Abstract
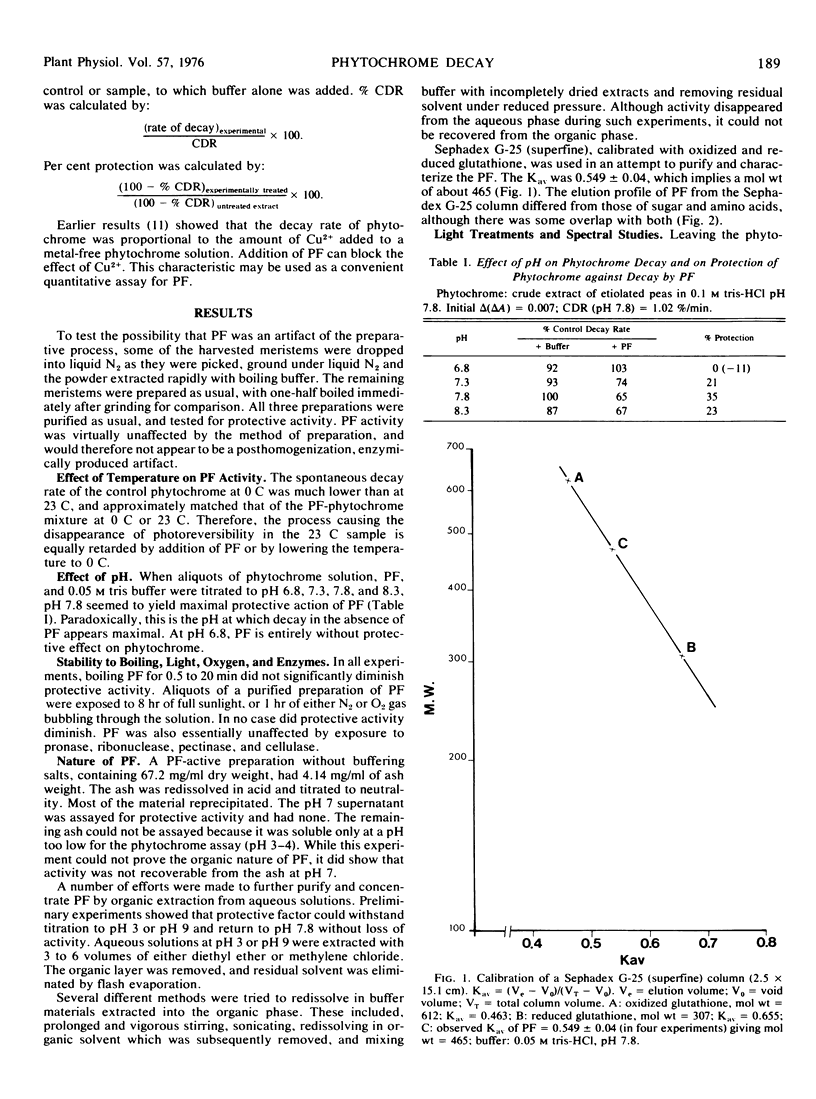
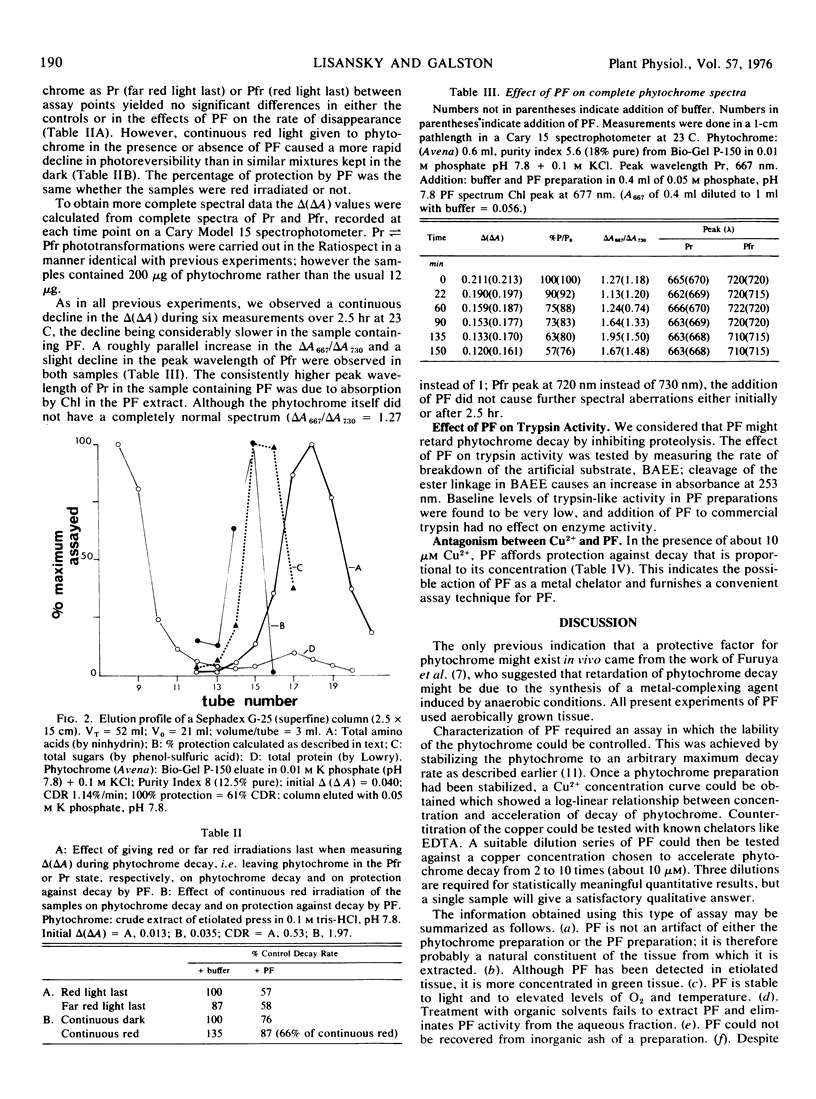
The decay in vitro of crude and purified phytochrome preparations, measured as decline in spectrophotometrically detectable photoreversibility, can be retarded by the addition of an as yet unidentified “protective factor” from the green tissue of peas. The factor has an apparent molecular weight of 465, is stable at elevated temperatures and to oxygen and high light intensity. It confers optimal protection at pH about 7.8 and is not greatly affected by treatment with hydrolytic enzymes. Protective factor also protects phytochrome against accelerated decay resulting from the addition of Cu2+; it may therefore function as a metal chelator.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs W. R., Siegelman H. W. Distribution of Phytochrome in Etiolated Seedlings. Plant Physiol. 1965 Sep;40(5):934–941. doi: 10.1104/pp.40.5.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox L. R. Loss of phytochrome photoreversibility in vitro: I. Extraction and partial purification of killer. Plant Physiol. 1975 Feb;55(2):386–389. doi: 10.1104/pp.55.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hillman W. S. Rapid Destruction of the P(FS) Form of Phytochrome by a Substance in Extracts of Pisum Tissue. Plant Physiol. 