Abstract
Background
To evaluate the impact of depression prior to autologous and allogeneic HCT on clinical outcomes post-transplant.
Methods
We analyzed data from the Center for International Blood and Marrow Transplant Research to compare outcomes after autologous (n=3786) or allogeneic (n=7433) HCT for adult patients with hematologic malignancies with an existing diagnosis of pre-HCT depression requiring treatment vs. those without pre-HCT depression. Using Cox regression models, we compared OS between patients with or without depression. We compared the number of days-alive-and-out-of-the-hospital in the first 100 days post-HCT using Poisson models. We also compared the incidence of grade II-IV acute and chronic GVHD in allogeneic HCT.
Results
1116 (15%) patients with pre-transplant depression and 6317 (85%) without depression underwent allogeneic HCT in 2008-2012 were included. Pre-transplant depression was associated with lower OS (HR=1.13, 95%CI1.04-1.23, P=0.004) and higher incidence of grade II-IV acute GVHD (HR=1.25, 95%CI 1.14-1.37, P<0.0001), but similar incidence of chronic GVHD. Pre-transplant depression was associated with fewer days alive and out-of-the hospital (Means-Ratio (MR)=0.97, 95%CI0.95-0.99, P=0.004). There were 512 (13.5%) patients with pre-transplant depression and 3274 (86.5%) without depression who underwent autologous HCT. Pre-transplant depression in autologous HCT was not associated with OS (HR=1.15, 95%CI0.98-1.34, P=0.096), but was associated with fewer days-alive-and-out-of-the-hospital (MR=0.98, 95%CI0.97-0.99, P=0.002).
Conclusions
Pre-transplant depression was associated with lower OS and higher risk of acute GVHD among allogeneic HCT recipients, and fewer days-alive-and-out-of-the-hospital during the first 100 days after autologous and allogeneic HCT. Patients with pre-transplant depression represent a vulnerable population at risk for post-transplant complications.
Keywords: Depression, autologous HCT, Transplant Outcomes, pre-HCT depression, GVHD
Introduction
Depression is associated with increased mortality among otherwise healthy individuals and patients with various medical conditions including cardiovascular disease, cancer, and solid organ transplantation.1-4 Hematopoietic cell transplantation (HCT) represents a physically and emotionally challenging therapy, often with a prolonged period necessary for recovery.5, 6 The psychological burden associated with HCT is well-documented with increasing rates of depression and anxiety seen up to 5 years post-HCT.7-9 However, the relationship between a diagnosis of depression prior to HCT and clinical outcomes of patients after autologous and allogeneic HCT is not fully understood.
While a diagnosis of post-transplant depression has been linked to increased mortality,10 studies exploring the impact of an existing diagnosis of depression prior to HCT on overall survival (OS) in this population have yielded conflicting results.10-15 The inconsistent nature of these findings is partly due to limitations of prior work including mostly single-institution studies with small sample sizes, the inclusion of autologous and allogeneic HCT recipients collectively, inconsistent case ascertainment and timing of such ascertainment, and failure to account for significant confounders.11-13, 16 Therefore, larger multi-center studies with robust sample sizes that enable us to examine the association between pre-HCT depression and OS of autologous and allogeneic HCT are necessary to answer this question.
Beyond mortality, the impact of a pre-HCT depression on other outcomes including hospital-length-of-stay, and incidence of acute and chronic graft-versus-host disease (GVHD) is currently unknown. Hospital length-of-stay during the first 100 days post-HCT is an important marker of health care utilization and has clinical implications for both patients and families.17 Acute and chronic GVHD are major post-transplant complications in patients undergoing allogeneic HCT with resulting functional limitations, morbidity, and mortality.12, 13,9, 18, 19 Importantly, GVHD can often be prevented and treated with the administration and adherence to immunosuppressive therapy to ensure adequate disease control.12, 13, 20-22 Given the association between depression and low medical adherence,23 it is plausible that pre-HCT depression may impact hospital length-of-stay during the first 100 days post-HCT and the incidence of GVHD.
Defining the effects of pre-HCT depression on post-transplant outcomes including OS, hospital length-of-stay, and incidence of acute and chronic GVHD among allogeneic HCT recipients has important implications regarding the diagnosis, management, and psychological support provided for depressed patients undergoing such intensive therapy.
In this study, we utilized data from the Center for International Blood and Marrow Transplant Research (CIMBTR) to examine the relationship between pre-HCT depression and OS in a large cohort of patients with hematologic malignancies undergoing autologous and allogeneic HCT. We also analyzed the effect of pre-HCT depression on hospital length-of-stay during the first 100 days post-HCT. Finally, we assessed whether pre-HCT depression is associated with the incidence of acute and chronic GVHD in patients undergoing allogeneic HCT. We hypothesized that pre-HCT depression would be associated with lower OS and higher hospital length-of-stay during the first 100 days in both the autologous and allogeneic HCT settings. Moreover, we hypothesized that pre-HCT depression is associated with higher rates of acute and chronic GVHD.
Methods
Participants
We analyzed data from the CIBMTR registry to compare outcomes of patients with hematologic malignancies undergoing autologous and allogeneic HCT who have a diagnosis of pre-HCT depression requiring treatment and those without pre-HCT depression. The CIBMTR® is a research collaboration between the National Marrow Donor Program®/Be The Match® and the Medical College of Wisconsin. It comprises a voluntary working group of more than 450 transplant centers worldwide that contribute detailed data on allogeneic and autologous HCT. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits and patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. The CIBMTR collects an internationally accepted standard data-set that contains a number of key variables for all consecutive transplant recipients. Data are collected pre-transplant, 100 days and six months post-transplant, annually until year 6 post-transplant and biannually thereafter until death.
