Abstract
The purpose of this study was to evaluate sympathetic skin response (SSR) and heart rate variability (HRV) in determining autonomic nervous system (ANS) involvement in patients with Parkinson disease (PD). Forty-eight idiopathic PD patients and 30 healthy controls participated in this study. SSR, HRV, Unified Parkinson's Disease Rating Scale (UPDRS) III, the Scales for outcomes in Parkinson's Disease-Autonomic (SCOPA-AUT), Hoehn and Yahr (H&Y) scale were evaluated. Absent lower limb SSR was determined unilaterally in 2, bilaterally in 1 of 3 advanced PD patients; there was significant difference between PD and control groups in terms of the SSR (P < 0.01), significant prolonged SSR latencies and decreased SSR amplitudes from bilateral hands and feet. Significant difference was noted in HRV between PD and control groups except for root mean square of successive differences (rMSSD) and high-frequency (HF) power (P < 0.05). There was a significant different correlation between the parameters of SSR and the SCOPA-AUT, and between the parameters (except HF power) of HRV and the SCOPA-AUT. Some parameters of SSR were relevantly associated with HRV. The right hand SSR amplitude correlated positively with the (SD) of all R-R interval, total spectral power, very low frequency. The right foot amplitude correlated positively with total spectral power. Both SSR and HRV parameters are sensitive in determining ANS dysfunction not only in late but also in the early stage of PD, which can be used for early detection of autonomic dysfunction in patients with PD and have the potential to serve as electrophysiological markers of dysautonomia of PD.
Keywords: autonomic disorders, heart rate variability, Parkinson disease, sympathetic skin response
1. Introduction
Parkinson disease (PD) is an irreversible neurodegenerative disorder characterized by motor dysfunction and various nonmotor symptoms. Nonmotor symptoms are common in PD, especially autonomic symptoms, and almost all the functional autonomic symptoms can be involved in PD.[1,2] Autonomic dysfunction can present in the early stages of PD, even before motor symptoms occur, and impact the quality of patients’ life.[3,4] However, it is still underrecognized in daily clinical practice. The wide range of autonomic dysfunctions of PD encompasses cardiovascular, gastrointestinal, urogenital, thermoregulatory, and pupil domains.[1] It is important to recognize autonomic impairment because symptomatic treatment is frequently effective.
Sympathetic skin response (SSR) is being used to assess the autonomic dysfunctions such as PD, spinal cord injury, and stroke.[5–7] It is a noninvasive paraclinical electrophysiological test, which is an assessment of sympathetic cholinergic psudomotor function. SSR, detecting the potential generated by the skin sweat glands and adjacent skin, is a dependable test recording of sympathetic efferent fibers stimulation and is produced by electrical stimulation of median or posterior tibial nerve afferents. In addition, heart rate variability (HRV), variation of the time interval between heartbeats, has also been used to evaluate autonomic cardiac physiology in PD.[8] When used as noninvasive and routine electrocardiography, HRV may help differentiate essential tremor from early-stage tremor-dominant PD.[9]
Therefore, to confirm the relationship between the noninvasive methods (SSR and HRV) and the autonomic dysfunction of PD, we evaluated the correlation between, first, PD patients and healthy controls; second, SSR and HRV parameters and other clinical autonomic disorder parameters regularly occurring in PD and to find out whether SSR and HRV parameters may have the potential to serve as electrophysiological markers of dysautonomia of PD.
2. Methods
2.1. Study population
Data were obtained from the outpatient and inpatient clinic at the Neurology Department of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University. Forty-eight PD patients with confirmed PD diagnosis and 30 healthy controls having no history of a neurological disease were recruited to participate in the study. None of the patients had arrhythmia, hypertension, ischemic or other heart diseases, diabetes mellitus, chronic obstructive airway disease, alcoholism hypothyroidism, or other central nervous system diseases. The study was approved by the research ethics committee of the Second Affiliated Hospital and Yuying Children's Hospital of Wenzhou Medical University. All subjects signed an informed consent, and all the information was confidential.
All of the PD subjects underwent clinical examinations including the motor part (Part III) of the Unified Parkinson's Disease Rating Scale (UPDRS), the scales for outcomes in PD-Autonomic (SCOPA-AUT) and the Hoehn and Yahr (H&Y) Scale.
