (SAM), one of the main cellular methyl donors, and is present at high levels in adipose tissue in obesity (Kraus et al., 2014). Therefore, this enzyme may link NAD metabolism and changes in DNA methylation during aging. In particular, it is possible that increased NAD degradation during aging may increase the availability of NAM to NNMT, increasing the consumption of SAM (Kraus et al., 2014). This decrease in the availability of SAM to methylate DNA may explain the changes in methylation observed during aging (Figure 3). In addition, SAM is also involved in the synthesis of polyamines that are key regulators of chromatin structure (Kraus et al., 2014). Interestingly, N-methylnicotinamide has been implicated as a longevity factor in the worm C. elegans (Schmeisser et al., 2013). However, to date, the role of NNMT and methyl-nicotinamide in the aging process in mammalians has not been explored. It would be interesting to understand how degradation of NAD via CD38 and PARPs integrates and regulates the process of nicotinamide metabolism and DNA methylation in mammals.
INTERVENTIONS TO PREVENT AGE-RELATED NAD DECLINE
Caloric restriction
CR extends lifespan and prevents chronic diseases in a range of organisms, including yeast, worms, flies, mice, rats, and perhaps in monkeys and humans. It has been hypothesized that CR, by increasing the levels of intracellular NAD, could stimulate SIRTUIN activity, and then extend the lifespan of organisms, (Lin et al., 2000; Longo et al., 2015; Lu and Lin, 2010). Recently, it was reported that a CR mimetic, 2-deoxyglucose (2-DG), can extend the replicative lifespan of Hs68 cells by increasing intracellular NAD and SIRT1 activity (Yang et al., 2011). However, several other mechanisms, independent of SIRTUINS, have also been implicated in the biological effects of CR, including inhibition of the IGF-1 pathway and modulation of the AMPK-mTOR pathway (Longo et al., 2015). A recent study indicates that even though CR not always correlates with lifespan extension, it consistently improved health across mice strains and sexes (Mitchell et al, 2016).
Supplementation with NAD precursors
It is now clear that NAMPT-mediated NAD biosynthesis regulates SIRUTIN activity, particularly SIRT1 activity, in a number of different cellular and physiological conditions. Therefore, it is conceivable that activating SIRT1 by promoting NAD biosynthesis could be an efficient anti-aging intervention (Imai and Guarente, 2010). One way to promote NAD biosynthesis and SIRT1 activity in mammals is to use key NAD intermediates, such as NR and NMN (Cantó and Auwerx, 2011). Therefore, pharmacological approaches able to improve NAD availability have been investigated as potential therapeutic treatments for different human disorders (Houtkooper and Auwerx, 2012). NR has been investigated as an NAD-boosting molecule because of its ability to cross the plasma membrane and, different than NAM, it does not inhibit NAD-hydrolyzing enzymes such as PARP-1 and SIRTUINS (Chi and Sauve., 2013). Recently, diet supplementation with NR was shown to increase NAD levels in mammalian cells and mice tissue, and activate SIRT1 and SIRT3. This supplementation ameliorated metabolic defects of obese mice, boosted their energy expenditure and oxidative performance of skeletal muscle and brown adipose tissue, and, overall, reverted metabolic impairment (Cantó et al., 2012). In pre-diabetic mice NR improved glucose tolerance, reduced weight gain, liver damage and the development of hepatic steatosis, while protecting against sensory neuropathy (Trammell et al., 2016). Additionally, NR was shown to prevent and reverse the development of nonalcoholic fatty liver disease (NAFLD) by inducing a SIRT1- and SIRT3-dependent mitochondrial unfolded protein response (Gariani et al., 2016). NR supplementation also increased mitochondrial NAD levels and improved mitochondria function of old mice (Gomes et al., 2013), and rejuvenated and prevented senescence of muscle stem cells in aged mice (Zhang et al., 2016). Furthermore, NR supplementation was sufficient to increase longevity in mice (Zhang et al., 2016).
Another key NAD intermediate, NMN, a product of the NAMPT reaction, was shown to ameliorate glucose intolerance by restoring NAD levels in HFD-induced Type 2 diabetic mice. NMN administration enhanced hepatic insulin sensitivity and restored gene expression related to oxidative stress, inflammatory response, and circadian rhythm, partly through SIRT1 activation (Yoshino et al., 2011). Additionally, NMN improved glucose intolerance and lipid profiles in age-induced T2D mice. Treatment of mice for 1 week with NMN reduced lactate level, and increased ATP and mitochondrial encoded OXPHOS transcripts, improving mitochondrial function in aging mice, in a SIRT1-dependent manner (Gomes et al., 2013). In another study, 8 weeks of NMN supplementation restored arterial SIRT1 activity and ameliorated age-associated endothelial dysfunction and large elastic artery stiffening in mice. These improvements restored nitric oxide bioavailability, reduced oxidative stress, and promoted complete or partial normalization of structural proteins in the arterial wall (de Picciotto et al., 2016).
Beta cell-specific SIRT1-overexpressing (BESTO) transgenic mice exhibited enhanced glucose-stimulated insulin secretion and improved glucose tolerance compared to controls at both 3 and 8 months of age. However, in old BESTO mice there was loss of the improved glucose tolerance and insulin secretion (Ramsey et al., 2008). In these mice SIRT1 protein levels remain overexpressed, but its activity decreases. The loss of SIRT1 activity in old BESTO islets appears to be due to an age-associated decrease in NAMPT-mediated systemic NAD biosynthesis, since these animals have significantly reduced NMN levels. Administration of NMN in old BESTO mice restored the metabolic phenotype and improved glucose tolerance (Ramsey et al., 2008). These findings strongly suggest that an age-associated decline in systemic NAD biosynthesis indeed accounts for the reduced activity of SIRT1 in aged pancreatic β cells (Ramsey et al., 2008). The restoration of increased insulin secretion and improvement of glucose tolerance by NMN administration have also been observed in female NAMPT heterozygous mice.
