Supplemental Digital Content is available in the text
Keywords: acute ischemic stroke, desmoteplase, thrombolysis
Abstract
Background:
Pending results from double-blind, multicenter, parallel-group, randomized trials, the benefit and safety of the novel plasminogen activator, desmoteplase remain undetermined. The aim of this meta-analysis was to help evaluate desmoteplase's efficacy and safety.
Methods:
A thorough search was performed of the Cochrane Library, PubMed, and Embase from the inception of electronic data to March 2017, and double-blind, multicenter, parallel-group, randomized trials were chosen. We conducted a meta-analysis of studies investigating intravenous desmoteplase treatment of acute ischemic stroke patients 3 to 9 hours after symptom onset. Asymptomatic intracerebral hemorrhage, good clinical outcome at 90 days, and reperfusion 4 to 8 hours posttreatment were variables assessing efficacy; symptomatic intracerebral hemorrhage and death rates were measures of safety.
Results:
Six trials involving 1071 patients thrombolyzed >3 hours postonset were included (600 received intravenous desmoteplase, 471 placebo). Desmoteplase was associated with increased reperfusion (odds ratio [OR] 1.57; 95% confidence interval [CI], 1.10–2.24; P = .01 vs control) and showed a tendency to increase asymptomatic intracerebral hemorrhage (OR 1.25; 95% CI, 0.97–1.62; P = .09 vs control), whereas there was no increase in symptomatic intracerebral hemorrhage and death rate with desmoteplase. However, there was no difference in the clinical response at 90 days (OR 1.14; 95% CI, 0.88–1.49; P = .31 vs control). Subgroup analysis showed that desmoteplase 90 μg/kg (OR 1.53; 95% CI, 1.07–2.21; P = .02 vs control) and 125 μg/kg (OR 4.07; 95% CI, 1.16–14.24; P = .03 vs control) were associated with an increase in reperfusion. Also, we found desmoteplase 90 μg/kg showed a tendency to increase asymptomatic intracerebral hemorrhage (OR 1.25; 95% CI, 0.95–1.63; P = .11 vs control).
Conclusion:
Intravenous desmoteplase is associated with a favorable reperfusion efficacy and acceptable safety in ischemic stroke treatment >3 hours after symptom onset. Well-designed randomized controlled trials with larger patient cohorts and a moderate dose of drugs are needed to further evaluate the true efficacy of desmoteplase in stroke patients.
Trial Registration:
URL: http://www.crd.york.ac.uk/PROSPERO; PROSPERO registration number: CRD42016037667).
1. Introduction
Stroke is one of the leading causes of premature mortality worldwide.[1] With an aging population, the prevalence of stroke around the world is projected to increase significantly. Treatment with intravenous recombinant tissue-type plasminogen activator (r-tPA), the only approved drug treatment for acute ischemic stroke (AIS), within 0 to 4.5 hours can effectively reduce disability after AIS.[2] Considering the gold standard of therapy, r-tPA can greatly improve patient clinical function as measured by National Institutes of Health stroke scale (NIHSS), the Modified Rankin Scale (mRS) score, and the Barthel index (BI). However, r-tPA has also many disadvantages, including its narrow time window for thrombolysis (0–4.5 h), its inability to fully lyse large thrombi, and its associated high risk of cerebral and systemic hemorrhage.[3] A major issue with r-tPA is that it is not useful for the treatment of patients who present beyond 4.5 hours of symptom onset.
Desmoteplase, a fibrin-dependent plasminogen activator, has characteristics which make it a promising thrombolytic drug: high fibrin specificity,[4] nonactivation by β-amyloid,[5,6] and the absence of neurotoxicity.[7] Some published clinical data from AIS patients show that desmoteplase is associated with a higher rate of reperfusion and better clinical outcome.[8–11] However, data from other published trials of desmoteplase do not demonstrate efficacy.[12,13]
To help determine the safety (symptomatic intracranial hemorrhage [SICH], mortality, and asymptomatic intracranial hemorrhage [AICH]) and efficacy (good clinical outcome at 90 days and reperfusion) of desmoteplase in patients aged 18 to 85 years beyond 3 hours with AIS, we conducted a comprehensive meta-analysis of all available case series.
2. Materials and methods
The present meta-analysis strictly followed the Systematic Reviews and Meta-Analyses (PRISMA) statement in File 1.