2.2. Materials and procedure
All participants were interviewed using electrophysiological tests (SSR and HRV) and various scales and were examined neurologically by the authors. Motor symptoms were assessed using Part III of the UPDRS,[10] and all items were scored by a scale of 0 to 4 (normal/slight/mild/moderate/severe). As assessed by SCOPA-AUT questionnaire, autonomic subscores, cardiovascular, gastrointestinal, urinary, thermoregulatory, pupillomotor, and sexual were tested in patients with PD, and all items were scored by a scale of 0 to 3 (never/sometimes/regular/frequent).[11] A modified H&Y was used for disease staging.
In electrophysiological studies, SSR test was recorded by an Oxford synergy electromyography device with disk surface electrodes using sweep speed of 300 to 500 ms/div, sensitivity of 500 to 1000 uv/div, filtering of 0.5 to 500 Hz, frequency of stimulation equals to 1 stimulus/20–30 seconds, and intensity of stimulation equals to 20 milliampere. The examination was conducted under standard condition, in a quiet and semidark room at temperature of 25°C to let the skin temperature maintain above 32°C. SSR were obtained in all the subjects’ limbs. The subjects were kept awake and relaxed. For recording the median nerve SSR, the active electrode was placed at the palm of the hand and the reference electrode at dorsum of the hand. For recording the tibial nerve SSR, the active electrode was placed at the sole of the foot and the reference electrode at the dorsum of the foot. SSR parameter included the latency, which was indicated by the first continuous deflection from the baseline and the amplitude, which was measured from peak to peak. Absent responses were defined when no consistent change in the baseline usually larger than 50 mV was observed in any 1 of the recordings in the 2 seconds following the stimulus.
HRV test was recorded by 24-hour continuous recording electrocardiography (marquette), power spectral analyses of which have been proposed as a means to evaluate cardiovascular autonomic function.[12] The following time domain indexes were gathered: the standard deviation (SD) of all R-R intervals (SDNN), the SD of the averages of R-R intervals during all 5-minute periods that constitute the 24-hour day (SDANN), mean of the standard deviations of all R-R intervals for all 5-minute segments of the 24-hour recording (SDNN index), percentage of R-R intervals differing more than 50 ms from each other (pNN50), and the root mean square of successive differences (rMSSD). The frequency domain analyses were also obtained: total spectral power, defined as the power between the 0.0 and 0.40 Hz; low frequency (LF) power, the power between the 0.04 to 0.15 Hz; high-frequency (HF) power, the power between the 0.15 and 0.40 Hz, and very low frequency (VLF) power, the power ≤0.04 Hz. Commonly, most of these parameters were analyzed from 24-hour continuous recording electrocardiography when the subjects were at rest.
2.3. Statistical analysis
The Shapiro-Wilk test was applied to evaluate the normality of the distribution for continuous data before statistical analysis. Two independent samples t test was used to assess the differences between the 2 groups for continuous variables met normal or similar normal distribution. Mann-Whitney U test was performed if the distributions were skewed. Chi-square tests (or Fisher exact test) were performed to compare the differences of the proportion of categorical variables between the 2 groups. Spearman rank correlation was used to test the significance of correlations. All tests were 2-sided and P ≤0.05 was set as the significant level. Data management and all statistical analyses including figures drawing were performed using Rstudio Version 1.0.44 (RStudio, Inc.).
3. Results
The basic characteristics of the patients included the following: mean age (±SD) equal to 68.90 (±9.09) years (range: 38–81 years), duration of the disease was 5.4 ± 4.2 years (range: 0.3–14 years). The control group (63.33 ± 6.94 years, range: 48–76 years) were statistically different with respect to age and sex.
