Abstract
Noninvasive and convenient tests to assess pulmonary fibrosis and disease progression in interstitial lung diseases (ILDs) are currently unavailable. The extracellular matrix molecules, laminin (LN), type IV collagen (IVC), procollagen III N-terminal peptide (PIIINP), and hyaluronic acid (HA) are involved in ILD development and progression. This study aims to investigate the association of disease progression and serum levels of LN, IVC, PIIINP, and HA in patients with ILD. This retrospective study included 323 patients (162 cases of idiopathic pulmonary fibrosis [IPF] and 161 cases of connective tissue diseases ILD [CTD-ILD]) treated in Shanghai Pulmonary Hospital between January 2013 and January 2015 and 160 healthy controls. Serum LN, IVC, PIIINP, and HA were analyzed by radioimmunoassay. Data of the percentage of forced vital capacity in the prediction value (FVC%pred), the percentage of diffusing capacity of the lung for carbon monoxide in the prediction value (DLCO%pred), high resolution computed tomography (HRCT) score, and patient mortality were collected. Serum LN, IVC, PIIINP, and HA were significantly increased in the patients with IPF or CTD-ILD compared with the healthy controls (all P < .05) and were further elevated in the acute exacerbation cases (all P < .05). Serum LN, IVC, PIIINP, and HA positively correlated with HRCT score and negatively correlated with FVC%pred and DLCO%pred significantly in the patients (all P < .05). The survived patients had significantly lower serum LN, IVC, PIIINP, and HA than the dead patients (all P < .05). Serum levels of LN, IVC, PIIINP, and HA may reflect ILD progression and may be indicators for the severity of ILDs.
Keywords: hyaluronic acid, interstitial lung diseases, laminin, procollagen III N-terminal peptide, type IV collagen
1. Introduction
Interstitial lung diseases (ILDs) represent a heterogeneous group of nontumor pulmonary disorders characterized by inflammation in the alveoli and eventual scarring of lung tissue.[1] Idiopathic pulmonary fibrosis (IPF) and CTD-ILD are the most common types of ILD. IPF is associated with alveolar epithelial dysfunction, persistent fibroblast proliferation, and excessive extracellular matrix (ECM) deposition.[2] IPF is progressive and often leads to poor prognosis. Most patients with IPF will develop pulmonary hypertension, pulmonary heart disease, and ultimate right heart failure; the approximate survival is only 3 to 5 years.[2] CTDs are a group of diseases that have the connective tissues of the body as a primary target of pathology, such as rheumatoid arthritis, systemic sclerosis, Sjögren syndrome, systemic lupus erythematosus, and polymyositis/dermatomyositis. Connective tissue is a major component of the bronchi, blood vessels, interstitium, and pleura in the lung. Thus, the lung is often involved in CTDs.[3] Although different ILDs show diverse clinical and histopathological feature, disorders in ECM protein metabolism appear to be common contributors to the pathogenesis of ILDs.[1]
In patients with ILDs, the balance between collagen synthesis and degradation in the lung is disrupted, and an increase in collagen synthesis and/or a decrease in collagen removal result in excessive ECM deposition in the lung interstitium.[1] In the lung tissue of mice with bleomycin-induced pulmonary fibrosis and patients with systemic sclerosis-related ILD presenting fibrotic nonspecific interstitial pneumonia, collagen I deposition increases substantially compared with that in the lung tissue of control mice and healthy individuals.[4] In addition to collage I, laminin (LN), and type IV collage (IVC), which are also major components of the basemen membrane and ECM of the lung, have also been found to contribute to the development of pulmonary fibrosis.[5,6] Type III procollagen N-terminal peptide (PIIINP) is released during the conversion of type III procollagen to type III collagen and enters circulation after being released.[7] Thus, serum PIIINP levels may reflect collagen synthesis. Increased PIIINP levels have been detected in the serum and bronchoalveolar lavage fluid of patients with IPF.[8] Besides the ECM proteins, hyaluronic acid (HA), which is the major nonsulfated glycosaminoglycan in the lung, is also implicated to play a role in the development of lung diseases such as fibrotic interstitial pneumonia.[9,10] Therefore, serum levels of the 4 ECM molecules LN, IVC, PIIINP, and HA may indicate the disease progression of ILDs.
Serum ECM molecule levels have been used to evaluate the severity of liver fibrosis and patient prognosis.[11–13] El-Mezayen et al[11] combined serum IVC and LN levels with liver biochemical indexes to develop a fibrosis discriminant scoring system and found that the scoring system estimated significant liver fibrosis with 79% sensitivity and 88% specificity in patients with chronic hepatitis C. Incorporation of serum levels of PIIINP into conventional assessment approaches, such as the Knodell index, has been found to improve the accuracy of prediction of the survival prognosis in patients with hepatitis B virus liver cirrhosis.[12] In addition, serum HA has also been tested as a biomarker to evaluate the severity of liver fibrosis.[13]
Similar to liver fibrosis, serum levels of the 4 ECM molecules LN, IVC, PIIINP, and HA in patients with ILD may also correlate with the severity of pulmonary fibrosis and survival prognosis. This study aims to test this hypothesis. Here, we analyzed the correlation of serum levels of LN, IVC, PIIINP, and HA with pulmonary function parameters and high resolution computed tomography (HRCT) score in patients with IPF or CTD-ILD and examined the association of serum levels of the 4 ECM molecules with the severity of the diseases and survival prognosis of patients with IPF or CTD-ILD.