2.1. Clinical trial registration
The protocol of this systematic review and meta-analysis was registered with the international prospective register of systematic reviews. (URL: http://www.crd.york.ac.uk/PROSPERO; PROSPERO registration number: CRD42016037667.)
2.2. Data sources and search strategy
We searched the Cochrane Library, PubMed, and Embase from the inception of electronic data to March 2017 with search terms for relevant studies. The search was restricted to English language trials. The search terms and strategies are shown in File 2.
2.3. Study selection and data extraction
Study selection was based on the following inclusion criteria: The study design was a double-blind, multicenter, parallel-group, randomized type. The study evaluated patients aged 18 to 85 years with a NIHSS score of 4 to 24 within 3 to 9 hours of symptom onset. The study compared desmoteplase with placebo and reported outcomes of interest. A study was excluded if it was unpublished or lacked a control group.
The titles and abstracts obtained from the online searches were independently screened by 2 reviewers (XL and CL) to identify articles meeting the exclusion criteria. The remaining articles were then reviewed and retained only if they met the inclusion criteria. If relevant data in a selected study were unreported or unclear, we sought further information from the journal's website and corresponding author. Any conflicting opinions about an article's inclusion were discussed by the 2 reviewers and, if necessary, a third reviewer (LL) was consulted.
The study characteristics, quality, and outcome data were extracted using a standardized data extraction form. Study characteristics included country (and centers) of origin, study time period, study group sizes, patient ages and sex ratio, mean baseline NIHSS score and timing relative to symptom onset, penumbral tissue volume, and patient glucose level or history of diabetes.
2.4. Risk of bias
The quality of included studies was assessed using the Cochrane risk of bias assessment tools: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The quality of each item was assigned with 1 of the 3 degrees of risk: low, unclear, or high.[14] Two reviewers independently assessed each item. Disagreement was settled through discussion or by consultation with the corresponding author.
The meta-analysis compared desmoteplase and placebo groups’ rates of specific outcomes: AICH, good clinical function at 90 days (an NIHSS score improvement of ≥8 points or an NIHSS score ≤1 and an mRS score ≤2, and a BI of 75–100), reperfusion (defined as either a 30% or more reduction in mean-transit-time volume of abnormality or by a 2 point or more improvement on the adapted thrombolysis in myocardial infarction grading scheme using magnetic resonance angiography) was assessed 4 to 24 hours posttreatment, symptomatic intracerebral hemorrhage (SICH, defined as intracerebral hemorrhage confirmed by computerized tomography or other imaging associated with an increase of 4 or more points on the NIHSS within 72 hours of treatment), and mortality at 90 days (all causes of death).[15] The authors’ definition was used if a preferred definition was not used in the studies.[16]
2.5. Statistical analysis
For studies pooled for the meta-analysis, we performed the between-study heterogeneity test by using the I2 index. The lack of significant heterogeneity and obvious bias suggest that the results of this meta-analysis are reliable and accurate. The effect of desmoteplase on the outcome was expressed as an odds ratio (OR) with a 95% confidence interval (CI) by using the random-effects size model if significant heterogeneity was found, otherwise a fixed-effect size model was used. A P value of <0.05 was considered as significant for all tests. Data were analyzed using the Cochrane Collaboration's Review Manager software package (RevMan, version 5.3; The Nordic Cochrane Centre and the Cochrane Collaboration, Copenhagen, Denmark).
3. Results
3.1. Literature search
PubMed, Embase, and Cochrane Library searches yielded 17, 190, and 17 studies, respectively. After removing duplicates using ENDNOTE software (version 7.5; Niles Software), the titles and abstracts of the remaining 193 studies were screened carefully: 162 studies were excluded. The excluded studies included 103 that were reviews or commentaries or lacked a comparison group; 26 whose focus was on imaging; 14 that did not study the drug desmoteplase; and 19 that were excluded for only ancillary analysis of the drug of desmoteplase. Out of 31 articles that were read in full, 25 studies were not true randomized controlled trials (RCTs), and 6 were retained for meta-analysis. All of the included studies had a double-blind, multicenter, parallel-group, randomized design. The flow diagram shown in File 3 shows the study selection process.
3.2. Description of studies
Data from 1071 of the 1117 patients from the 6 included studies were available for inclusion in the meta-analysis.[8–13] (The DIAS study was conducted in 2 parts; the part 2 protocol was amended due to a concern regarding dosing and the standards of included people in part 1. Therefore, we excluded part 1 of the DIAS study from our meta-analysis.) Of these patients, 600 received intravenous desmoteplase, and 471 received placebo. The patient sample sizes ranged from 37 to 492, with an average of 178 per trial.