We examined outcomes of patients undergoing autologous and allogeneic HCT separately. Inclusion criteria for the allogeneic HCT cohort were adult (≥ 18) patients with hematologic malignancies undergoing their first allogeneic HCT between 2008-2012. For the autologous HCT cohort, we included adult (≥ 18) patients with non-Hodgkin’s lymphoma, Hodgkin’s disease, or multiple myeloma undergoing their first autologous HCT between 2007-2012. For participants with multiple myeloma, we only included those receiving melphalan conditioning given that it was used in > 97% of such cases. For both autologous and allogeneic HCT, we excluded patients with a prior history of HCT.
A diagnosis of pre-HCT depression
We determined the presence of pre-transplant depression based on a question reported in the CIBMTR pre-HCT form specifically asking whether there is “clinically significant depression requiring treatment (yes vs. no).” The answer to this question was obtained based on a review of the medical record at each transplant center. We performed an audit to ensure the quality of the data obtained from this question at transplant centers, which showed 85% accuracy. To further ensure high-quality data were used, we excluded transplant centers with case volume > 10 transplants who did not report both patients with and without pre-HCT depression.
Definition of Outcomes
The primary objective of this study was to compare OS between patients with or without pre-HCT depression undergoing allogeneic and autologous HCT. Secondary outcomes include length of stay during the first 100 days, and the incidence of acute and chronic GVHD among patients receiving allogeneic HCT.
OS was defined as the time from the date of HCT to date of death, with survivors censored at the time of last contact. We evaluated the incidence of grades II-IV acute GVHD according to the Glucksberg grading criteria,24 and chronic GVHD according to the NIH chronic GVHD consensus criteria25 as reported to the CIBMTR. We censored patients at date of subsequent HCT or last follow-up. Length-of-stay is captured as total days of hospitalization (initial admission and any readmission) between day 0 (day of HCT) and day +100 post-HCT. Patients who die early after HCT have less time at risk for hospitalization and shorter length-of-stay than those who survive to day +100. To account for this association of early mortality with shorter hospitalization, we used the number of days alive and out of the hospital as the metric for comparing length-of-stay in the first 100 days. For patients who died within 100 days post-HCT, we evaluated the number of days of survival out of the hospital. For patients who survived through day 100, we censored follow-up at that time point. We then evaluated the proportion of days alive and out of the hospital. The CIBMTR has utilized this approach to examine hospital length-of-stay during first 100 days after allogeneic HCT in prior studies.17
Statistical analysis
We analyzed data of patients undergoing autologous and allogeneic HCT separately. We compared clinical characteristics for patients with and without pre-HCT depression using chi-square test for categorical variables and the Wilcoxon rank sum test for continuous variables. We estimated the probability of OS using the Kaplan-Meier method with log-rank test for univariate comparisons. We used a cumulative incidence function to account for competing risks when estimating the incidence of grades II-IV acute GVHD and chronic GVHD. Death without GVHD was treated as a competing risk.
We used Cox proportional hazards regression models to compare OS between patients with or without pre-HCT depression while controlling for significant patient-, disease-, and treatment-related variables. We forced a main effect of pre-HCT depression into the model. The remaining covariates were included using a backward selection technique with a value of P ≤ 0.05 as the criterion for inclusion in the final models.
In the multivariate OS allogeneic HCT analysis, the variables that were considered in the model included: recipient age, gender, region of HCT center, performance status, race, education, income, marital status, work status, smoking history, diagnosis, disease risk index,26 time from diagnosis to HCT, conditioning regimen intensity, receipt of TBI, year of HCT, graft type, donor source, donor age, degree of HLA match, donor-recipient sex match, recipient CMV status and GVHD prophylaxis regimen. We also examined whether comorbidities (obesity, cardiovascular disease, pulmonary disease, combination of comorbidities) may moderate outcomes between patients with or without pre-HCT depression. We built the models with or without comorbid conditions and we examined the interaction between pre-HCT depression and the comorbidities of interest, but found no significant interactions.
In the multivariate OS autologous HCT analysis, the variables that were considered in the model included: recipient age, gender, region of HCT, performance status, race, education, income, marital status, work status, smoking history, diagnosis, disease status at the time of HCT, time from diagnosis to HCT, conditioning regimen, and year of HCT. We also built the models with or without comorbidities and explored the interaction of pre-HCT and comorbidities of interest.
In a similar fashion, we used Cox proportional hazards regression models to examine the effect of pre-HCT depression on acute and chronic GVHD among allogeneic HCT recipients. Model building proceeded as described previously for OS analysis. We again built the models with or without comorbid conditions and examined the interaction between pre-HCT depression and the comorbidities of interest, but found no significant interaction.
We used multivariate analysis using Poisson regression to compare mean time (in days) that patients were alive and out of the hospital within the first 100 days between patients with or without pre-HCT depression while controlling for significant patient-, disease-, and treatment-related variables. Total number of days alive (up to 100) was used as an offset term in Poisson regression to account for differential exposure time of the patients. The same variables that were described in OS analysis were considered in this model. We constructed two separate models for autologous and allogeneic HCT, respectively. Results are summarized as means ratios for comparing groups; a means ratio > 1 indicates more days alive and out of the hospital. We performed all analyses using the SAS version 9.3. All p-values are 2-sided with a value of 0.05 considered statistically significant.