2. Materials and methods
2.1. Study design
This single-center retrospective study was conducted in Shanghai Pulmonary Hospital of Tongji University in Shanghai China. A total of 323 patients with ILD treated in Shanghai Pulmonary Hospital from January 2013 to December 2015 were included in this study. Shanghai Pulmonary Hospital ethics committee has approved this study (the approval number: K13-108). Data collection and handling are in accordance with relevant ethical guidelines. All the data were kept anonymous. Written informed consent was obtained from each study participants.
2.2. Study participants
The patient inclusion criteria were as follows: had available test results for serum LN, IVC, PIIINP, and HA; had a confirmed diagnosis of IPF or CTD-ILD. Of the 323 participating patients with ILD, 162 were diagnosed with IPF according to the American Thoracic Society/European Respiratory Society 2013 revised diagnostic criteria for IPF[14]; 161 were diagnosed with CTD-ILD. The diagnostic criteria for CTD followed the guidelines recommended by the American Rheumatism Association and the American College of Rheumatology.[15–20] The patients that had ILD and a confirmed diagnosis of 1 type of CTDs by rheumatologists were diagnosed as CTD-ILD. A total of 160 age-matched healthy individuals, who had routine physical examination in Shanghai Pulmonary Hospital, were included as controls. Patients or control individuals with the following clinical characteristics were excluded from the study: had a malignancy during the study or a history of malignancy; were using glucocorticoid and/or immunosuppressive (including azathioprine, cyclophosphamide, mycophenolate mofetil, and cyclosporin A); and had severe diseases or functional disorder in other organs. The flowchart for patient screening is displayed in Fig. 1. Acute exacerbation (AE) of ILD (AE-ILD) was defined as a continuous exacerbation of shortness of breath for ≤30 days, a PaO2/FIO2 ratio ≤225, a reduction in PaO2 >10 mm Hg, and increased shadows on chest imaging. Patients with confirmed pulmonary infection such as pneumonia were excluded. The diagnosis criteria for infection are the presence of fever (body temperature >38.5 °C), elevated white blood cell count >15,000, and pulmonary infiltrates that can be resolved by antibiotic therapies. The definition of AE-ILD was based on the diagnostic criteria for AE of IPF (AE-IPF).[14]
Figure 1.
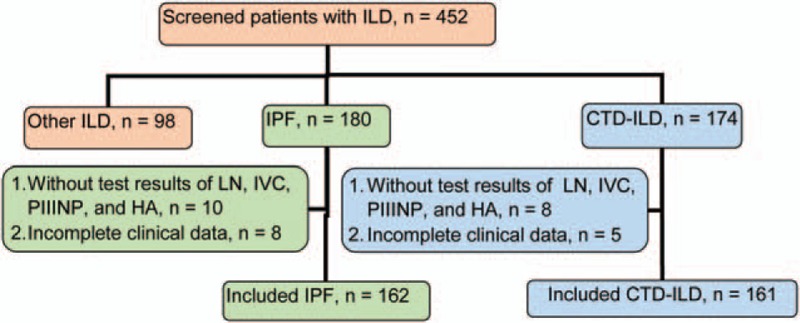
Flowchart of patient screening. CTD-ILD = connective tissue diseases interstitial lung disease, ILD = interstitial lung disease, IPF = idiopathic pulmonary fibrosis.
2.3. Measurement of serum levels of laminin, type IV collagen, type III procollagen N-terminal peptide, and hyaluronic acid
Five milliliter venous blood was withdrawn from each participant after an overnight fast, and serum was separated from the whole blood and stored at −25 °C for future use. The serum samples were thawed at room temperature and then centrifuged at 1500 to 2000×g for 15 minutes. The levels of LN, IVC, PIIINP, and HA in the supernatant were measured using a radioimmunoassay analysis kit (Beijing Kemei Biotechonology Ltd., Beijing, China) according to the protocol provided by the manufacturer in a gamma radioimmunoassay analyzer (SN-695B, Shanghai Nuclear Research Rihuan Electro-Optic Instrument Ltd., Shanghai, China).