DIAS and DIAS-J studies[8,10] used totally 4 different doses of desmoteplase—62.5, 70, 90, and 125 μg/kg. The other studies[9,11–13] used the 2 highest doses of desmoteplase, 90 and 125 μg/kg, or used only 90 μg/kg. Therefore, we performed subgroup analysis by categorizing patients into 3 subgroups for comparison based upon the desmoteplase dose for comparison: <90, 90, and 125 μg/kg.
3.3. Study quality evaluation
If we were unable to find information concerning the risk of bias, we described the grade as unclear. We defined the study as having low bias if there was a clear description in the text of studies. Any disagreements were discussed by the 2 reviewers, and a third reviewer was consulted if needed. Figure 1 shows that the studies included in the meta-analysis had a low risk of bias based on the Cochrane risk of bias assessment tools.
Figure 1.
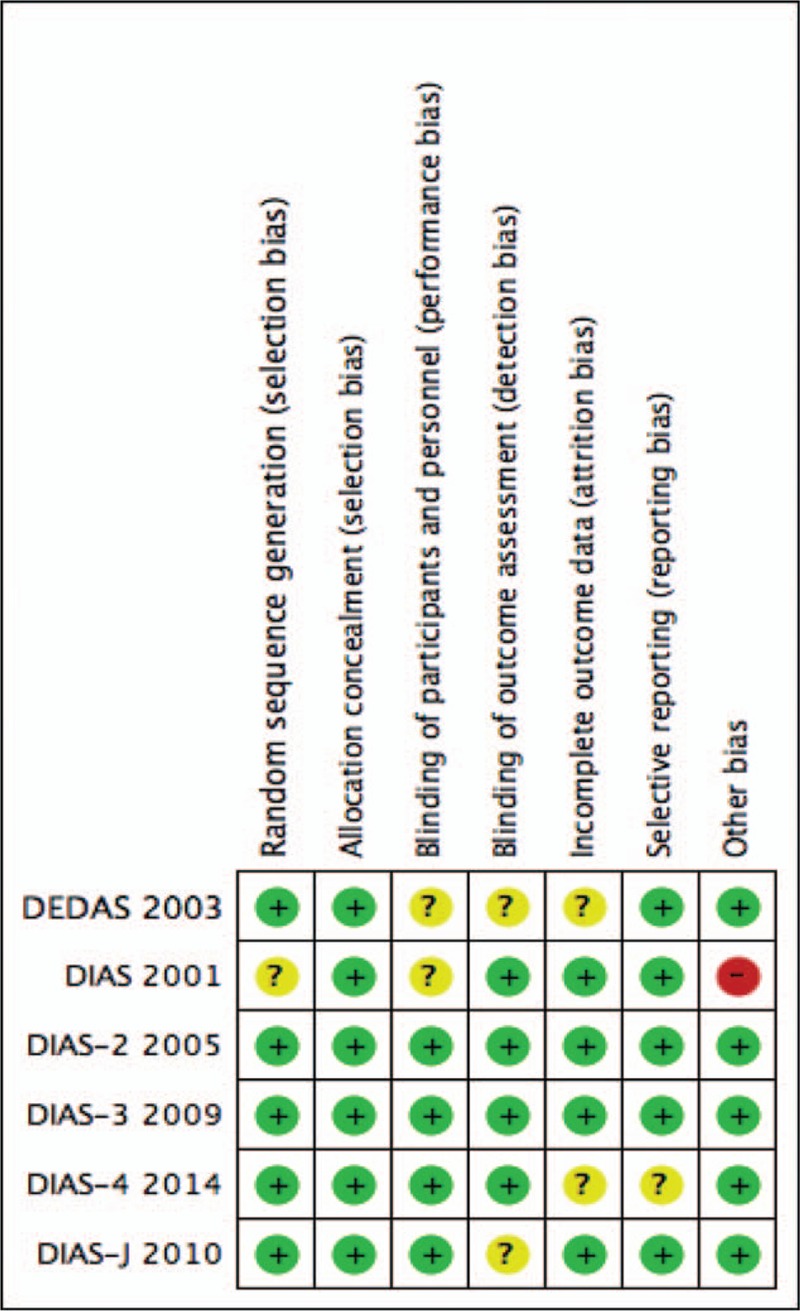
Risk of bias summary review: authors’ judgments on each risk of bias item for each included study.