Mice overexpressing the mitotic checkpoint kinase gene BubR1 live longer, whereas mice hypomorphic for BubR1 (BubR1(H/H)) live shorter and show signs of accelerated aging. During aging, BubR1 levels decline in many tissues of wild-type mice. Loss of BubR1 levels with age is due to a decline in NAD, and treatment of mice with the NAD precursor NMN increases BubR1 abundance in vivo (North et al., 2014). These studies together imply that NAD precursors do in fact ameliorate metabolic and age-related disorders.
CD38 and PARP1 inhibitors
Several CD38 inhibitors (CD38i) have been developed, including the natural products apigenin and quercetin (Escande et al., 2013), and the thiazoloquin(az)olin(on)es developed by GlaxoSmithKline (Haffner et al., 2015). All of these compounds can increase cellular NAD levels, and at least apigenin and quercetin have been shown to have beneficial metabolic effects (Escande et al., 2013). However, the precise role of CD38i on the regulation of NAD degradation and the generation of CD38-derived second messengers such as cADPR and NAADP is not known. CD38i may serve as pharmacological tools for several conditions including age-related metabolic dysfunction, obesity, diabetes, Alzheimer disease, and cancers, and for inflammatory conditions such asthma, COPD, and arthritis (Chini, 2009) (Figure 5). Interestingly, anti-CD38 antibodies have been recently introduced as therapeutic tools in multiple myeloma and are being tested for other hematological malignancies (Lokhorst et al., 2015; van de Donk et al., 2016). It would be relevant to determine the impact of these antibodies on CD38 activity, NAD decline, and metabolic dysfunction during the aging process.
Similar data has been generated for PARP1 inhibitors (Bai et al., 2011; Pirinen et al., 2014). Pharmacological inhibition of PARP in vitro and in vivo increased NAD+ content, SIRT1 activity, and enhanced oxidative metabolism (Bai et al., 2011). A PARP inhibitor also improved mitochondrial function in skeletal muscle, enhanced endurance performance, and protected against HFD-induced metabolic complications (Pirinen et al., 2014), suggesting that these inhibitors can be used to treat metabolic dysfunction. Interestingly, to date, the effect of combination therapies with CD38i, PARP inhibitors, and NAD precursors have not been reported. It is likely that a combination of these agents may provide a superior therapeutic approach to prevent age-related NAD decline.
CLOSING REMARKS
In conclusion, NAD metabolism is very complex and includes several pathways and metabolites that need to be integrated in our understanding of the aging process. Future research will determine how mechanisms involved in aging, such as reactive oxygen radicals, DNA damage, inflammation, and cellular senescence, regulate and are regulated by NAD metabolism. In addition, it is important to determine the precise enzymes involved on the age-related NAD decline. Our recent work highlights the role of the enzyme CD38 as a key NADase involved on the process of age-related NAD decline. Metabolic dysfunctions of aging maybe treated by targeted NAD-replacement therapies with NAD precursors and/or NAD degradation inhibitors. In particular, it is also important to notice that CD38 and its homologous gene BST-1 are involved on the degradation of not only NAD itself, but also its precursors such as NMN and NR. Thus, CD38i and PARP inhibitors may be used in combination with NMN and NR to enhance the potential of NAD-replacement therapy for several diseases. Future studies on the role of NAD metabolism in the aging process will be essential for the development of drugs that target age-related diseases.
Highlights.
NAD plays a key role in energy metabolism, cell signaling and energy sensing
Cellular NAD levels decrease during the process of chronological aging
NAD decline during aging leads to decrease in SIRTUINS activity, mitochondrial and metabolic dysfunction
NAD decline could be cause by increased in its catabolism or decrease in its synthesis
The enzyme CD38 is the main NADase in tissues and plays a key role on the age-related NAD decline
NAD replacement therapy with NAD precursors, activators of NAD synthesis and/or inhibitors of its degradation may serve as target for age-related metabolic dysfunction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. 2008;105:14447–14452. doi: 10.1073/pnas.0803790105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksoy P, Escande C, White TA, Thompson M, Soares S, Benech JC, Chini EN. Regulation of SIRT 1 mediated NAD dependent deacetylation: a novel role for the multifunctional enzyme CD38. Biochem. Biophys Res Com. 2006a;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- Aksoy P, White TA, Thompson M, Chini EN. Regulation of intracellular levels of NAD: a novel role for CD38. Biochem. Biophys. Res. Commun. 2006b;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avalos JL, Bever KM, Wolberger C. Mechanism of sirtuin inhibition by nicotinamide: altering the NAD(+) cosubstrate specificity of a Sir2 enzyme. Mol. Cell. 2005;17:855–868. doi: 10.1016/j.molcel.