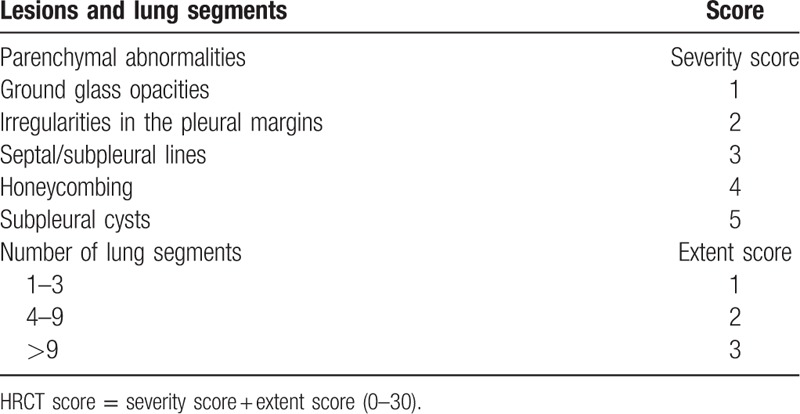
2.4. Chest high resolution computed tomography score
One radiologist and 2 physicians scored chest HRCT according to the criteria in Table 1,[21] and the average HRCT score was used for the study.
Table 1.
HRCT scoring criteria.
2.5. Pulmonary function evaluation
The pulmonary function of the patients with ILD was evaluated by the percentage of forced vital capacity in the prediction value (FVC%pred) and the percentage of diffusing capacity of the lung for carbon monoxide in the prediction value (DLCO%pred). FVC%pred and DLCO%pred represent pulmonary ventilation capacity and pulmonary diffusing capacity, respectively, are considered as the key indicators to evaluate the pulmonary function of patients with ILD.[22] Patients with AE-ILD were not able to complete the pulmonary function test because of their severe lung condition.
2.6. Follow-up
The 323 patients with ILD were followed up in the outpatient clinic of Shanghai Pulmonary Hospital or by telephone calls. The follow-up ended on December 31, 2015. Overall mortality and mortality within 1 year was recorded. The serum levels of LN, IVC, PIIINP, and HA were compared in dead versus survived patients. Kaplan–Meier survival in the patients with IPF and the patients with CTD-ILD was analyzed.
2.7. Statistical analysis
Statistical analysis was performed with the statistical analysis software SPSS 16.0 (Chicago, IL). Continuous variables are presented as mean ± standard deviation. Two-group comparison was analyzed by Student t test, and P < .05 was considered statistically significantly different. The correlation of the serum levels of LN, IVC, PIIINP, and HA with FVC%pred, DLCO%pred, and HRCT score was analyzed by bivariate linear regression.
3. Results
3.1. Clinical characteristics
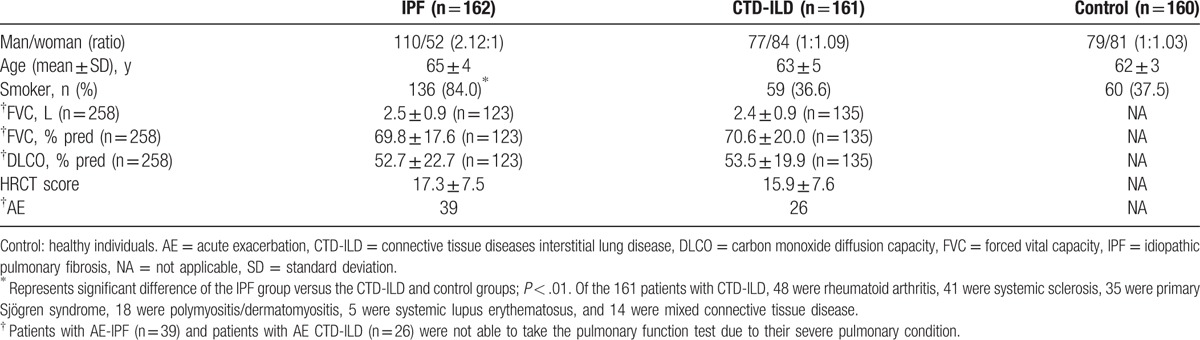
The flow chart for patient screening is illustrated in Fig. 1. In total, 452 patients with ILD treated in Shanghai Pulmonary Hospital between January 2013 and January 2015 were screened. A total of 323 patients including 162 cases of IPF and 161 cases of CTD-ILD were included in the study. The control group included 160 age-matched healthy individuals. Age was similar in the IPF, CTD-ILD, and control groups. Compared with the CTD-ILD and control groups, the IPF group showed higher ratio of man/woman (Table 2). The proportion of smokers in the IPF group (84.0%) was significantly higher than that in the CTD-ILD group (36.6%) and the control group (37.5%, P < .01). Of the 65 cases of AE-ILD, 39 were AE-IPF and 26 were AE CTD-ILD. All of the other patients (123 cases of IPF and 135 cases of CTD-ILD) showed stable disease.
Table 2.
Demographics and clinical characteristics of study participants.