3.4. Outcome results
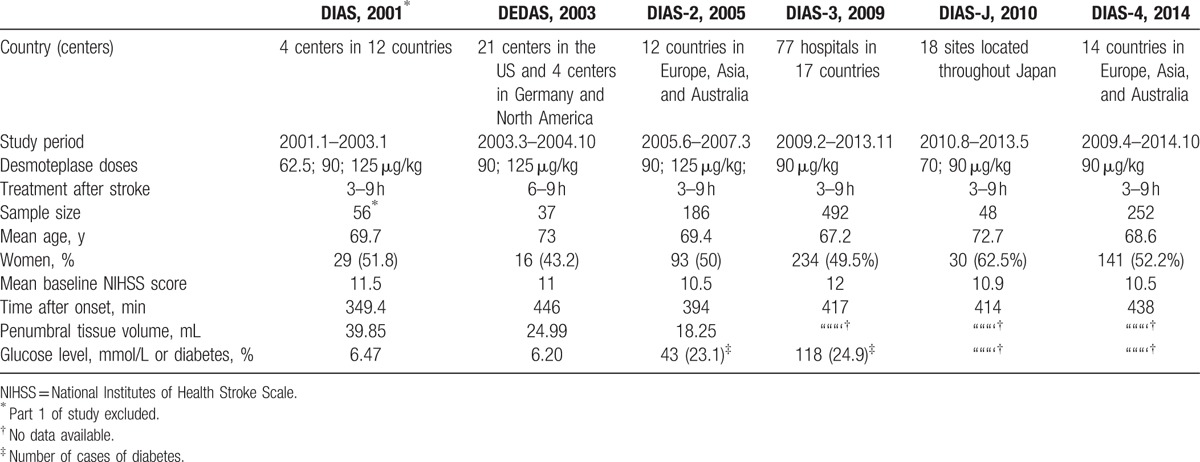
The included studies were relevant continuous trials, so patients’ characteristics were similar. The median age of patients was 67 to 73 years, and women made up 43.2% to 62.5% of the study populations. The average NIHSS scores ranged from 10.5 to 12, and the mean time to treatment varied from 349.4 to 446 minutes. The mismatch volume varied among studies, with DIAS having the largest average volume (39.85 mL) (Table 1).
Table 1.
Baseline characteristics of included studies.
3.5. Safety
3.5.1. Symptomatic intracerebral hemorrhage
All 6 RCTs evaluated the rate of SICH.[8–13] SICH within 72 hours was observed in 3.2% (19/595) of patients in the desmoteplase groups and 2.1% (10/467) of placebo-treated patients. There was no significant difference between the desmoteplase and placebo groups (OR 1.43; 95% CI, 0.67–3.04; P = .35; Fig. 2), and no significant heterogeneity was found (I2 = 0%).
Figure 2.
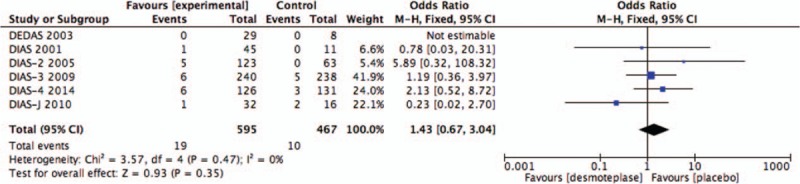
Forest plot of symptomatic intracranial hemorrhage outcome data for the desmoteplase and placebo groups for comparison.
3.5.2. Mortality
All 6 RCTs studies were included in the analysis of mortality.[8–13] A total of 109 deaths occurred within 90 days, 61 from the treatment group (n = 595; 10.2%), and 48 from the placebo-treated group (n = 467; 10.3%). No significant increase in mortality was found in patients treated with desmoteplase versus placebo (OR 1.05; 95% CI, 0.70–1.59; P = .80; Fig. 3), and there was no significant heterogeneity (I2 = 0%).
Figure 3.
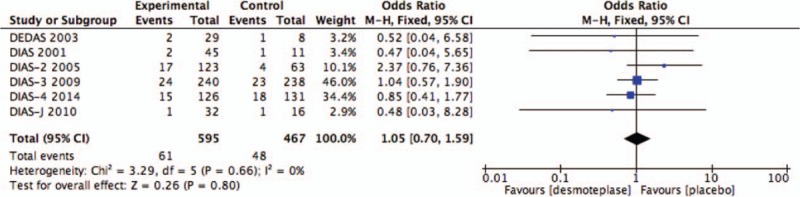
Forest plot of mortality outcome data for the desmoteplase and placebo groups.