Results
Allogeneic HCT Participants
Among 7433 allogeneic HCT patients, 1116 (15%) had a history of depression requiring treatment [Table 1]. Patients with pre-transplant depression were more likely to be female, and had a lower performance status compared to those without pre-HCT depression. Patients with pre-HCT depression had a higher prevalence of obesity, smoking, cardiovascular, and pulmonary disease. No differences between the two groups were noted in terms of diagnosis, disease risk, time from diagnosis to HCT, year of HCT, graft type, or donor source.
Table 1.
Characteristics of allogeneic HCT recipients
| Variables | No depression (n=6317) | History of Depression (n=1116) | P-value |
|---|---|---|---|
| Age at transplant, median-(range) | 52(18-80) | 52(18-78) | 0.97 |
|
| |||
| Age at transplant-years | 0.01 | ||
| 18-29 | 821(13) | 121(11) | |
| 30-39 | 772(12) | 128(11) | |
| 40-49 | 1202(19) | 235(21) | |
| 50-59 | 1933(31) | 381(34) | |
| ≥60 | 1589(25) | 251(22) | |
|
| |||
| Male gender | 3761(60) | 486(44) | <0.001 |
|
| |||
| Karnofsky score-(<90) | 2106(33) | 468(42) | <0.001 |
|
| |||
| Region | 0.004 | ||
| West | 1395(22) | 240(22) | |
| Midwest | 1898(30) | 344(31) | |
| South | 1827(29) | 365(33) | |
| Northeast | 1197(19) | 167(15) | |
|
| |||
| Race | <0.001 | ||
| White | 5492(87) | 1045(94) | |
| African-American | 432(7) | 42(4) | |
| Asian/Pacific | 230(4) | 13(1) | |
| Native American | 26(<1) | 1(<1) | |
| Missing | 137(2) | 15(1) | |
|
| |||
| Education | <0.001 | ||
| High school/or lower | 1816(29) | 386(35) | |
| College | 854(14) | 160(14) | |
| Graduate School | 1755(28) | 295(26) | |
| Missing | 1892(30) | 275(25) | |
|
| |||
| Marital status | <0.001 | ||
| Single | 1070(17) | 167(15) | |
| Married | 4378(69) | 745(67) | |
| Separated | 78(1) | 21(2) | |
| Divorced | 480(8) | 129(12) | |
| Widowed | 113(2) | 24(2) | |
| Missing | 198(3) | 30(3) | |
|
| |||
| Work status | <0.001 | ||
| Full time | 1932(31) | 268(24) | |
| Part time | 331(5) | 62(6) | |
| Unemployed | 889(14) | 203(18) | |
| Medical disability | 1388(22) | 320(29) | |
| Retired | 954(15) | 156(14) | |
| Missing | 823(13) | 107(10) | |
|
| |||
| Obesity | 0.02 | ||
| BMI ≤30 | 4322(68) | 720(65) | |
| BMI >30 | 1990(32) | 394(35) | |
| Missing | 5(<1) | 2(<1) | |
|
| |||
| Smoking-(Yes) | 2565(41) | 561(50) | <0.001 |
|
| |||
| Cardiovascular disease-(yes) | 1883(30) | 453(41) | <0.001 |
|
| |||
| Pulmonary disease-(Yes) | 1069(17) | 281(25) | <0.001 |
|
| |||
| Disease | 0.25 | ||
| AML | 2724(43) | 513(46) | |
| ALL | 692(11) | 107(10) | |
| CML/MPN | 597(9) | 102(9) | |
| MDS | 1151(18) | 211(19) | |
| NHL | 637(10) | 95(9) | |
| HL | 92(1) | 19(2) | |
| Multiple myeloma | 100(2) | 22(2) | |
| Other | 324(5) | 47(4) | |
|
| |||
| Disease risk index | 0.54 | ||
| Low | 739(12) | 145(13) | |
| Intermediate | 3332(53) | 583(52) | |
| High | 1629(26) | 276(25) | |
| Very high | 257(4) | 53(5) | |
| Missing | 360(6) | 59(5) | |
|
| |||
| Time from diagnosis to HCT, median in-months-(range) | 8(<1-203) | 9(<1-188) | 0.31 |
|
| |||
| Conditioning intensity | 0.14 | ||
| MAC-TBI | 1921(30) | 304(27) | |
| MAC-chemo | 2120(34) | 392(35) | |
| RIC/NST-TBI | 919(15) | 177(16) | |
| RIC/NST-chemo | 1347(21) | 243(22) | |
| Missing | 10(<1) | 0 | |
|
| |||
| TBI-(yes) | 2793(44) | 475(43) | 0.54 |
|
| |||
| HCT Year | 0.11 | ||
| 2008 | 1833(29) | 294(26) | |
| 2009 | 1711(27) | 296(27) | |
| 2010 | 1125(18) | 217(19) | |
| 2011 | 878(14) | 149(13) | |
| 2012 | 770(12) | 160(14) | |
|
| |||
| Graft type | 0.42 | ||
| Bone marrow | 712(11) | 123(11) | |
| Peripheral blood | 4502(71) | 815(73) | |
| Cord blood | 1103(17) | 178(16) | |
|
| |||
| Donor source | 0.17 | ||
| Cord blood | 1103(17) | 178(16) | |
| HLA identical sibling | 1828(29) | 325(29) | |
| Well-matched unrelated | 2011(32) | 383(34) | |
| Other unrelated | 1039(16) | 182(16) | |
| Other relatives | 302(5) | 39(3) | |
| Missing | 34(<1) | 9(<1) | |
|
| |||
| Unrelated donor age, median-(range) | 31(18-61) | 29(19-59) | 0.01 |
|
| |||
| Donor sex match | <0.001 | ||
| Male into male | 2299(36) | 310(28) | |
| Male into female | 1436(23) | 353(32) | |
| Female into male | 1368(22) | 162(15) | |
| Female into female | 1067(17) | 264(24) | |
| Missing | 142(2) | 27(2) | |
|
| |||
| Recipient CMV-positive | 3825(61) | 683(61) | 0.13 |
|
| |||
| GVHD prophylaxis | 0.08 | ||
| T cell depletion+/-other | 153(2) | 16(1) | |
| FK506+/-other | 4735(75) | 861(77) | |
| Cyclosporine+/-other | 1139(18) | 198(18) | |
| Other | 171(3) | 29(3) | |
| Missing | 119(2) | 12(1) | |
Autologous HCT Participants
Among 3786 patients, 512 (13.5%) had pre-HCT depression requiring treatment [Table 2]. There were no differences between the two groups in terms of age, region of HCT, diagnosis, disease status at the time of HCT, time from diagnosis to HCT, conditioning regimen, or year of HCT. Patients with pre-HCT depression (vs. without depression) were more likely to be female and had a lower performance status. They were also more likely to have comorbidities including smoking, obesity, cardiovascular, and pulmonary disease.