3.2. Serum levels of LN, IVC, PIIINP, and HA, pulmonary function, and HRCT score
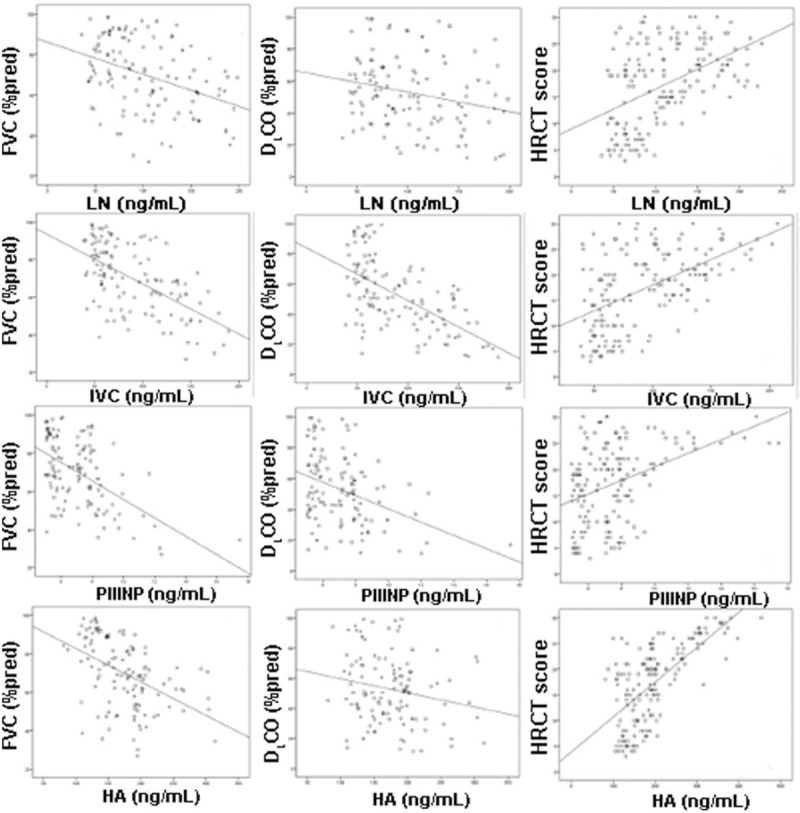
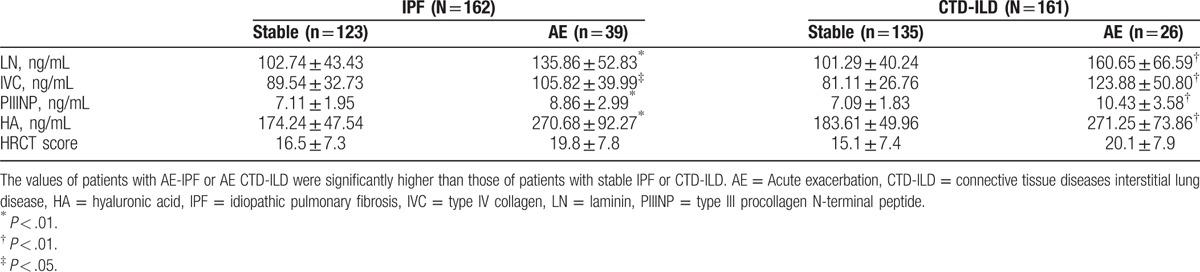
The serum levels of LN, IVC, PIIINP, and HA in the IPF and CTD-ILD groups were similar, whereas they were significantly higher than those in the control group (All P < .05, Table 3). Notably, serum PIIINP and HA in the patients with IPF or CTD-ILD were approximately 1.6 and 2.8-folds of those in the controls, respectively, whereas serum LN and IVC in the IPF and CTD-ILD groups were approximately 20% higher than those in the control group (Table 3). The serum levels of LN, IVC, PIIINP, and HA negatively correlated with FVC (%pred) and DLCO (%pred) significantly (All P < .01, Table 4), whereas positively correlated with HRCT score significantly (P < .01, Table 4) in patients with IPF (Fig. 2) and patients with CTD-ILD (Fig. 3). The patients with AE-IPF had significantly higher serum levels of LN, IVC, PIIINP, and HA than the patients with stable IPF (all P < .05, Table 5). Similarly, the AE CTD-ILD cases also showed significantly higher serum levels of the 4 molecules than the stable CTD-ILD cases (all P < .05, Table 5). The serum levels of LN, IVC, PIIINP, and HA were not significantly different in AE-IPF versus AE CTD-ILD cases.
Table 3.
Comparison of the levels of LN, IVC, PIIINP, and HA.
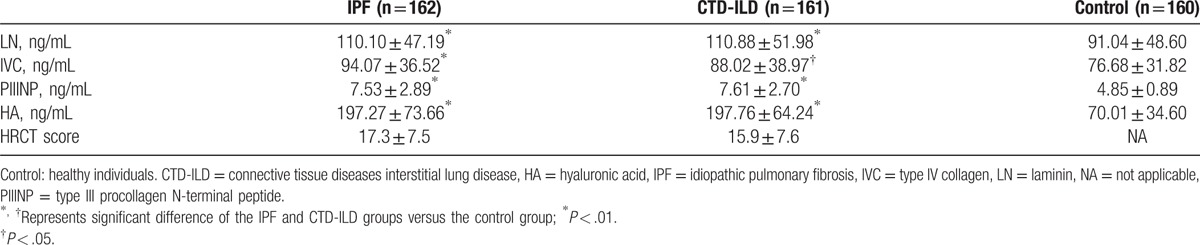
Table 4.