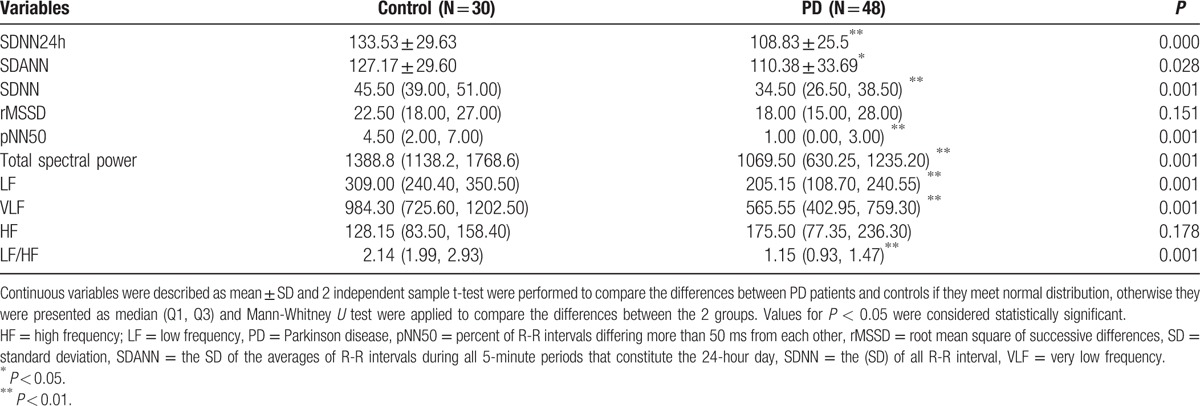
Absent lower limb SSR was determined unilaterally in 2, bilaterally in 1 of 3 advanced PD patients; there was significant difference between PD and control groups in terms of the of SSR (P < 0.01), significant prolonged SSR latencies and decreased SSR amplitudes from both hands and feet. There were significant differences between PD and control groups in HRV, with analysis of SDNN, SDANN, SDNN index, LF (mediated by baroreflex feedback), LF/HF (an index of sympatho-parasympathetic balance) and total spectral power. The parameters of HRV were found decreased in patients with PD when compared with the control subjects except for rMSSD and HF power (P < 0.05). Tables 1 and 2 provided information about electrophysiological autonomic parameters of the PD and healthy control subjects.
Table 1.
Latency and SSR amplitude in the extremities of PD patients and the controls.
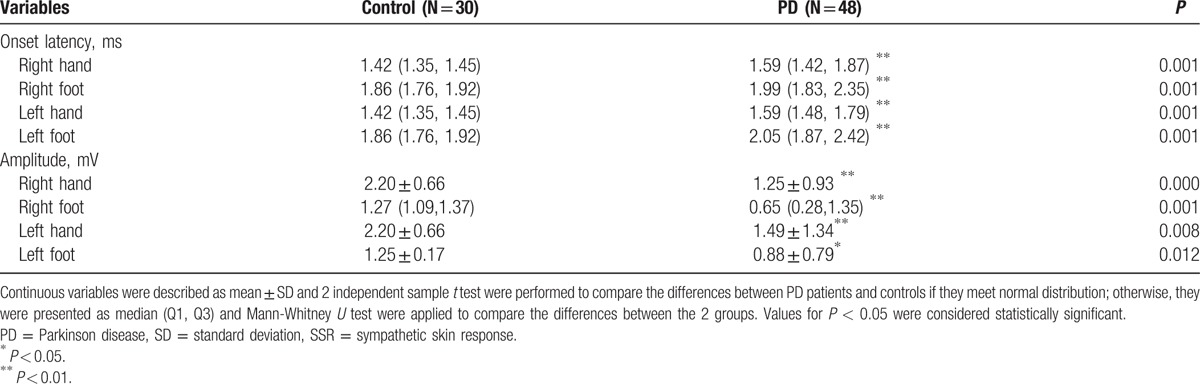
Table 2.
Comparisons of the parameters of HRV data from PD patients and controls.
Correlation between electrophysiological autonomic parameters and clinical PD scales demonstrated: SSR latencies correlated positively with the SCOPA-AUT score, SSR amplitudes and HRV parameters correlated negatively with the SCOPA-AUT score; and the SCOPA-AUT score correlated positively with the UPDRS III score and H&Y score (Table 3, Fig. 1). The left hand SSR latency correlated negatively with LF/HF (r = −0.366, P = 0.031). The right hand SSR amplitude correlated positively with SDNN (r = 0.320, P = 0.028), total spectral power (r = 0.311, P = 0.034), LF (r = 0.355, P = 0.014), and VLF (r = 0.315, P = 0.031). The left foot amplitude correlated positively with rMSSD (r = 0.349, P = 0.043), total spectral power (r = 0.461, P = 0.006), LF (r = 0.472, P = 0.005), and VLF (r = 0.447, P = 0.008). The right foot amplitude correlated positively with total spectral power (r = 0.442, P = 0.035).