1966 Sep;41(7):1242–1244. doi: 10.1104/pp.41.7.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya M., Hopkins W. G., Hillman W. S. Effects of metal-complexing and sulfhydryl compounds on nonphotochemical phytochrome changes in vivo. Arch Biochem Biophys. 1965 Oct;112(1):180–186. doi: 10.1016/0003-9861(65)90026-3. [DOI] [PubMed] [Google Scholar]
- Kidd G. H., Pratt L. H. Phytochrome destruction: an apparent requirement for protein synthesis in the induction of the destruction mechanism. Plant Physiol. 1973 Oct;52(4):309–311. doi: 10.1104/pp.52.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lisansky S. G., Galston A. W. Phytochrome stability in vitro: I. Effect of metal ions. Plant Physiol. 1974 Mar;53(3):352–359. doi: 10.1104/pp.53.3.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. H., Kidd G. H., Coleman R. A. An immunochemical characterization of the phytochrome destruction reaction. Biochim Biophys Acta. 1974 Sep 13;365(1):93–107. doi: 10.1016/0005-2795(74)90253-0. [DOI] [PubMed] [Google Scholar]
- Wetherell D. F. Phytochrome in cultured wild carrot tissue. I. Synthesis. Plant Physiol. 1969 Dec;44(12):1734–1737. doi: 10.1104/pp.44.12.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]