Table 2.
Characteristics of autologous HCT recipients
| Variables | No depression (n=3274) | History of Depression (n= 12) | P-value |
|---|---|---|---|
| Age at transplant, median-(range) | 58(18-81) | 57(19-80) | 0.03 |
|
| |||
| Age at transplant-years | 0.35 | ||
| 18-29 | 155(5) | 23(4) | |
| 30-39 | 177(5) | 34(7) | |
| 40-49 | 478(15) | 83(16) | |
| 50-59 | 1056(32) | 174(34) | |
| ≥60 | 1408(43) | 198(39) | |
|
| |||
| Male gender | 1998(61) | 247(48) | <0.001 |
|
| |||
| Karnofsky score-(<90%) | 1179(36) | 234(46) | <0.001 |
|
| |||
| Region | 0.10 | ||
| West | 415(13) | 81(16) | |
| Midwest | 1122(34) | 183(36) | |
| South | 1312(40) | 194(38) | |
| Northeast | 425(13) | 54(11) | |
|
| |||
| Race | <0.001 | ||
| White | 2648(81) | 458(89) | |
| African-American | 490(15) | 42(8) | |
| Asian/Pacific | 60(2) | 2(<1) | |
| Native American | 16(<1) | 1(<1) | |
| Other | 4(<1) | 2(<1) | |
| Missing | 56(2) | 7(1) | |
|
| |||
| Education | 0.009 | ||
| High school/or lower | 992(30) | 180(35) | |
| College | 571(17) | 96(19) | |
| Graduate School | 839(26) | 134(26) | |
| Missing | 872(27) | 102(20) | |
|
| |||
| Marital status | 0.02 | ||
| Single | 379(12) | 55(11) | |
| Married | 2347(72) | 350(68) | |
| Separated | 36(1) | 6(1) | |
| Divorced | 318(10) | 72(14) | |
| Widowed | 117(4) | 23(4) | |
| Missing | 77(2) | 6(1) | |
|
| |||
| Work status | <0.001 | ||
| Full time | 973(3) | 115(22) | |
| Part time | 150(5) | 26(5) | |
| Unemployed | 267(8) | 57(11) | |
| Medical disability | 498(15) | 115(22) | |
| Retired | 920(28) | 147(29) | |
| Missing | 465(14) | 52(10) | |
|
| |||
| Obesity | 0.02 | ||
| BMI ≤30 | 2022(62) | 285(56) | |
| BMI >30 | 1247(38) | 227(44) | |
| Missing | 5(<1) | 0 | |
|
| |||
| Smoking-(Yes) | 1410(43) | 251(49) | 0.004 |
|
| |||
| Cardiovascular disease-(Yes) | 1322(40) | 274(54) | <0.001 |
|
| |||
| Pulmonary disease-(Yes) | 606(19) | 154(30) | <0.001 |
|
| |||
| Disease | 0.86 | ||
| NHL | 1070(33) | 161(31) | |
| HL | 329(10) | 52(10) | |
| Multiple myeloma | 1875(57) | 299(58) | |
|
| |||
| Disease status | 0.23 | ||
| Early | 559(17) | 78(15) | |
| Intermediate | 2483(76) | 389(76) | |
| Advanced | 225(7) | 42(8) | |
|
| |||
| Time from diagnosis to HCT, median in months-(range) | 10(2-203) | 11(3-189) | 0.40 |
|
| |||
| Conditioning intensity | 0.54 | ||
| BEAM/BEAM-like | 807(25) | 127(25) | |
| Bu +Cy+/-other | 203(6) | 34(7) | |
| Bu+/-Other | 56(2) | 7(1) | |
| TBI+/-Other | 154(5) | 29(6) | |
| CBV/CBV-like | 120(4) | 10(2) | |
| Other | 58(2) | 6(1) | |
| Melphalan | 1875(57) | 299(58) | |
|
| |||
| HCT Year | 0.07 | ||
| 2007 | 608(19) | 103 (20) | |
| 2008 | 1304(40) | 191 (37) | |
| 2009 | 513(16) | 69 (13) | |
| 2010 | 289(9) | 45 (9) | |
| 2011 | 325(10) | 49 (10) | |
| 2012 | 235(7) | 55 (11) | |
Allogeneic HCT Outcomes
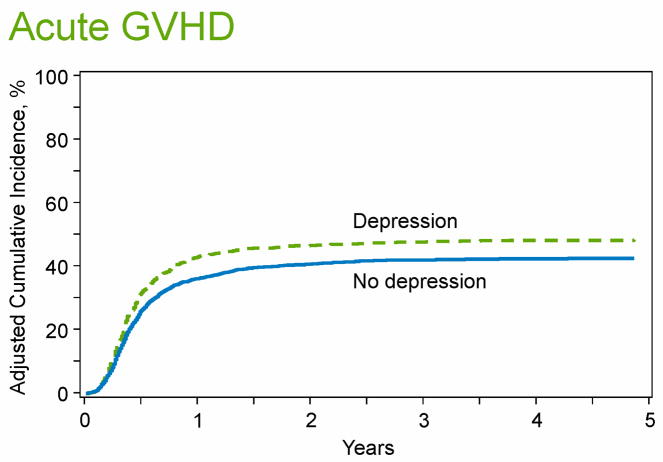
Table 3 summarizes the unadjusted analyses comparing allogeneic HCT outcomes between those with and without pre-HCT depression. Participants with pre-HCT depression had lower OS compared to those without pre-HCT depression [Figure 1A]. The three-year OS was 40% (95%CI 37-43%) for those with pre-HCT depression vs. 45% (95%CI 44-47%) for patients without depression (P=0.002). Patients with pre-HCT depression also had a higher 100-day incidence of grade II-IV acute GVHD (46% (95%CI 43-49) vs. 39% (95%CI 38-40), P<0.001) [Figure 1B], but no difference in chronic GVHD at 3 years (P=0.80).