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Bai P, Cantó C, Oudart H, Brunyánszki A, Cen Y, Thomas C, Yamamoto H, Huber A, Kiss B, Houtkooper RH, et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakondi E, Catalgol B, Bak I, Jung T, Bozaykut P, Bayramicli M, Ozer NK, Grune T. Age-related loss of stress-induced nuclear proteasome activation is due to low PARP-1 activity. Free Radic. Biol. Med. 2011;50:86–92. doi: 10.1016/j.freeradbiomed.2010.10.700. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutiérrez-Juárez R, Rossetti L, Gu W, Accili D. SirT1 gain-of-function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa MTP, Soares SM, Novak CM, Sinclair D, Levine JA, Aksoy P, Chini EN. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A, et al. Resveratrol improves health and survival of mice on a high calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beers KW, Chini EN, Dousa TP. All-trans-retinoic acid stimulates synthesis of cyclic ADP-ribose in renal LLC-PK1 cells. J. Clin. Invest. 1995;95:2385–2390. doi: 10.1172/JCI117932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieganowski P, Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/s0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 2002;277:45099–107. doi: 10.1074/jbc.M205670200. [DOI] [PubMed] [Google Scholar]
- Bogan KL, Brenner C. Nicotinic acid, nicotinamide, and nicotinamide riboside: A molecular evaluation of NAD+ precursor vitamins in human nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- Boily G, Seifert EL, Bevilacqua L, He XH, Sabourin G, Estey C, Moffat C, Crawford S, Saliba S, Jardine K, et al. SirT1 Regulates Energy Metabolism and Response to Caloric Restriction in Mice. PLoS One. 2008;3:e1759. doi: 10.1371/journal.pone.0001759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradaile NM, Pickering JG. Nicotinamide phosphoribosyltransferase imparts human endothelial cells with extended replicative lifespan and enhanced angiogenic capacity in a high glucose environment. Aging Cell. 2009;8:100–112. doi: 10.1111/j.1474-9726.2009.00453.x. [DOI] [PubMed] [Google Scholar]
- Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age-related changes in NAD+ metabolism oxidative stress and SIRT1 activity in wistar rats. PLoS One. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, Zhang D, Scadden DT, Chen D. SIRT3 Reverses Aging-associated Degeneration. Cell Rep. 2013;3:319–327. doi: 10.1016/j.celrep.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho-Pereira J, Tarragó MG, Chini CCS, Nin V, Escande C, Warner GM, Puranik AS, Schoon RA, Reid JM, Galina A, et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambronne XA, Stewart ML, Kim D, Jones-Brunette AM, Morgan RK, Farrens DL, Cohen MS, Goodman RH. Biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cang C, Zhou Y, Navarro B, Seo YJ, Aranda K, Shi L, Battaglia-Hsu S, Nissim I, Clapham DE, Ren D. mTOR regulates lysosomal ATP-sensitive two-pore Na(+) channels to adapt to metabolic state. Cell. 2013;152:778–790. doi: 10.1016/j.cell.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Auwerx J. NAD+ as a signaling molecule modulating metabolism. Cold Spring Harb Symp. Quant. Biol. 2011;76:291–298. doi: 10.1101/sqb.2012.76.010439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Jiang LQ, Deshmukh AS, Mataki C, Coste A, Lagouge M, Zierath JR, Auwerx J. Interdependence of AMPK and SIRT1 for metabolic adaptation to fasting and exercise in skeletal muscle. Cell Metab. 2010;11:213–219. doi: 10.1016/j.cmet.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Menzies KJ, Auwerx J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti R, Pirinen E, Lamperti C, Marchet S, Sauve AA, Li W, Leoni V, Schon EA, Dantzer F, Auwerx J, et al. NAD+-Dependent Activation of Sirt1 Corrects the Phenotype in a Mouse Model of Mitochondrial Disease. Cell Metab. 2014;19:1042–1049. doi: 10.1016/j.cmet.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P, Weill JD, Mandel P. Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem. Biophys. Res. Commun. 1963;11:39–43. doi: 10.1016/0006-291x(63)90024-x. [DOI] [PubMed] [Google Scholar]
- Chang HC, Guarente L. SIRT1 and other sirtuins in Metabolism. Trends Endocrinol. Metab. 2014;25:138–145. doi: 10.1016/j.tem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Cheng J, Yusufi AN, Thompson MA, Chini EN, Grande JP. Nicotinic acid adenine dinucleotide phosphate: a new Ca2+ releasing agent in kidney. J. Am. Soc. Nephrol. 2001;12:54–60. doi: 10.1681/ASN.V12154. [DOI] [PubMed] [Google Scholar]
- Chi Y, Sauve AA. Nicotinamide riboside, a trace nutrient in foods, is a Vitamin B3 with effects on energy metabolism and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:657–661. doi: 10.1097/MCO.0b013e32836510c0. [DOI] [PubMed] [Google Scholar]
- Chiang SH, Harrington WW, Luo G, Milliken NO, Ulrich JC, Chen J, Rajpal DK, Qian Y, Carpenter T, Murray R, et al. Genetic Ablation of CD38 Protects against Western Diet-Induced Exercise Intolerance and Metabolic Inflexibility. PLoS One. 2015;10:e0134927. doi: 10.1371/journal.pone.0134927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN. CD38 as a regulator of cellular NAD: a novel potential pharmacological target for metabolic conditions. Curr. Pharm. Des. 2009;15:57–63. doi: 10.2174/138161209787185788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN, Beers KW, Dousa TP. Nicotinate adenine dinucleotide phosphate [NAADP] triggers a specific calcium release system in sea urchin eggs. J. Biol. Chem. 1995;270:3216–3223. doi: 10.1074/jbc.270.7.3216. [DOI] [PubMed] [Google Scholar]