3.5.3. Asymptomatic intracerebral hemorrhage
AICH data were reported in all 6 RCTs.[8–13] There were 385 cases of AICH observed: 227 of patients treated with desmoteplase (n = 595; 38.2%) and 158 of placebo-treated patients (n = 467; 33.8%). Intravenous desmoteplase showed a tendency to increase in the occurrence of AICH in AIS patients compared with placebo (OR 1.25; 95% CI, 0.97–1.62; I2 = 9%; P = .09; Fig. 4).
Figure 4.
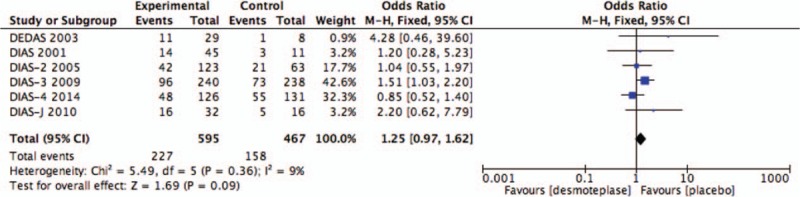
Forest plot of asymptomatic intracranial hemorrhage outcome data for the desmoteplase and placebo groups for comparison.
3.6. Efficacy
3.6.1. Good clinical outcome at 90 days
Five RCTs evaluated the rate of good clinical outcome at 90 days.[8,9,11–13] Good clinical outcome was seen within 90 days in 41.3% (230/557) of the treatment groups, and 38.0% (170/447) of placebo-treated patients. There was no significant difference between patients receiving placebo and desmoteplase-treated patients in this outcome measure (OR 1.14; 95% CI, 0.88–1.49; P = .31; Fig. 5). Significant heterogeneity was not detected (I2 = 0%).
Figure 5.
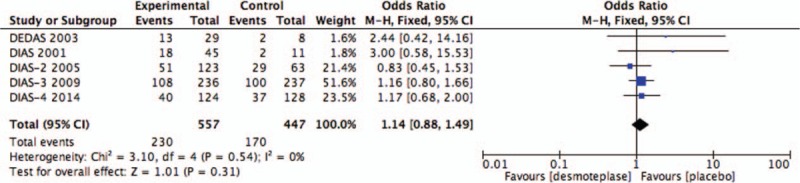
Forest plot of good clinical outcome at 90 days outcome data for the desmoteplase and placebo groups for comparison.
3.6.2. Reperfusion
Five of the included studies analyzed rates of reperfusion 4 to 24 hours after treatment.[8–11,13] Reperfusion 4 to 8 hours after treatment occurred in 143 of 302 in the treatment groups (47.4%), and in 90 of 240 placebo-treated patients (37.5%). Desmoteplase showed a significant increase in the occurrence of reperfusion between the desmoteplase and placebo groups (OR 1.57; 95% CI, 1.10–2.24; P = .01 vs control; Fig. 6). No significant heterogeneity was found (I2 = 0%).
Figure 6.
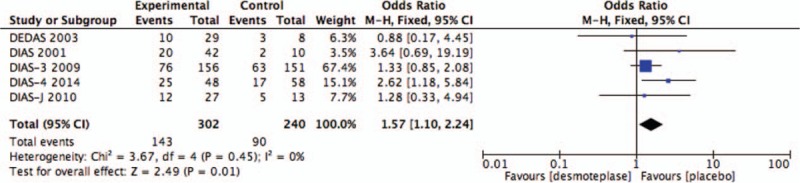
Forest plot of reperfusion outcome data for the desmoteplase and placebo groups for comparison.
3.6.3. Subgroup analysis
As before, subgroup analysis of the 3 groups was based upon the desmoteplase dose for comparison: <90, 90, and 125 μg/kg. Desmoteplase therapy was associated with a high occurrence of reperfusion when the data were restricted to the subgroup of patients who were treated with a desmoteplase dose of 90 μg/kg (OR 1.53; 95% CI, 1.07–2.21; P = .02; Fig. 11) or 125 μg/kg (OR 4.07; 95% CI, 1.16–14.24; P = .03; Fig. 11). Also, we found 90 μg/kg subgroup showed a tendency to increased AICH compared with placebo-treated patients (OR 1.25; 95% CI, 0.95–1.63; P = .11; Fig. 9). There was no difference between placebo-treated and desmoteplase-treated patients in other outcomes (Figs. 7–11).