Table 3.
Unadjusted Analyses of pre-transplant depression and clinical outcomes of autologous and allogeneic HCT recipients
| Outcome | No Depression | History of depression | P-value | ||
|---|---|---|---|---|---|
| N | (95% CI) | N | (95% CI) | ||
| Allogenic HCT | |||||
|
| |||||
| Overall-survival | 6317 | 1116 | *<0.001 | ||
| 1-year | 60 (59-62) | 57 (54-60) | 0.03 | ||
| 3-year | 45 (44-47) | 40 (37-43) | 0.002 | ||
|
| |||||
| II-IV acute GVHD | 6299 | 1115 | <0.001 | ||
| 100-day | 39 (38-40) | 46 (43-49) | |||
|
| |||||
| III-IV acute GVHD | 6306 | 1115 | 0.19 | ||
| 100-day | 17 (16-18) | 19 (17-21) | |||
|
| |||||
| Chronic GVHD | 6200 | 1109 | *0.57 | ||
| 1-year | 43 (42-44) | 43 (40-46) | 0.93 | ||
| 3-year | 49 (48-51) | 50 (47-53) | 0.80 | ||
|
| |||||
| Moderate-severe chronic GVHD | 6234 | 1112 | *0.05 | ||
| 1-year | 23 (22-24) | 25 (23-28) | 0.13 | ||
| 3-year | 26 (25-27) | 28 (26-31) | 0.07 | ||
|
| |||||
| Autologous HCT | N | (95% CI) | N | (95% CI) | P-value |
|
| |||||
| Overall-survival | 3274 | 512 | *0.04 | ||
| 1-year | 89 (87-90) | 87 (84-90) | 0.30 | ||
| 3-year | 73 (72-75) | 67 (63-72) | 0.01 | ||
| 4-year | 67 (65-69) | 62 (57-66) | 0.04 | ||
Overall p-value produced by log-rank or Gray’s test.
Figure 1. Outcomes of allogeneic HCT recipients with or without history of pre-transplant depression.
1A: Overall Survival; 1B: Cumulative incidence of acute graft-versus-host disease.
In multivariable models, pre-HCT depression was associated with lower OS [RR=1.13, 95%CI 1.04-1.23, P=0.003), higher incidence of grades II-IV acute GVHD [RR=1.24, 95%CI 1.14-1.37, P<0.0001], and fewer days alive and out of the hospital during the first 100 days [MR=0.97, 95%CI 0.95-0.99, P=0.004]. Pre-transplant depression was not associated with chronic GVHD in multivariate analyses [Table 4].
Table 4.
Multivariable analyses of pre-transplant depression and clinical outcomes of autologous and allogeneic HCT recipients
| Allogenic HCT | N | HR | 95% CI | P-value |
|---|---|---|---|---|
|
| ||||
| Overall-survival | ||||
| History of depression | 1095 | 1.13 | 1.04-1.23 | 0.003 |
|
| ||||
| II-IV acute GVHD | ||||
| History of depression | 1115 | 1.25 | 1.14-1.37 | <0.0001 |
|
| ||||
| Chronic GVHD | ||||
| History of Depression | 1109 | 1.06 | 0.96-1.16 | 0.26 |
|
| ||||
| Length of stay | ||||
| History of depression | 1093 | 0.97 | 0.95-0.99 | 0.002 |
|
| ||||
| Autologous HCT | N | HR | 95% CI | P-value |
|
| ||||
| Overall-survival | ||||
| History of depression | 512 | 1.15 | 0.98-1.34 | 0.09 |
|
| ||||
| Length-of-stay | ||||
| History of depression | 444 | 0.98 | 0.97-0.99 | 0.002 |
Similar results were obtained when controlling for comorbidities in the multivariate analyses. There were no significant interactions between comorbidities (obesity, cardiovascular, or pulmonary disease) and pre-HCT depression.