- Chini EN, Chini CC, Kato I, Takasawa S, Okamoto H. CD38 is the major enzyme responsible for synthesis of nicotinic acid-adenine dinucleotide phosphate in mammalian tissues. Biochem J. 2002;362:125–130. doi: 10.1042/0264-6021:3620125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chini EN, Dousa TP. Enzymatic synthesis and degradation of nicotinate adenine dinucleotide phosphate [NAADP], a Ca[2+]-releasing agonist, in rat tissues. Biochem. Biophys. Res. Commun. 1995;205:167–174. doi: 10.1006/bbrc.1995.1485. [DOI] [PubMed] [Google Scholar]
- Cho SH, Chen JA, Sayed F, Ward ME, Gao F, Nguyen TA, Krabbe G, Sohn PD, Lo I, Minami S, et al. SIRT1 Deficiency in Microglia Contributes to Cognitive Decline in Aging and Neurodegeneration via Epigenetic Regulation of IL-1β. J. Neurosci. 2015;35:807–818. doi: 10.1523/JNEUROSCI.2939-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury M, Jonscher KR, Friedman JE. Reduced mitochondrial function in obesity-associated fatty liver: SIRT3 takes on the fat. Aging (Albany NY) 2011;3:175–178. doi: 10.18632/aging.100289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapper DL, Walseth TF, Dargie PJ, Lee HC. Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 1987;262:9561–9568. [PubMed] [Google Scholar]
- Collins PB, Chaykin S. The management of nicotinamide and nicotinic acid in the mouse. J. Biol. Chem. 1972;247:778–783. [PubMed] [Google Scholar]
- Costford SR, Bajpeyi S, Pasarica M, Albarado DC, Thomas SC, Xie H, Church TS, Jubrias SA, Conley KE, Smith SR, et al. Skeletal muscle NAMPT is induced by exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2010;298:E117–E126. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devalaraja-Narashimha K, Padanilam BJ. PARP1 deficiency exacerbates diet-induced obesity in mice. J. Endocrinol. 2010;205:243–52. doi: 10.1677/JOE-09-0402. [DOI] [PubMed] [Google Scholar]
- van de Donk NW, Janmaat ML, Mutis T, Lammerts van Bueren JJ, Ahmadi T, Sasser AK, Lokhorst HM, Parren PW. Monoclonal antibodies targeting CD38 in hematological malignancies and beyond. Immunol. Rev. 2016;270:95–112. doi: 10.1111/imr.12389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Euler-Chelpin H. Fermentation of sugars and fermentative enzymes: Nobel Lecture. Nobel Foundation; May 23, 1930. 1930. Retrieved August 12, 2008. [Google Scholar]
- Escande C, Nin V, Price NL, Capellini V, Gomes AP, Barbosa MT, O’Neil L, White TA, Sinclair DA, Chini EN. Flavonoid apigenin is an inhibitor of the NAD+ ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Campisi J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69(Suppl 1):S4–9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- Feige JN, Lagouge M, Canto C, Strehle A, Houten SM, Milne JC, Lambert PD, Mataki C, Elliott PJ, Auwerx J. Specific SIRT1 Activation Mimics Low Energy Levels and Protects against Diet-Induced Metabolic Disorders by Enhancing Fat Oxidation. Cell Metab. 2008;8:347–358. doi: 10.1016/j.cmet.2008.08.017. [DOI] [PubMed] [Google Scholar]
- Frederick DW, Loro E, Liu l., Davila A, Jr, Chellappa K, Silverman IM, Quinn WJ, 3rd, Gosai SJ, Tichy ED, Davis JG, et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016;24:269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. Glucose Restriction Inhibits Skeletal Myoblast Differentiation by Activating SIRT1 through AMPK-Mediated Regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariani K, Menzies KJ, Ryu D, Wegner CJ, Wang X, Ropelle ER, Moullan N, Zhang H, Perino A, Lemos V, et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garten A, Schuster S, Penke M, Gorski T, de Giorgis T, Kiess W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015;11:535–546. doi: 10.1038/nrendo.2015.117. [DOI] [PubMed] [Google Scholar]
- Gerdts J, Brace EJ, Sasaki Y, DiAntonio A, Milbrandt J. SARM1 activation triggers axon degeneration locally via NAD? destruction. Science. 2015;348:453–457. doi: 10.1126/science.1258366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhart-Hines Z, Rodgers JT, Bare O, Lerin C, Kim SH, Mostoslavsky R, Alt FW, Wu Z, Puigserver P. Metabolic control of muscle mitochondrial function and fatty acid oxidation through SIRT1/PGC-1α. EMBO J. 2007;26:1913–1923. doi: 10.1038/sj.emboj.7601633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes AP, Price NL, Ling AJY, Moslehi JJ, Montgomery MK, Rajman L, White JP, Teodoro JS, Wrann CD, Hubbard BP, et al. Declining NAD(+) induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozio A, Sociali G, Sturla L, Caffa I, Soncini D, Salis A, Raffaelli N, De Flora A, Nencioni A, Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube K, Bürkle A. Poly(ADP-ribose) polymerase activity in mononuclear leukocytes of 13 mammalian species correlates with species-specific life span. Proc. Natl. Acad. Sci. USA. 1992;89:11759–11763. doi: 10.1073/pnas.89.24.11759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner AV, Dai J, Gomes AP, Xiao CY, Palmeira CM, Rosenzweig A, Sinclair DA. Regulation of the mPTP by SIRT3-mediated deacetylation of CypD at lysine 166 suppresses age-related cardiac hypertrophy. Aging (Albany NY) 2010;2:914–923. doi: 10.18632/aging.100252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffner CD, Becherer JD, Boros EE, Cadilla R, Carpenter T, Cowan D, Deaton DN, Guo Y, Harrington W, Henke BR, et al. Discovery, Synthesis, and Biological Evaluation of Thiazoloquin(az)olin(on)es as Potent CD38 Inhibitors. J. Med. Chem. 2015;58:3548–3571. doi: 10.1021/jm502009h. [DOI] [PubMed] [Google Scholar]