Correlation of serum levels of LN, IVC, PIIINP, and HA to percentage of forced vital capacity in the prediction value, percentage of diffusing capacity of the lung for carbon monoxide in the prediction value, and HRCT score.
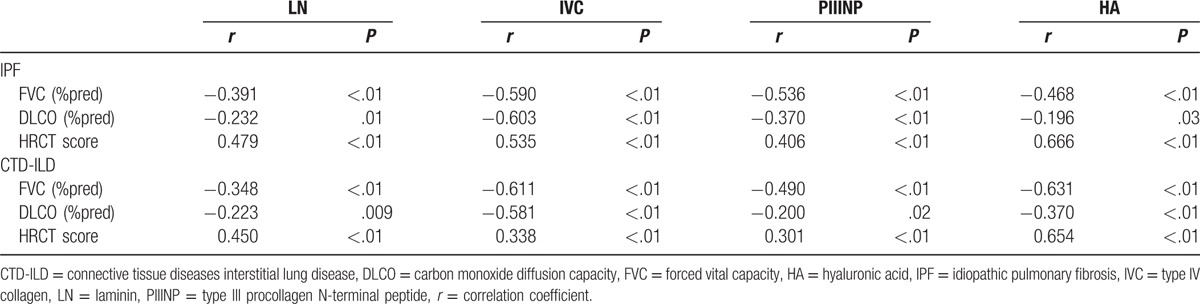
Figure 2.
Correlation of serum levels of laminin, type IV collagen, type III procollagen N-terminal peptide, and hyaluronic acid to percentage of forced vital capacity in the prediction value, percentage of diffusing capacity of the lung for carbon monoxide in the prediction value, and high-resolution computed tomography score in the patients with idiopathic pulmonary fibrosis. HA = hyaluronic acid, IVC = type IV collagen, LN = laminin, PIIINP = type III procollagen N-terminal peptide.
Figure 3.
Correlation of serum levels of laminin, type IV collagen, type III procollagen N-terminal peptide, and hyaluronic acid to percentage of forced vital capacity in the prediction value, percentage of diffusing capacity of the lung for carbon monoxide in the prediction value, and high-resolution computed tomography score in the patients with connective tissue diseases interstitial lung disease. CTD-ILD = connective tissue diseases interstitial lung disease, HA = hyaluronic acid, IPF = idiopathic pulmonary fibrosis, IVC = type IV collagen, LN = laminin, PIIINP = type III procollagen N-terminal peptide.
Table 5.
Comparison of serum levels of LN, IVC, PIIINP, and HA in AE versus stable cases.
3.3. Correlation of serum levels of LN, IVC, PIIINP, and HA to survival prognosis
The 162 patients with IPF were followed up for 4 to 156 weeks, and the median follow-up time was 80 weeks. The overall mortality in the IPF group was 33.95% (55/162), and the 1-year mortality was 15.4% (25/162). The 161 cases of CTD-ILD were followed for 8 to 156 weeks with a median of 109 weeks. The overall mortality and 1-year mortality was 13.66% (22/161) and 9.9% (16/161), respectively. The serum levels of LN, IVC, PIIINP, and HA were significantly higher in the patients who died in 1 year than in the patients who survived 1 year (all P < .05, Table 6), suggesting that serum levels of the 4 ECM molecules may be associated with survival prognosis. Kaplan–Meier survival analysis revealed that the IPF group had significantly higher overall mortality (33.95%) than the CTD-ILD group (13.66%) (Fig. 4), whereas the 1-year mortality was similar in the AE-IPF cases (25/39, 64.1%) and AE CTD-ILD cases (16/26, 61.5%).
Table 6.
Comparison of the serum levels of LN, IVC, PIIINP, and HA in patients who died within 1 year versus patients who survived 1 year.
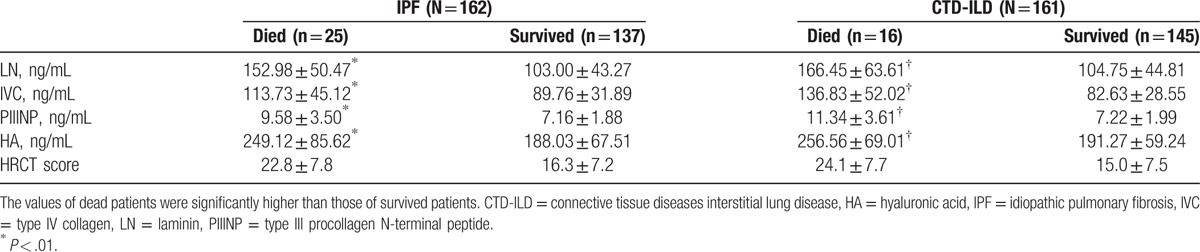
Figure 4.