Autologous HCT Outcomes
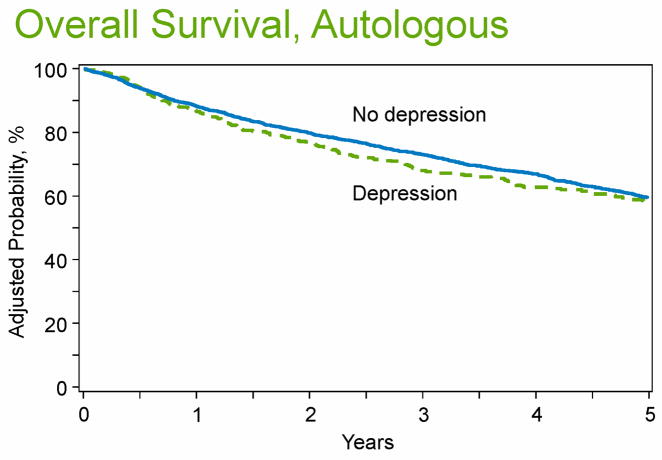
In the unadjusted analyses, pre-HCT depression was associated with lower OS among autologous HCT recipients. The 3-year OS between patients with pre-HCT depression vs. those without pre-HCT depression was 67% (95%CI 63-72%) vs. 73% (95%CI 72-75%) (P=0.01) [Table 3, Figure 2]. However, after adjusting for significant variables, pre-HCT depression was not associated with OS in multivariate analyses [RR=1.15, 95%CI 0.98-1.34, P=0.09] [Table 4]. Pre-HCT depression was associated with fewer days alive and out of the hospital during the first 100 days [MR=0.98, 95%CI 0.97-0.99, P=0.002].
Figure 2. Overall survival of autologous HCT recipients with or without history of pre-transplant depression.
Discussion
Among patients undergoing allogeneic HCT, a diagnosis of pre-transplant depression was associated with lower OS, higher risk of acute GVHD, and lower proportion of days spent alive and out-of-the hospital during the first 100 days post-HCT. Among autologous HCT recipients, pre-transplant depression was not associated with OS, but was associated with lower proportion of days spent alive and out-of-the hospital. Therefore, patients with a diagnosis of depression prior to HCT represent a highly vulnerable population at risk for post-HCT complications, and they may benefit from more intensive interventions to improve their post-HCT outcomes.
This is the largest, multi-center study of allogeneic HCT recipients that confirms an association between pre-transplant depression and lower OS. The difference in OS between patients with pre-HCT depression and those without depression is clinically relevant (5% absolute difference) and it persists despite controlling for many potential confounders and comorbidities. Interestingly, we did not detect a difference in OS among autologous HCT recipients with or without pre-HCT depression. This may partly explain the discrepant findings reported in the literature as outcomes of patients undergoing autologous and allogeneic HCT are often analyzed collectively.10, 14-16, 27
To our knowledge, we are the first to report an association between pre-HCT depression and higher incidence of acute GVHD in patients undergoing allogeneic HCT. It is unclear how pre-HCT depression could affect the incidence of acute GVHD. Possible explanations include differences in health behaviors such as adherence and medical follow-up among depressed vs. non-depressed individuals,23 and/or psychobiological processes resulting in systematic shift in immune activation favoring the development of acute GVHD.28-30 Prior research has shown an association between depression and elevation in inflammatory cytokines such as TNF-alpha, and a shift towards a T-helper-1 immune response,31 which in turn is thought to play a role in the pathophysiology of acute GVHD.32 Future research should focus on understanding the mechanism of association between pre-HCT depression and the increased risk of acute GVHD.
We also report higher health care utilization, as measured by days spent alive and out of the hospital, among patients with pre-HCT depression compared to those without depression in both the allogeneic and autologous HCT settings. In one prior single-center study, a diagnosis of any mood, anxiety, or adjustment disorder was associated with longer hospital length-of-stay during HCT admission.6 Our findings illustrate that differences in resource utilization among depressed and non-depressed individuals persist up to 100 days post-HCT. Possible explanations include the tendency for depressed individuals to present with multiple physical complaints,6, 33, 34 health behaviors such as non-adherence resulting in more hospitalizations,23 and/or psychobiological processes having an adverse effect on immunologic function post-HCT.28, 29 While our findings do not establish a causal relationship between pre-HCT depression and higher resource utilization, they are hypotheses-generating and point to the need for future research.
Our study has several limitations. First, we did not utilize a validated measure to assess the presence of pre-HCT depression. However, the question regarding pre-HCT depression reported to the CIBMTR has inherent face and content validity. To overcome reporter bias, we excluded transplant centers with > 10 transplants who did not report both depressed and non-depressed patients to the registry. Notably, the ascertainment of these cases through the CIBMTR would bias towards the null hypothesis with potential cross-contamination between the two groups. Given the lack of large multi-center studies examining this topic, this study represents the first attempt to better understand the relationship between pre-HCT depression and important clinical outcomes after HCT. Second, we are unable to ascertain whether patients with a history of pre-HCT depression were actively receiving or had received any therapy in the past. Third, it is possible that differences in illness severity and other factors could confound the relationship between pre-HCT depression and OS. Nonetheless, pre-HCT depression remained a significant predictor of OS after adjusting for potential confounders. Fourth, while we report on the association between pre-HCT depression and worse post-transplant outcomes, we are unable to determine the mechanism of this association. Lastly, we are unable to comment on the association between pre-HCT depression and risk of relapse or non-relapse mortality. Given the heterogeneity of the population and since the disease risk index26 has only been validated when examining OS to control for such heterogeneity, we made an a priori decision to not explore the association between pre-HCT depression and relapse or non-relapse mortality.