- Harden A, Young WJ. The alcoholic ferment of yeast-juice Part II.--The coferment of yeast-juice. Proc. R. Soc. London. Series B. 1906;78:369–375. [Google Scholar]
- Hellmich MR, Strumwasser F. Purification and characterization of a molluscan egg-specific NADase, a second-messenger enzyme. Cell Regul. 1991;2:193–202. doi: 10.1091/mbc.2.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata Y, Kimura N, Sato K, Ohsugi Y, Takasawa S, Okamoto H, Ishikawa J, Kaisho T, Ishihara K, Hirano T. ADP ribosyl cyclase activity of a novel bone marrow stromal cell surface molecule, BST-1. FEBS Lett. 1994;356:244–248. doi: 10.1016/0014-5793(94)01279-2. [DOI] [PubMed] [Google Scholar]
- Houtkooper RH, Auwerx J. Exploring the therapeutic space around NAD+ J. Cell Biol. 2012;199:205–209. doi: 10.1083/jcb.201207019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocrinol. Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y, Wang H, Wang Q, Deng H. Overexpression of CD38 decreases cellular NAD levels and alters the expression of proteins involved in energy metabolism and antioxidant defense. J. Proteome Res. 2014;13:786–795. doi: 10.1021/pr4010597. [DOI] [PubMed] [Google Scholar]
- Imai S, Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: implications for metabolic diseases. Trends Pharmacol. Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Guarente L. NAD+ and Sirtuins in Aging and Disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai S, Yoshino J. The importance of NAMPT/NAD/SIRT1 in the systemic regulation of metabolism and aging. Diabetes Obes. Metab. Suppl. 2013;3:26–33. doi: 10.1111/dom.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iqbal J, Zaidi M. TNF regulates cellular NAD+ metabolism in primary macrophages. Biochem. Biophys. Res. Commun. 2006;342:1312–1318. doi: 10.1016/j.bbrc.2006.02.109. [DOI] [PubMed] [Google Scholar]
- Jackson TM, Rawling JM, Roebuck BD, Kirkland JB. Large supplements of nicotinic acid and nicotinamide increase tissue NAD+ and poly(ADP-ribose) levels but do not affect diethylnitrosamine-induced altered hepatic foci in Fischer-344 rats. J. Nutr. 1995;125:1455–1461. doi: 10.1093/jn/125.6.1455. [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. The sirtuin SIRT6 regulates lifespan in male mice. Nature. 2012;483:218–221. doi: 10.1038/nature10815. [DOI] [PubMed] [Google Scholar]
- Kato M, Lin S-J. Regulation of NAD+ metabolism, signaling and compartmentalization in the yeast Saccharomyces cerevisiae. DNA repair. 2014;23:49–58. doi: 10.1016/j.dnarep.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, de Cabo R, et al. Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett. 2008;582:543–548. doi: 10.1016/j.febslet.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, Kim UH. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008;57:868–878. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- Kim H, Jacobson EL, Jacobson MK. Synthesis and degradation of cyclic ADP-ribose by NAD glycohydrolases. Science. 1993;261:1330–1333. doi: 10.1126/science.8395705. [DOI] [PubMed] [Google Scholar]
- Kim KA, Jeong JJ, Yoo SY, Kim DH. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. J. BMC Microbiol. 2016;16:9. doi: 10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 2013;5:48. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koltai E, Szabo Z, Atalay M, Boldogh I, Naito H, Goto S, Nyakas C, Radak Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech Ageing Dev. 2010;131:21–28. doi: 10.1016/j.mad.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus D, Yang Q, Kong D, Banks AS, Zhang L, Rodgers JT, Pirinen E, Pulinilkunnil TC, Gong F, Wang YC, et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CU, Song EK, Yoo CH, Kwak YK, Han MK. Lipopolysaccharide Induces CD38 Expression and Solubilization in J774 Macrophage Cells. Mol. Cells. 2012;34:573–576. doi: 10.1007/s10059-012-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Aarhus R. ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 1991;3:203–209. doi: 10.1091/mbc.2.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC. A unified mechanism of enzymatic synthesis of two calcium messengers: cyclic ADP-ribose and NAADP. J. Biol. Chem. 1999;380:785–793. doi: 10.1515/BC.1999.098. [DOI] [PubMed] [Google Scholar]
- Lee HC. Cyclic ADP-ribose and nicotinic acid adenine dinucleotide phosphate (NAADP) as messengers for calcium mobilization. J. Biol. Chem. 2012;287:31633–31640. doi: 10.1074/jbc.R112.349464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner F, Niere M, Ludwig A, Ziegler M. Structural and functional characterization of human NAD kinase. Biochem. Biophys. Res. Commun. 2001;288:69–74. doi: 10.1006/bbrc.2001.5735. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 2004;18:12–16. doi: 10.1101/gad.1164804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Nam M, Fan W, Akie TE, Hoaglin DC, Gao G, Keaney JF, Jr, Cooper MP. Nutrient sensing by the mitochondrial transcription machinery dictates oxidative phosphorylation. J. Clin. Invest. 2014;124:768–784. doi: 10.1172/JCI69413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokhorst HM, Plesner T, Laubach JP, Nahi H, Gimsing P, Hansson M, Minnema MC, Lassen U, Krejcik J, Palumbo A, et al. Targeting CD38 with Daratumumab Monotherapy in Multiple Myeloma. N. Engl. J. Med. 2015;373:1207–1219. doi: 10.1056/NEJMoa1506348. [DOI] [PubMed] [Google Scholar]