Table 3.
Correlation analysis between the autonomic data assessed by SCOPA-AUT scale and variables.
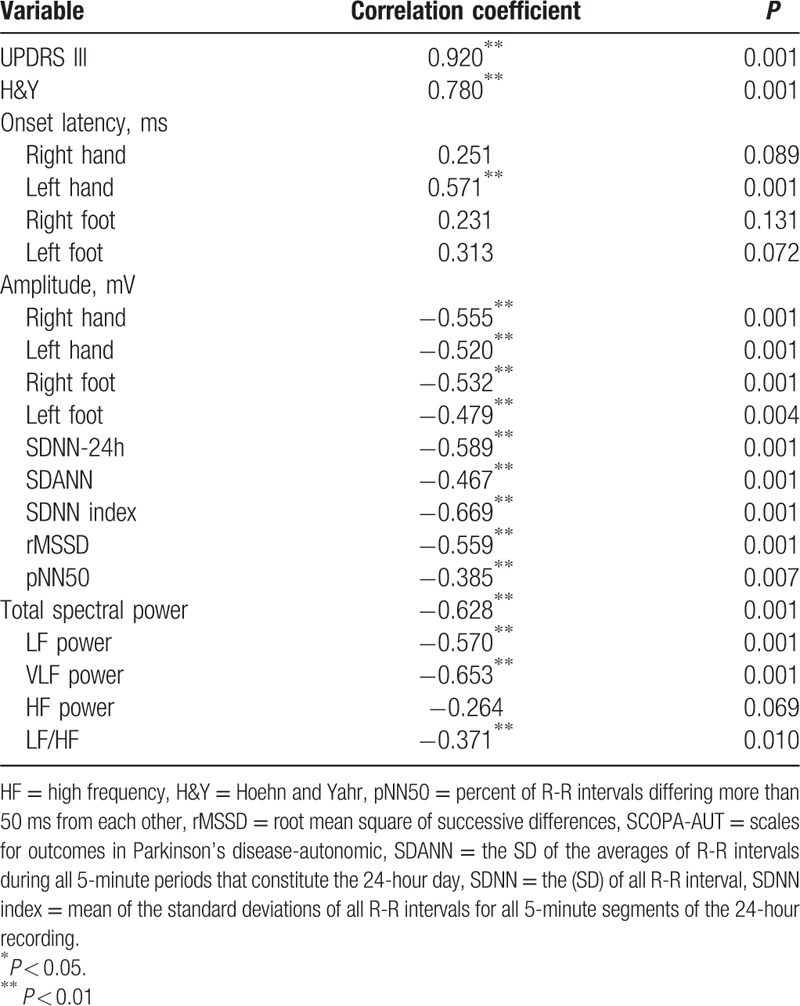
Figure 1.
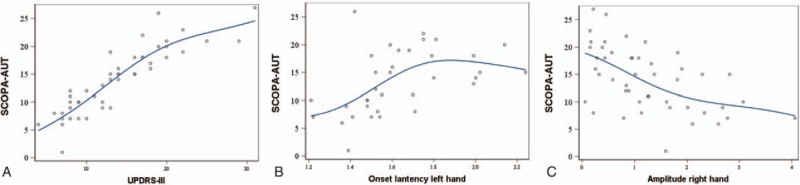
The relationship between SCOPA-AUT and UPDRS-III, Onset lantency left hand and Amplitude right hand. (A) The relationship between SCOPY-AUT and UPDRS-III. (B) The relationship between SCOPA-AUT and Onset lantency left hand; (C) The relationship between SCOPA-AUT and Amplitude right hand. UPDRS = Unified Parkinson's Disease Rating Scale.
4. Discussion
A great impact of autonomic dysfunction on the quality of PD patients’ life has been widely acknowledged.[1,3,12] The autonomic nervous system (ANS) can be divided into the sympathetic and the parasympathetic nervous systems. To shed more light on the involvement of the ANS in patients with PD, the SSR and HRV tests were used in this study. When compared with signs of the ANS involvement and clinical symptoms, the data analysis revealed the usefulness of both tests on the assessment of the dysautonomia.