In conclusion, patients with a reported diagnosis of depression prior to allogeneic HCT have lower OS, higher incidence of grades II-IV acute GVHD, and they spend fewer days alive and out-of-the hospital during the first 100 days post-HCT. Among autologous HCT recipients, pre-transplant depression is not associated with OS, but it is associated with fewer days spent alive and out-of-the hospital during the first 100 days post-HCT. These findings highlight the potential impact of pre-transplant depression on important post-transplant outcomes including mortality, post-transplant complications, and health care utilization. Importantly, we identify patients with pre-HCT depression as a vulnerable population, who may benefit from specialized interventions to improve their post-transplant outcomes.
Acknowledgments
CIBMTR Support List
The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement 5U24-CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-13-1-0039 and N00014-14-1-0028 from the Office of Naval Research; and grants from Alexion; *Amgen, Inc.; Anonymous donation to the Medical College of Wisconsin; Be the Match Foundation; *Bristol Myers Squibb Oncology; *Celgene Corporation; *Chimerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Genentech, Inc.; Genzyme Corporation; *Gilead Sciences, Inc.; Health Research, Inc. Roswell Park Cancer Institute; HistoGenetics, Inc.; Incyte Corporation; *Jazz Pharmaceuticals, Inc.; Jeff Gordon Children’s Foundation; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Mesoblast; *Millennium: The Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Onyx Pharmaceuticals; Optum Healthcare Solutions, Inc.; Otsuka America Pharmaceutical, Inc.; Otsuka Pharmaceutical Co, Ltd. – Japan; Oxford Immunotec; Perkin Elmer, Inc.; Pharmacyclics; *Sanofi US; Seattle Genetics; Sigma-Tau Pharmaceuticals; *Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Telomere Diagnostics, Inc.; TerumoBCT; Therakos, Inc.; University of Minnesota; and *Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, Health Resources and Services Administration (HRSA) or any other agency of the U.S. Government. *Corporate Members
Footnotes
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data. All were involved in drafting the article or revising it critically for important intellectual content. All provided final approval of the manuscript and agree to be accountable for all aspects of the work.
Conflict of Interest Disclosures: None
References
- 1.Blumenthal JA, Lett HS, Babyak MA, et al. Depression as a risk factor for mortality after coronary artery bypass surgery. Lancet. 2003;362:604–609. doi: 10.1016/S0140-6736(03)14190-6. [DOI] [PubMed] [Google Scholar]
- 2.Wulsin LR, Vaillant GE, Wells VE. A systematic review of the mortality of depression. Psychosom Med. 1999;61:6–17. doi: 10.1097/00006842-199901000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Ganguli M, Dodge HH, Mulsant BH. Rates and predictors of mortality in an aging, rural, community-based cohort: the role of depression. Arch Gen Psychiatry. 2002;59:1046–1052. doi: 10.1001/archpsyc.59.11.1046. [DOI] [PubMed] [Google Scholar]
- 4.Spiegel D, Giese-Davis J. Depression and cancer: mechanisms and disease progression. Biol Psychiatry. 2003;54:269–282. doi: 10.1016/s0006-3223(03)00566-3. [DOI] [PubMed] [Google Scholar]
- 5.Fife BL, Huster GA, Cornetta KG, Kennedy VN, Akard LP, Broun ER. Longitudinal study of adaptation to the stress of bone marrow transplantation. J Clin Oncol. 2000;18:1539–1549. doi: 10.1200/JCO.2000.18.7.1539. [DOI] [PubMed] [Google Scholar]
- 6.Prieto JM, Blanch J, Atala J, et al. Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol. 2002;20:1907–1917. doi: 10.1200/JCO.2002.07.101. [DOI] [PubMed] [Google Scholar]
- 7.Wolcott DL, Wellisch DK, Fawzy FI, Landsverk J. Adaptation of adult bone marrow transplant recipient long-term survivors. Transplantation. 1986;41:478–484. doi: 10.1097/00007890-198604000-00013. [DOI] [PubMed] [Google Scholar]
- 8.Vose JM, Kennedy BC, Bierman PJ, Kessinger A, Armitage JO. Long-term sequelae of autologous bone marrow or peripheral stem cell transplantation for lymphoid malignancies. Cancer. 1992;69:784–789. doi: 10.1002/1097-0142(19920201)69:3<784::aid-cncr2820690328>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 9.Syrjala KL, Chapko MK, Vitaliano PP, Cummings C, Sullivan KM. Recovery after allogeneic marrow transplantation: prospective study of predictors of long-term physical and psychosocial functioning. Bone Marrow Transplant. 1993;11:319–327. [PubMed] [Google Scholar]
- 10.Loberiza FR, Jr, Rizzo JD, Bredeson CN, et al. Association of depressive syndrome and early deaths among patients after stem-cell transplantation for malignant diseases. J Clin Oncol. 2002;20:2118–2126. doi: 10.1200/JCO.2002.08.757. [DOI] [PubMed] [Google Scholar]
- 11.Pillay B, Lee SJ, Katona L, Burney S, Avery S. Psychosocial factors associated with quality of life in allogeneic stem cell transplant patients prior to transplant. Psychooncology. 2014;23:642–649. doi: 10.1002/pon.3462. [DOI] [PubMed] [Google Scholar]