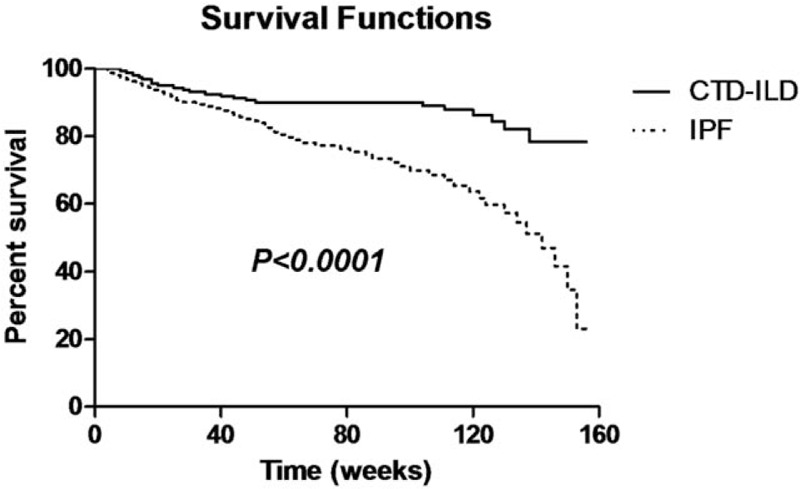
Overall survival curve. CTD-ILD = connective tissue diseases interstitial lung disease, IPF = idiopathic pulmonary fibrosis.
4. Discussion
Accumulative evidence supports that the 4 ECM molecules LN, IVC, PIIINP, and HA are associated with ILD development and progression.[5,6,23,24] The alveolar epithelium secrets LNs into the basement membrane. Patients with pulmonary fibrosis exhibit abnormal α3 LN subunit distribution in the basement membrane of the fibrotic regions in the lung.[5] Transgenic mice lacking the α3 LN subunit in the lung epithelium have worse mortality and pulmonary fibrosis than wild-type mice after the intratracheal administration of bleomycin.[5] In a rat model of bleomycin-induced pulmonary fibrosis, the expression of collagen I and IV in the lung is increased, and both types of collagen deposit around the new pulmonary blood vessels.[6] Immunohistochemical staining has shown that collagen XII and XIV were expressed in the fibrotic areas of the lung tissue specimen of patients with IPF, and collagen I was overexpressed in the fibrotic area.[23] Bjermer et al[24] compared the PIIINP and HA levels in the bronchoalveolar lavage fluid of 22 patients with IPF versus those of 21 healthy controls and found that the patients with IPF had significantly higher PIIINP and HA levels than healthy controls. In addition, among the patients with IPF, those with deteriorated pulmonary function and radiographic progression showed higher PIIINP and HA levels than those with stable disease.[24]
Serum levels of the 4 ECM molecules have been tested as biomarkers to evaluate liver fibrosis and predict prognosis in patients with liver disease.[11–13] However, whether they could also be used to assess the severity of pulmonary fibrosis is still unknown, although previous studies have shown that the 4 ECM molecules are involved in ILD development.[5,6,23,24] In the present study, we found that serum levels of the 4 ECM molecules were all significantly higher in the patients with IPF or CTD-ILD than in the healthy individuals and were all further increased in the AE-IPF and AE CTD-ILD cases. These results suggest that serum levels of the 4 ECM molecules may be continuously increased during IPF and CTD-ILD development and progression and thus may be biological indicators of the disease stage of IPF and CTD-ILD.
Serum PIIINP and HA levels have been analyzed previously in patients with pulmonary fibrosis.[8,10,25,26] Milman et al[25] investigated 27 patients with pulmonary fibrosis and 24 healthy controls and found that serum PIIINP and HA levels were not significantly different between the 2 groups but were higher in the patients with progressive disease than the patients with stable disease. Low et al[8] measured the PIIINP levels in serum and bronchoalveolar lavage fluid of 24 patients with IPF and 29 healthy individuals and found that the patients with IPF had significantly elevated PIIINP in serum and bronchoalveolar lavage fluid. In a dog model of IPF, serum PIIINP level was similar to that in healthy dogs.[26] Inokoshi et al[10] reported that serum HA was significantly increased in patients with chronic interstitial pneumonia compared with healthy controls and was further elevated in patients with AE of interstitial pneumonia. Compared with the previous studies, which included small number of patients and only focused on 1 or 2 of the 4 ECM molecules,[8,10,24,25] the present study investigated serum levels of the 4 ECM molecules together in 323 patients and 160 healthy controls. The results of the present study suggest that serum levels of the 4 ECM molecules may reflect disease progression of ILDs. This present study also demonstrated that serum levels of the 4 ECM molecules were similar in patients with IPF and patients with CTD-ILD and remained similar in AE cases of IPF and CTD-ILD. These data indicate that mechanisms underlying the AE of different types of ILD may share some common feature.