Most patients with PD describe autonomic symptoms detailed at the time of diagnosis suggesting that clinical characteristics may have potential sensitivity as clinical markers of the premotor phase.[13] Autonomic symptoms in PD are better evaluated with the SCOPA-AUT scale.[14] The SCOPA-AUT questionnaire is divided into 6 subscores for the following domains: cardiovascular, thermoregulatory, gastrointestinal, urinary, pupillomotor, and sexual. Although the items in the questionnaire, such as SCOPA-AUT scale for assessing the disorder of ANS in PD patients are reliable and valid,[15,16] they are more complicated and subjective. Objective measurements of autonomic function are needed.
SSR and HRV tests are recommended since these tests are objective quantitative tests. SSR and HRV analyses are noninvasive and easily applicable electrophysiological tests are used in the measure of sympathetic and parasympathetic nervous system function, respectively. SSR provides information on cholinergic sympathetic function of the ANS. SSR is commonly used for the evaluation of ANS function for its easy application and reliable results.[17] The SSR wave reflects temporary reaction in electrical potential generated in the skin's deep layers, the results of internal or external stimuli. The wave is considered to originate from sweat glands’ synchronized activation in response to discharges by efferent sympathetic nerve fibers. Though 1 study found SSR responses were not elicited only in a minority of individuals, and without related difference between PD patients and controls,[18] another study found that the SSR might provide valuable information on cholinergic sympathetic function in patients with PD.[19] In our study, we found that there was significant difference between PD and control groups in terms of the SSR (P < 0.01), significant prolonged SSR latencies and decreased SSR amplitudes from bilateral hands and both feet, and the absent lower limb SSR was determined in advanced PD patients.
The activity of the sympathetic and parasympathetic (vagal) components of the ANS on the sinus node,[20] which is a cardiovascular autonomic function was reflected well by the use of HRV. Most generally used time domain parameters in PD research are the SDNN, the rMSSD, and pNN50. SDNN represents a general measurement of ANS balance, whereas rMSSD and pNN50 predominantly reflects parasympathetic activity. In frequency domain analyses recordings, there are LF, VLF, and HF power components in recordings. The LF power is modulated by both the sympathetic and parasympathetic systems, whereas the HF power is predominantly under the influence of the parasympathetic nervous system. The LF/HF ratio may reflect sympathetic modulations or sympathovagal balance.[21] Prior data have indicated that the rMSSD was lower in PD compared to control subjects and the rMSSD might be the most promising linear parameter that reflected ANS disturbance in PD.[18] Other previous studies showed HRV change could not only present early in the disease process but also predicted the motor symptom onset,[3,22,23] and decreased HRV was associated with an increased risk of PD.[23,24] Time-domain (SDNN, rMSSD) but not frequency-domain measures, such as HF, LF, LF/HF of HRV were associated with the risk of PD.[23] On the contrary, another study did not see an association between lower HRV and a risk of PD diagnosis, suggesting they might not predate PD diagnosis.[25] The parameters of HRV were found decreased in patients with PD when compared with the control subjects except for rMSSD and HF power (P < 0.05) in our study.
Most studies focusing on autonomic dysfunction in PD by describing differences of SSR or HRV parameters between PD patients and healthy controls.[26,27] In contrast, the progressions of the HRV and SSR parameters over time, as well as their associations with various PD-related symptoms have only been partly reported. Previous studies found that SSR parameters were demonstrated to be correlated with UPDRS score, disease duration, disease stage, bradykinesia, and rigidity.[5,28] There still has not been any studies related to whether the parameters of the SSR or HRV have associations with SCOPA-AUT in PD. In our study, correlation between electrophysiological autonomic parameters and clinical PD scales demonstrated that there was a significant different correlation between the parameters of SSR and the SCOPA-AUT, the clinical autonomic disorder parameter. The left hand SSR latency correlated positively with the SCOPA-AUT score, SSR amplitudes correlated negatively with the SCOPA-AUT score and the SCOPA-AUT score correlated positively with the UPDRS III score and H&Y score. Therefore, we suggested that SSR was useful for the assessment of ANS function in patients with PD. Although there was no significant difference been noted in rMSSD between PD patients and controls, the rMSSD of the PD patient correlated negatively with the SCOPY-AUT score, and we also found there was a significant different correlation between the parameters (except HF power) of HRV and the SCOPA-AUT scale. We thus believed that HRV was beneficial for the assessment of ANS function in patients with PD.