- 12.Socie G, Stone JV, Wingard JR, et al. Long-term survival and late deaths after allogeneic bone marrow transplantation. Late Effects Working Committee of the International Bone Marrow Transplant Registry. N Engl J Med. 1999;341:14–21. doi: 10.1056/NEJM199907013410103. [DOI] [PubMed] [Google Scholar]
- 13.Duell T, van Lint MT, Ljungman P, et al. Health and functional status of long-term survivors of bone marrow transplantation. EBMT Working Party on Late Effects and EULEP Study Group on Late Effects. European Group for Blood and Marrow Transplantation. Ann Intern Med. 1997;126:184–192. doi: 10.7326/0003-4819-126-3-199702010-00002. [DOI] [PubMed] [Google Scholar]
- 14.Broers S, Hengeveld MW, Kaptein AA, Le Cessie S, van de Loo F, de Vries T. Are pretransplant psychological variables related to survival after bone marrow transplantation? A prospective study of 123 consecutive patients. J Psychosom Res. 1998;45:341–351. doi: 10.1016/s0022-3999(98)00003-8. [DOI] [PubMed] [Google Scholar]
- 15.Prieto JM, Atala J, Blanch J, et al. Role of depression as a predictor of mortality among cancer patients after stem-cell transplantation. J Clin Oncol. 2005;23:6063–6071. doi: 10.1200/JCO.2005.05.751. [DOI] [PubMed] [Google Scholar]
- 16.Hoodin F, Weber S. A systematic review of psychosocial factors affecting survival after bone marrow transplantation. Psychosomatics. 2003;44:181–195. doi: 10.1176/appi.psy.44.3.181. [DOI] [PubMed] [Google Scholar]
- 17.Ballen KK, Joffe S, Brazauskas R, et al. Hospital Length of Stay in the First 100 Days after Allogeneic Hematopoietic Cell Transplantation for Acute Leukemia in Remission: Comparison among Alternative Graft Sources. Biol Blood Marrow Transplant. 2014 doi: 10.1016/j.bbmt.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutherland HJ, Fyles GM, Adams G, et al. Quality of life following bone marrow transplantation: a comparison of patient reports with population norms. Bone Marrow Transplant. 1997;19:1129–1136. doi: 10.1038/sj.bmt.1700806. [DOI] [PubMed] [Google Scholar]
- 19.Lee SJ, Flowers ME. Recognizing and managing chronic graft-versus-host disease. Hematology Am Soc Hematol Educ Program. 2008:134–141. doi: 10.1182/asheducation-2008.1.134. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan KM, Witherspoon RP, Storb R, et al. Prednisone and azathioprine compared with prednisone and placebo for treatment of chronic graft-v-host disease: prognostic influence of prolonged thrombocytopenia after allogeneic marrow transplantation. Blood. 1988;72:546–554. [PubMed] [Google Scholar]
- 21.Wingard JR, Piantadosi S, Vogelsang GB, et al. Predictors of death from chronic graft-versus-host disease after bone marrow transplantation. Blood. 1989;74:1428–1435. [PubMed] [Google Scholar]
- 22.Loughran TP, Jr, Sullivan K, Morton T, et al. Value of day 100 screening studies for predicting the development of chronic graft-versus-host disease after allogeneic bone marrow transplantation. Blood. 1990;76:228–234. [PubMed] [Google Scholar]
- 23.DiMatteo MR, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160:2101–2107. doi: 10.1001/archinte.160.14.2101. [DOI] [PubMed] [Google Scholar]
- 24.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 25.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 26.Armand P, Kim HT, Logan BR, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–3671. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang G, Orav EJ, Tong MY, Antin JH. Predictors of 1-year survival assessed at the time of bone marrow transplantation. Psychosomatics. 2004;45:378–385. doi: 10.1176/appi.psy.45.5.378. [DOI] [PubMed] [Google Scholar]
- 28.McDaniel JS, Musselman DL, Porter MR, Reed DA, Nemeroff CB. Depression in patients with cancer. Diagnosis, biology, and treatment. Arch Gen Psychiatry. 1995;52:89–99. doi: 10.1001/archpsyc.1995.03950140007002. [DOI] [PubMed] [Google Scholar]
- 29.Hodes GE, Kana V, Menard C, Merad M, Russo SJ. Neuroimmune mechanisms of depression. Nat Neurosci. 2015;18:1386–1393. doi: 10.1038/nn.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Knight JM, Lyness JM, Sahler OJ, Liesveld JL, Moynihan JA. Psychosocial factors and hematopoietic stem cell transplantation: potential biobehavioral pathways. Psychoneuroendocrinology. 2013;38:2383–2393. doi: 10.1016/j.psyneuen.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brunoni AR, Machado-Vieira R, Sampaio-Junior B, et al. Plasma levels of soluble TNF receptors 1 and 2 after tDCS and sertraline treatment in major depression: Results from the SELECT-TDCS trial. J Affect Disord. 2015;185:209–213. doi: 10.1016/j.jad.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 32.El-Jawahri A, Chen YB. Pleiotropic approach to graft-versus-host disease. J Clin Oncol. 2013;31:4462–4464. doi: 10.1200/JCO.2013.52.8182. [DOI] [PubMed] [Google Scholar]
- 33.Simon GE, VonKorff M, Piccinelli M, Fullerton C, Ormel J. An international study of the relation between somatic symptoms and depression. N Engl J Med. 1999;341:1329–1335. doi: 10.1056/NEJM199910283411801. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K, Spitzer RL, Williams JB, et al. Physical symptoms in primary care. Predictors of psychiatric disorders and functional impairment. Arch Fam Med. 1994;3:774–779. doi: 10.1001/archfami.3.9.774. [DOI] [PubMed] [Google Scholar]