To further verify the association between serum levels of the 4 ECM molecules and ILD progression, we analyzed the correlation of the serum levels of the 4 ECM molecules with the parameters that are considered to reflect ILD progression, including pulmonary function parameters and HRCT score.[27,28] To our best knowledge, the present study first showed that the serum levels of the 4 ECM molecules had a significant positive association with HRCT score and a significant negative association with FVC%pred and DLCO%pred in patients with IPF or CTD-ILD. These results suggest that deterioration of pulmonary function during ILD progression may be accompanied with increases in the serum levels of the 4 ECM molecules. Serum PIIINP levels have been shown to negatively correlate with total lung capacity in patients with pulmonary fibrosis.[25]
AE of ILD is a serious medical condition that is often associated with high mortality and poor patient prognosis.[14] The reported mortality associated with AE-IPF is 71% to 78%,[29,30] and the incidence rate of AE-IPF in patients with IPF is 35% to 57%.[29–31] Our previous study of 1044 patients with CTD-ILD has demonstrated that 16 of the 20 dead patients had AE CTD-ILD.[32] In the patient cohort of this present study, the incidence rate of AE-IPF and AE CTD-ILD was 24.1% and 16.1%, respectively, and the mortality of AE-IPF and AE CTD-ILD was 64.1% and 61.5%, respectively. In addition, we also found that the serum levels of the 4 ECM molecules were significantly higher in the dead patients than the survived patients. Thus, the serum levels of the 4 ECM molecules may indicate the survival prognosis of patients with ILD.
This study presented one time measurement of serum levels of LN, IVC, PIIINP, and HA in healthy individuals, patients with stable IPF or CTD-ILD, and patients with AE of the diseases. Although our results suggest that serum levels of the 4 ECM molecules appear to reflect the severity of IPF or CTD-ILD, a serial measurement of the molecules at different disease stage of same patients is required to further verify our conclusion. We will make such measurement in our future study.
5. Conclusion
Compared with healthy individuals, patients with IPF or CTD-ILD showed significantly higher serum levels of LN, IVC, PIIINP, and HA. Serum levels of the 4 ECM molecules were also significantly higher in patients with AE than in patients with stable disease. HRCT scores positively correlated with serum levels of the 4 ECM molecules, whereas FVC%pred and DLCO%pred negatively correlated with them. Survived patients had significantly lower serum LN, IVC, PIIINP, and HA than dead patients. These results suggest that serum levels of LN, IVC, PIIINP, and HA may reflect IPF and CTD-ILD progression and may be used as biomarkers to stage ILD.
Footnotes
Abbreviations: AE = acute exacerbation, CTD-ILD = connective tissue diseases interstitial lung disease, DLCO = carbon monoxide diffusion capacity, ECM = extracellular matrix, FVC = forced vital capacity, HA = hyaluronic acid, HRCT = high resolution computed tomography, ILD = interstitial lung disease, IPF = idiopathic pulmonary fibrosis, IVC = type IV collagen, LN = laminin, PIIINP = type III procollagen N-terminal peptide.
YS and HG have contributed equally to the article.
Authors’ contributions: HL, DW, YS, and HG participated in the conception, hypothesis, and design of the study. YZ, QL, FZ, YZ, LS, and YH participated in the molecular studies. YS, HG, and HL performed the statistical analysis and drafted the manuscript. All authors read and approved the final manuscript.
Funding/support: The present study was funded by grants from the National Science Foundation of China (nos.: 81670055, 81670056, 91442103, 81500052, and 81570057), Ministry of Science and Technology of the People's Republic of China (2016YFC1100200 and 2016YFC1100204), the Health Bureau Program of Shanghai Municipality (SHDC12014120 and 2013SY047), and Tongji University (1511219020).
The authors have no conflicts of interest to disclose.