One previous study[19] could not find that these SSR alterations were associated with the presence of orthostatic hypotension or R-R interval variations. On the contrary, we found that some parameters of SSR were relevantly associated with HRV. The left hand SSR latency correlated negatively with LF/HF. The right hand SSR amplitude correlated positively with SDNN, total spectral power, VLF. The left foot amplitude correlated positively with rMSSD, total spectral power, and VLF. The right foot amplitude correlated positively with total spectral power. Absent lower limb SSR was determined unilaterally in 2, bilaterally in 1 of 3 advanced PD patients, the 3 patients had a high score on the SCOPA-AUT scale. Nevertheless, the 2 patients who had unilaterally absent lower limb SSR had normal HRV, which may be because SSR and HRV detected the 2 different aspects of the ANS of PD.
In summary, quantitative changes in SSR and HRV analysis parameters were measured in PD patients. These data demonstrated that SSR and HRV could provide complementary information about the presence of dysautonomia in subjects with PD. Thus, SSR and HRV parameters may have the potential to serve as electrophysiological markers of dysautonomia of PD. Further study of this population and larger groups of subjects are needed.
Footnotes
Abbreviations: ANS = autonomic nervous system, H&Y = Hoehn and Yahr, HF = high frequency, HRV = heart rate variability, LF = low frequency, PD = Parkinson disease, pNN50 = percent of R-R intervals differing more than 50 ms from each other, rMSSD = root mean square of successive differences, SCOPA-AUT = Scales for outcomes in Parkinson's disease-Autonomic, SD = standard deviation, SDANN = the SD of the averages of R-R intervals during all 5-minute periods that constitute the 24-hour day, SDNN = the (SD) of all R-R interval, SDNN index = mean of the standard deviations of all R-R intervals for all 5-minute segments of the 24-hour recording, SSR = sympathetic skin response, UPDRS = Unified Parkinson's Disease Rating Scale, VLF = very low frequency.
This work was supported by Jiangsu Provincial Special Program of Medical Science (BL2014042); Suzhou Clinical Research Center of Neurological Disease (Szzx201503). This was also partly supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
The authors report no conflicts of interest.
References
- [1].Li K, Reichmann H, Ziemssen T. Recognition and treatment of autonomic disturbances in Parkinson's disease. Expert Rev Neurother 2015;15:1189–203. [DOI] [PubMed] [Google Scholar]
- [2].Kadastik-Eerme L, Muldmaa M, Lilles S, et al. Nonmotor features in Parkinson's disease: what are the most important associated factors. Parkinsons Dis 2016;2016:4370674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, et al. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson's disease. Mov Disord 2011;26:399–406. [DOI] [PubMed] [Google Scholar]
- [4].Goldstein DS. Dysautonomia in Parkinson disease. Compr Physiol 2014;4:805–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Giza E, Katsarou Z, Georgiadis G, et al. Sympathetic skin response in Parkinson's disease before and after mental stress. Neurophysiol Clin 2012;42:125–31. [DOI] [PubMed] [Google Scholar]
- [6].Hubli M, Krassioukov AV. How reliable are sympathetic skin responses in subjects with spinal cord injury. Clin Auton Res 2015;25:117–24. [DOI] [PubMed] [Google Scholar]
- [7].Çakır T, Evcik FD, Subaşı V, et al. Investigation of the H reflexes, F waves and sympathetic skin response with electromyography (EMG) in patients with stroke and the determination of the relationship with functional capacity. Acta Neurol Belg 2015;115:295–301. [DOI] [PubMed] [Google Scholar]
- [8].Jain S. Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism Relat Disord 2011;17:77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yoon JH, Kim MS, Lee SM, et al. Heart rate variability to differentiate essential tremor from early-stage tremor-dominant Parkinson's disease. J Neurol Sci 2016;368:55–8. [DOI] [PubMed] [Google Scholar]