- Lombard DB, Alt FW, Cheng HL, Bunkenborg J, Streeper RS, Mostoslavsky R, Kim J, Yancopoulos G, Valenzuela D, Murphy A, et al. Mammalian Sir2 Homolog SIRT3 Regulates Global Mitochondrial Lysine Acetylation. Mol Cell Biol. 2007;27:8807–8814. doi: 10.1128/MCB.01636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo VD, Antebi A, Bartke A, Barzilai N, Brown-Borg HM, Caruso C, Curiel TJ, de Cabo R, Franceschi C, Gems D, et al. Interventions to Slow Aging in Humans: Are We Ready? Aging Cell. 2015;14:497–510. doi: 10.1111/acel.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopatina O, Inzhutova A, Salmina AB, Higashida H. The Roles of Oxytocin and CD38 in Social or Parental Behaviors. Front. Neurosci. 2012;6:182. doi: 10.3389/fnins.2012.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S-P, Lin S-J. Regulation of Yeast Sirtuins by NAD+ Metabolism and Calorie Restriction. Biochim Biophys. Acta. 2010;1804:1567–1575. doi: 10.1016/j.bbapap.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Hao BX, Graeff R, Wong CW, Wu WT, Yue J. Two pore channel 2 (TPC2) inhibits autophagosomal-lysosomal fusion by alkalinizing lysosomal pH. J. Biol. Chem. 2013;288:24247–24263. doi: 10.1074/jbc.M113.484253. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Malavasi F, Deaglio S, Funaro A, Ferrero E, Horenstein AL, Ortolan E, Vaisitti T, Aydin S. Evolution and Function of the ADP Ribosyl Cyclase/CD38 Gene Family in Physiology and Pathology. Physiol. Rev. 2008;88:841–886. doi: 10.1152/physrev.00035.2007. [DOI] [PubMed] [Google Scholar]
- Massudi H, Grant R, Braidy N, Guest J, Farnsworth B, Guillemin GJ. Age-associated changes in oxidative stress and Sirt1 activity in wistar rats. PLoS one. 2012;7:e42357. doi: 10.1371/journal.pone.0042357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell E, Peterson BS, Bomze HM, Hirschey MD. SIRT3 regulates progression and development of diseases of aging. Trends Endocrinol. Metab. 2015;26:486–492. doi: 10.1016/j.tem.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, et al. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor RK, Baur JA, Gomes AP, Ward TM, Csiszar A, Mercken EM, Abdelmohsen K, Shin YK, Canto C, Scheibye-Knudsen M, et al. SRT1720 improves survival and healthspan of obese mice. Sci. Rep. 2011;1:70. doi: 10.1038/srep00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell SJ, Madrigal-Matute J, Scheibye-Knudsen M, Fang E, Aon M, González-Reyes J, Cortassa S, Kaushik S, Gonzalez-Freire M, Patel B, et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 2016;23:1093–1112. doi: 10.1016/j.cmet.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed JS, Wilson JC, Myers MJ, Sisson KJ, Alway SE. Dysregulation of SIRT-1 in aging mice increases skeletal muscle fatigue by a PARP-1-dependent mechanism. Aging (Albany NY) 2014;6:820–834. doi: 10.18632/aging.100696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan AJ, Davis LC, Ruas M, Galione A. TPC: the NAADP discovery channel? Biochem. Soc. Trans. 2015;43:384–389. doi: 10.1042/BST20140300. [DOI] [PubMed] [Google Scholar]
- Mouchiroud L, Houtkooper RH, Moullan N, Katsyuba E, Ryu D, Cantó C, Mottis A, Jo YS, Viswanathan M, Schoonjans K, et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430–441. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musso T, Deaglio S, Franco L, Calosso L, Badolato R, Garbarino G, Dianzani U, Malavasi F. CD38 expression and functional activities are up-regulated by IFN-gamma on human monocytes and monocytic cell lines. J. Leukoc. Biol. 2001;69:605–12. [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, Sassone-Corsi P. Circadian Control of the NAD+ Salvage Pathway by CLOCK-SIRT1. Science. 2009;324:654–657. doi: 10.1126/science.1170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikiforov A, Kulikova V, Ziegler M. The human NAD metabolome: Functions, metabolism and compartmentalization. Crit, Rev. Biochem. Mol. Biol. 2015;50:284–297. doi: 10.3109/10409238.2015.1028612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren Hooten N, Fitzpatrick M, Kompaniez K, Jacob KD, Moore BR, Nagle J, Barnes J, Lohani A, Evans MK. Coordination of DNA repair by NEIL1 and PARP-1: a possible link to aging. Aging (Albany NY) 2012;4:674–685. doi: 10.18632/aging.100492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, Rosenberg MA, Jeganathan KB, Hafner AV, Michan S, Dai J, Baker DJ, Cen Y, Wu LE, Sauve AA, et al. SIRT2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33:1438–1453. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne B, Bentley NL, Montgomery MK, Turner N. The role of mitochondrial sirtuins in health and disease. Free Rad. Biol. Med. 2016 doi: 10.1016/j.freeradbiomed.2016.04.197. doi: 10.1016/j.freeradbiomed.2016.04.197. [DOI] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1α in skeletal muscle. Aging (Albany NY) 2009;1:771–783. doi: 10.18632/aging.100075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade P. The hunt for an alternate way to generate NAADP. Focus on "NAADP as a second messenger: neither CD38 nor base exchange reaction are necessary for in vivo generation of NAADP in myometrial cells". Am. J Physiol. Cell Physiol. 2007;292:C4–C7. doi: 10.1152/ajpcell.00390.2006. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Cockayne DA, Monard S, Jacobson EL, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, et al. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vivo. Nat. Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschöp MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc. Natl. Acad. Sci. USA. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Picciotto NE, Gano LE, Johnson LC, Martens CR, Sindler AL, Mills KF, Imai S, Seals DR. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016 doi: 10.1111/acel.12461. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirinen E, Cantó C, Jo YS, Morato L, Zhang H, Menzies KJ, Williams EG, Mouchiroud L, Moullan N, Hagberg C, et al. Pharmacological Inhibition of Poly(ADP-Ribose) Polymerases Improves Fitness and Mitochondrial Function in Skeletal Muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preugschat F, Carter LH, Boros EE, Porter DJ, Stewart EL, Shewchuk LM. A pre-steady state and steady state kinetic analysis of the N-ribosyl hydrolase activity of hCD157. Arch. Biochem. Biophys. 2014;564:156–163. doi: 10.1016/j.abb.2014.09.008. [DOI] [PubMed] [Google Scholar]
- Quarona V, Zaccarello G, Chillemi A, Brunetti E, Singh VK, Ferrero E, Funaro A, Horenstein AL, Malavasi F. CD38 and CD157: A Long Journey from Activation Markers to Multifunctional Molecules. Cytometry B Clin. Cytom. 2013;84:207–217. doi: 10.1002/cyto.b.21092. [DOI] [PubMed] [Google Scholar]
- Quintas A, de Solís AJ, Díez-Guerra FJ, Carrascosa JM, Bogónez E. Age-associated decrease of SIRT1 expression in rat hippocampus: prevention by late onset caloric restriction. Exp Gerontol. 2012;47:198–201. doi: 10.1016/j.exger.2011.11.010. [DOI] [PubMed] [Google Scholar]
- Ramis MR, Esteban S, Miralles A, Tan DX, Reiter RJ. Caloric restriction, resveratrol and melatonin: Role of SIRT1 and implications for aging and related-diseases. Mech. Ageing Dev. 2015:146–148. doi: 10.1016/j.mad.2015.03.008. 28-41. [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Mills KF, Satoh A, Imai S. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, Hong HK, Chong JL, Buhr ED, Lee C, et al. Circadian Clock Feedback Cycle Through NAMPT-Mediated NAD+ Biosynthesis. Science. 2009;324:651–654. doi: 10.1126/science.1171641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappou E, Jukarainen S, Rinnankoski-Tuikka R, Kaye S, Heinonen S, Hakkarainen A, Lundbom J, Lundbom N, Saunavaara V, Rissanen A, Virtanen KA, et al. Weight Loss Is Associated With Increased NAD(+)/SIRT1 Expression But Reduced PARP Activity in White Adipose Tissue. J. Clin. Endocrinol. Metab. 2016;101:1263–1273. doi: 10.1210/jc.2015-3054. [DOI] [PubMed] [Google Scholar]
- Ruggieri S, Orsomando G, Sorci L, Raffaelli N. Regulation of NAD biosynthetic enzymes modulates NAD-sensing processes to shape mammalian cell physiology under varying biological cues. Biochem. Biophy. Acta. 2015;1854:1138–1173. doi: 10.1016/j.bbapap.2015.02.021. [DOI] [PubMed] [Google Scholar]
- Rusinko N, Lee HC. Widespread occurrence in animal tissues of an enzyme catalyzing the conversion of NAD+ into a cyclic metabolite with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 1989;264:11725–11731. [PubMed] [Google Scholar]
- Scheibye-Knudsen M, Mitchell SJ, Fang EF, Iyama T, Ward T, Wang J, Dunn CA, Singh N, Veith S, Hasan-Olive MM, et al. A high-fat diet and NAD(+) activate Sirt1 to rescue premature aging in cockayne syndrome. Cell Metab. 2014;20:840–855. doi: 10.1016/j.cmet.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeisser K, Mansfeld J, Kuhlow D, Weimer S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, et al. Role of Sirtuins in Lifespan Regulation is Linked to Methylation of Nicotinamide. Nat. Chem. Biol. 2013;9:693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Diecke S, Zhang WY, Lan F, He C, Mordwinkin NM, Chua KF, Wu JC. The Role of SIRT6 Protein in Aging and Reprogramming of Human Induced Pluripotent Stem Cells. J. Biol. Chem. 2013;288:18439–18447. doi: 10.1074/jbc.M112.405928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares S, Thompson M, White T, Isbell A, Yamasaki M, Prakash Y, Lund FE, Galione A, Chini EN. NAADP as a second messenger: neither CD38 nor baseexchange reaction are necessary for in vivo generation of NAADP in myometrial cells. Am. J. Physiol. Cell Physiol. 2007;292:C277–C239. doi: 10.1152/ajpcell.00638.2005. [DOI] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 2010;143:802–812. doi: 10.1016/j.cell.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- States DJ, Walseth TF, Lee HC. Similarities in amino acid sequences of Aplysia ADP-ribosyl cyclase and human lymphocyte antigen CD38. Trends Biochem. Sci. 1992;17:495. doi: 10.1016/0968-0004(92)90337-9. [DOI] [PubMed] [Google Scholar]