References
- [1].Virk RK, Fraire AE. Interstitial lung diseases that are difficult to classify: a review of bronchiolocentric interstitial lung disease. Arch Pathol Lab Med 2015;139:984–8. [DOI] [PubMed] [Google Scholar]
- [2].Loomis-King H, Flaherty KR, Moore BB. Pathogenesis, current treatments and future directions for idiopathic pulmonary fibrosis. Curr Opin Pharmacol 2013;13:377–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet 2012;380:689–98. [DOI] [PubMed] [Google Scholar]
- [4].Lee R, Reese C, Bonner M, et al. Bleomycin delivery by osmotic minipump: similarity to human scleroderma interstitial lung disease. Am J Physiol Lung Cell Mol Physiol 2014;306:L736–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Morales-Nebreda LI, Rogel MR, Eisenberg JL, et al. Lung-specific loss of α3 laminin worsens bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 2015;52:503–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Teles-Grilo ML, Leite-Almeida H, Martins dos Santos J, et al. Differential expression of collagens type I and type IV in lymphangiogenesis during the angiogenic process associated with bleomycin-induced pulmonary fibrosis in rat. Lymphology 2005;38:130–5. [PubMed] [Google Scholar]
- [7].Kivirikko KI, Myllylä R. Post-translational processing of procollagens. Ann N Y Acad Sci 1985;460:187–201. [DOI] [PubMed] [Google Scholar]
- [8].Low RB, Giancola MS, King TE, Jr, et al. Serum and bronchoalveolar lavage of N-terminal type III procollagen peptides in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1992;146:701–6. [DOI] [PubMed] [Google Scholar]
- [9].Lennon FE, Singleton PA. Role of hyaluronan and hyaluronan-binding proteins in lung pathobiology. Am J Physiol Lung Cell Mol Physiol 2011;301:L137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Inokoshi Y, Tanino Y, Wang X, et al. Clinical significance of serum hyaluronan in chronic fibrotic interstitial pneumonia. Respirology 2013;18:1236–43. [DOI] [PubMed] [Google Scholar]
- [11].El-Mezayen HA, Habib S, Marzok HF, et al. Diagnostic performance of collagen IV and laminin for the prediction of fibrosis and cirrhosis in chronic hepatitis C patients: a multicenter study. Eur J Gastroenterol Hepatol 2015;27:378–85. [DOI] [PubMed] [Google Scholar]
- [12].Babić Z, Bilić A, Babić D, et al. Predictive role of serum procollagen III peptide and Knodell's index in survival prognosis of patients with hepatitis B virus liver cirrhosis. Wien Klin Wochenschr 2003;115:302–8. [DOI] [PubMed] [Google Scholar]
- [13].Neuman MG, Cohen LB, Nanau RM. Hyaluronic acid as a non-invasive biomarker of liver fibrosis. Clin Biochem 2016;49:302–15. [DOI] [PubMed] [Google Scholar]
- [14].Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013;188:733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988;31:315–24. [DOI] [PubMed] [Google Scholar]
- [16].van den Hoogen F, Khanna D, Fransen J, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2013;65:2737–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med 1975;292:344–7. [DOI] [PubMed] [Google Scholar]
- [18].Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982;25:1271–7. [DOI] [PubMed] [Google Scholar]
- [19].Goules AV, Tzioufas AG, Moutsopoulos HM. Classification criteria of Sjogren's syndrome. J Autoimmun 2014;48–49:42–5. [DOI] [PubMed] [Google Scholar]
- [20].Sharp GC, Irvin WS, Tan EM, et al. Mixed connective tissue disease—an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med 1972;52:148–59. [DOI] [PubMed] [Google Scholar]
- [21].Warrick JH, Bhalla M, Schabel SI, et al. High resolution computed tomography in early scleroderma lung disease. J Rheumatol 1991;18:1520–8. [PubMed] [Google Scholar]
- [22].King TE, Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083–92. [DOI] [PubMed] [Google Scholar]
- [23].Tzortzaki EG, Koutsopoulos AV, Dambaki KI, et al. Active remodeling in idiopathic interstitial pneumonias: evaluation of collagen types XII and XIV. J Histochem Cytochem 2006;54:693–700. [DOI] [PubMed] [Google Scholar]
- [24].Bjermer L, Lundgren R, Hällgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax 1989;44:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Milman N, Kristensen MS, Bentsen K. Hyaluronan and procollagen type III aminoterminal peptide in serum and bronchoalveolar lavage fluid from patients with pulmonary fibrosis. APMIS 1995;103:749–54. [DOI] [PubMed] [Google Scholar]
- [26].Heikkila HP, Krafft E, Jespers P, et al. Procollagen type III amino terminal propeptide concentrations in dogs with idiopathic pulmonary fibrosis compared with chronic bronchitis and eosinophilic bronchopneumopathy. Vet J 2013;196:52–6. [DOI] [PubMed] [Google Scholar]
- [27].Johannson KA, de Boer K, Wolters PJ, et al. Diagnosis of idiopathic pulmonary fibrosis with high-resolution CT. Lancet Respir Med 2014;2:e5. [DOI] [PubMed] [Google Scholar]
- [28].Li X, Peng S, Wei L, et al. Relevance analysis of clinical and lung function parameters changing and prognosis of idiopathic pulmonary fibrosis. Int J Clin Exp Med 2014;7:4759–69. [PMC free article] [PubMed] [Google Scholar]
- [29].Kubo H, Nakayama K, Yanai M, et al. Anticoagulant therapy for idiopathic pulmonary fibrosis. Chest 2005;128:1475–82. [DOI] [PubMed] [Google Scholar]
- [30].Kim DS, Park JH, Park BK, et al. Acute exacerbation of idiopathic pulmonary fibrosis: frequency and clinical features. Eur Respir J 2006;27:143–50. [DOI] [PubMed] [Google Scholar]
- [31].Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356–63. [DOI] [PubMed] [Google Scholar]
- [32].Hu Y, Wang LS, Wei YR, et al. Clinical characteristics of connective tissue disease-associated interstitial lung disease in 1,044 Chinese patients. Chest 2016;149:201–8. [DOI] [PubMed] [Google Scholar]