- [10].Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society sponsored revision of the Unitied Parkinson's Disease Rating Scale (MDSUPDRS): scale presentation and climetric testing results. Mov Disord 2008;23:2129–70. [DOI] [PubMed] [Google Scholar]
- [11].Visser M, Marinus J, Stiggelbout AM, et al. Assessment of automomic dysfunction in Parkinson's disease: the SCOPA-AUT. Mov Disord 2004;19:1306–12. [DOI] [PubMed] [Google Scholar]
- [12].Jain S, Goldstein DS. Cardiovascular dysautonomia in Parkinson disease: from pathophysiology to pathogenesis. Neurobiol Dis 2012;46:572–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ziemssen T, Reichmann H. Cardiovascular autonomic testing in extrapyramidal disorders. J Neurol Sci 2011;310:129–32. [DOI] [PubMed] [Google Scholar]
- [14].Bonnet AM, Jutras MF, Czernecki V, et al. Nonmotor symptoms in Parkinson's disease in 2012: relevant clinical aspects. Parkinsons Dis 2012;2012:198316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Damian A, Adler CH, Hentz JG, et al. Autonomic function, as self-reported on the SCOPA-autonomic questionnaire, is normal in essential tremor but not in Parkinson's disease. Parkinsonism Relat Disord 2012;18:1089–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Forjaz MJ, Ayala A, Rodriguez-Blazquez C, et al. Assessing autonomic symptoms of Parkinson's disease with the SCOPA-AUT: a new perspective from Raschanalysis. Eur J Neurol 2010;17:273–9. [DOI] [PubMed] [Google Scholar]
- [17].Yerdelen D, Erol T, Karatas M. Selective autonomic screening in Guillain-Barré syndrome. Neurol India 2010;58:398–402. [DOI] [PubMed] [Google Scholar]
- [18].Maetzler W, Karam M, Berger MF, et al. Time- and frequency-domain parameters of heart rate variability and sympathetic skin response in Parkinson's disease. J Neural Transm (Vienna) 2015;122:419–25. [DOI] [PubMed] [Google Scholar]
- [19].Schestatsky P, Ehlers JA, Rieder CR, et al. Evaluation of sympathetic skin response in Parkinson's disease. Parinsonism Relate Disord 2006;12:486–91. [DOI] [PubMed] [Google Scholar]
- [20].Sztajzel J. Heart rate variability: a noninvasive electrocardiographic method to measure the autonomic nervous system. Swiss Med Wkly 2004;134:514–22. [DOI] [PubMed] [Google Scholar]
- [21].Brisinda D, Sorbo AR, Di Giacopo R. Cardiovascular autonomic nervous system evaluation in Parkinson disease and multiple system atrophy. J Neurol Sci 2014;336:197–202. [DOI] [PubMed] [Google Scholar]
- [22].Palma JA, Urrestarazu E, Alegre M, et al. Cardic autonomic impairment during sleep is linked with disease severity in Parkinson's disease. Clin Neurophysiol 2013;124:1163–8. [DOI] [PubMed] [Google Scholar]
- [23].Alonso A, Huang X, Mosley TH, et al. Heart rate variability and the risk of Parkinson disease: The Atherosclerosis Risk in Communities study. Ann Neurol 2015;77:877–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kallio M, Haapaniemi T, Turkka J, et al. Heart rate variability in patients with untreated Parkinson's disease. Eur J Neurol 2000;7:667–72. [DOI] [PubMed] [Google Scholar]
- [25].Jain S, Ton TG, Perera S, et al. Cardiovascular physiology in premotor Parkinson's disease: a neuroepidemiologic study. Mov Disord 2012;27:988–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reimann M, Schmidt C, Herting B, et al. Comprehensive autonomic assessment does not differentiate between Parkinson's disease, multiple systematrophy and progressive supranuclear palsy. J Neural Transm (Vienna) 2010;117:69–76. [DOI] [PubMed] [Google Scholar]
- [27].Koszewicz M, Mendak M, Konopka T, et al. The characteristics of autonomic nervous system disorders in burning mouth syndrome and Parkinson disease. J Orofac Pain 2012;26:315–20. [PubMed] [Google Scholar]
- [28].Del Tredici K, Braak H. Spinal cord lesions in sporadic Parkinson's disease. Acta Neuro-pathol 2012;124:643–64. [DOI] [PubMed] [Google Scholar]