- Stein LR, Imai S. Specific ablation of Nampt in adult neural stem cells recapitulates their functional defects during aging. EMBO J. 2014;33:1321–1340. doi: 10.1002/embj.201386917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Iqbal J, Zaidi S, Zhu LL, Zhang X, Peng Y, Moonga BS, Zaidi M. Structure and functional regulation of the CD38 promoter. Biochem. Biophys. Res. Commun. 2006;341:804–809. doi: 10.1016/j.bbrc.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Terakata M, Fukuwatari T, Kadota E, Sano M, Kanai M, Nakamura T. The niacin required for optimum growth can be synthesized from l-tryptophan in growing mice lacking tryptophan-2,3-dioxygenase. J. Nutr. 2013;143:1046–1051. doi: 10.3945/jn.113.176875. [DOI] [PubMed] [Google Scholar]
- Trammell SAJ, Weidemann BJ, Chadda A, Yorek MS, Holmes A, Coppey LJ, Obrosov A, Kardon RH, Yorek MA, Brenner C. Nicotinamide Riboside Opposes Type 2 Diabetes and Neuropathy in Mice. Sci. Rep. 2016;6:26933. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z, Labinskyy N, Gupte S, Chander PN, Edwards JG, Csiszar A. Dysregulation of mitochondrial biogenesis in vascular endothelial and smooth muscle cells of aged rats. Am. J. Physiol. Heart Circ. Physiol. 2008;294:H2121–2128. doi: 10.1152/ajpheart.00012.2008. [DOI] [PubMed] [Google Scholar]
- van der Veer E, Ho C, O'Neil C, Barbosa N, Scott R, Cregan SP, Pickering JG. Extension of human cell lifespan by nicotinamide phosphoribosyltransferase. J. Biol. Chem. 2007;282:10841–845. doi: 10.1074/jbc.C700018200. [DOI] [PubMed] [Google Scholar]
- Veith S, Mangerich A. RecQ helicases and PARP1 team up in maintaining genome integrity. Ageing Res. Rev. 2015;23:12–28. doi: 10.1016/j.arr.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]; Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- Warburg O, Christian W. Pyridin, the hydrogen-transferring component of the fermentation enzymes (pyridine nucleotide) Biochemische Zeitschrift. 1936;287:291. [Google Scholar]
- Wei W, Graeff R, Yue J. Roles and mechanisms of the CD38/cyclic adenosine diphosphate ribose/Ca2+ signaling pathway. World J. Biol. Chem. 2014;5:58–67. doi: 10.4331/wjbc.v5.i1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C, Campisi J. NAD+ controls neural stem cell fate in the aging brain. EMBO J. 2014;33:1289–1291. doi: 10.15252/embj.201488969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto-Katayama S, Ariyoshi M, Ishihara K, Hirano T, Jingami H, Morikawa K. Crystallographic studies on human BST-1/CD157 with ADP-ribosyl cyclase and NAD glycohydrolase activities. J. Mol Biol. 2002;316:711–23. doi: 10.1006/jmbi.2001.5386. [DOI] [PubMed] [Google Scholar]
- Yang H, Yang T, Baur JA, terzic E, Matsui T, Carmona JJ, Lamming DW, Souza-Pinto NC, Bohr VA, Rosenzweig A, et al. Nutrient-Sensitive Mitochondrial NAD+ Levels Dictate Cell Survival. Cell. 2007;130:1095–1107. doi: 10.1016/j.cell.2007.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang NC, Song TY, Chen HY, Hu ML. Effects of 2-deoxyglucose and dehydroepiandrosterone on intracellular NAD+ level, SIRT1 activity and replicative lifespan of human Hs68 cells. Biogerontology. 2011;12:527–536. doi: 10.1007/s10522-011-9342-7. [DOI] [PubMed] [Google Scholar]
- Yang Y, Sauve AA. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta. 2016 doi: 10.1016/j.bbapap.2016.06.014. doi.org/10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino J, Mills KF, Yoon MJ, Imai S. Nicotinamide mononucleotide, a key NAD+ intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young GS, Choleris E, Lund FE, Kirkland JB. Decreased cADPR and increased NAD+ in the Cd38−/− mouse. Biochem Biophys. Res. Commun. 2006;346:188–192. doi: 10.1016/j.bbrc.2006.05.100. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Cruzat VF, Newsholme P, Cheng J, Chen Y, Lu Y. Regulation of SIRT1 in aging: Roles in mitochondrial function and biogenesis. Mech. Ageing Dev. 2016;155:10–21. doi: 10.1016/j.mad.2016.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ryu D, Wu Y, Gariani K, Wang X, Luan P, D'Amico D, Ropelle ER, Lutolf MP, Aebersold R, et al. NAD? repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–43. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xiong Z-M, Cao K. Mechanisms controlling the smooth muscle cell death in progeria via down-regulation of poly(ADP-ribose) polymerase 1. Proc. Natl. Acad. Sci. 2014;111:E2261–E2270. doi: 10.1073/pnas.1320843111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Allison D, Condon B, Zhang F, Gheyi T, Zhang A, Ashok S, Russell M, MacEwan I, Qian Y, Jamison JA, Luz JG. The 2.5Å crystal structure of the SIRT1 catalytic domain bound to nicotinamide adenine dinucleotide (NAD+) and an indole (EX527 analogue) reveals a novel mechanism of histone deacetylase inhibition. J. Med. Chem. 2013;56:963–969. doi: 10.1021/jm301431y. [DOI] [PubMed] [Google Scholar]; Zhu XH, Lu M, Lee BY, Ugurbil K, Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M, Niere M. NAD+ surfaces again. Biochem. J. 2004;382:5–6. doi: 10.1042/BJ20041217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielinska W, Barata H, Chini EN. Metabolism of cyclic ADPribose: Zinc is an endogenous modulator of the cyclase/NAD glycohydrolase ratio of a CD38-like enzyme from human seminal fluid. Life Sci. 2004;74:1781–1790. doi: 10.1016/j.lfs.2003.08.033. [DOI] [PubMed] [Google Scholar]