Figure 11.
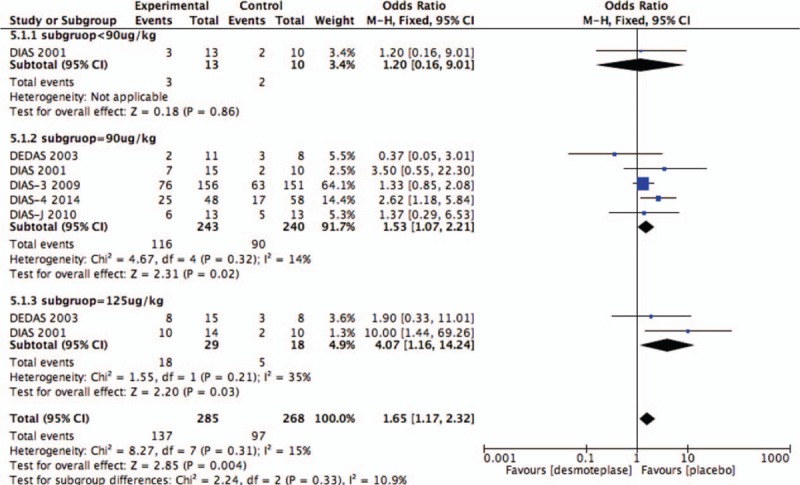
Forest plot of reperfusion outcome data for the desmoteplase subgroup and placebo group.
Figure 9.
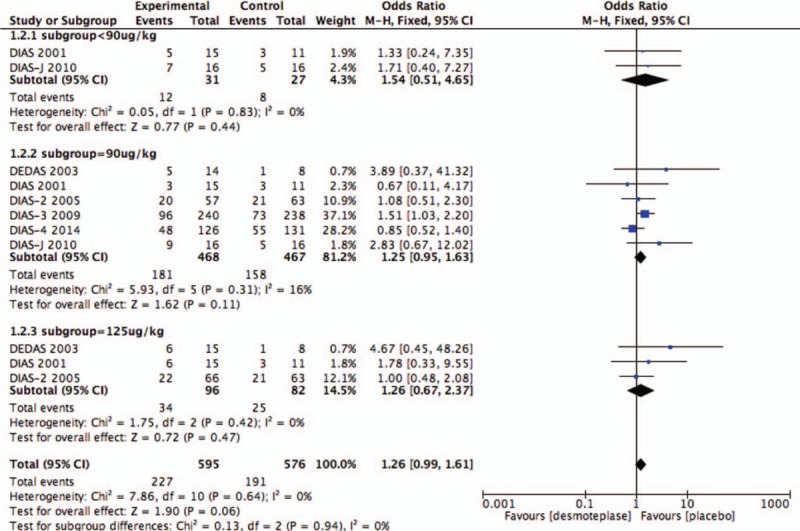
Forest plot of asymptomatic intracranial hemorrhage outcome data for the desmoteplase subgroup and placebo group.
Figure 7.
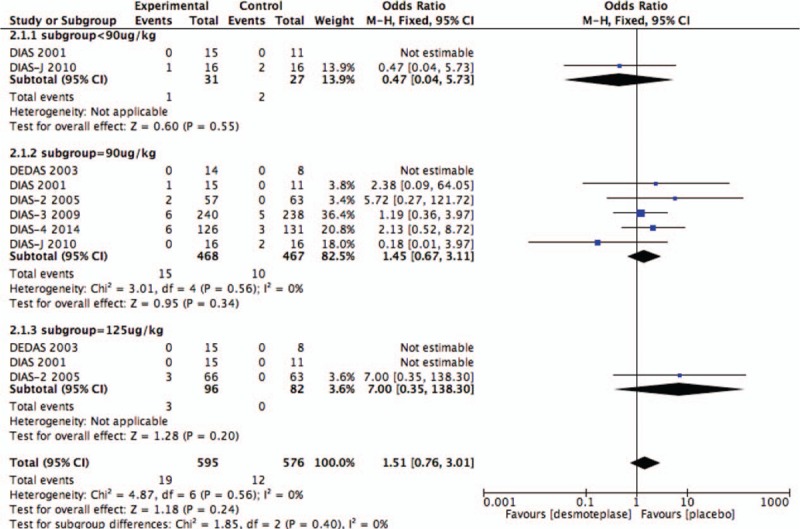
Forest plot of symptomatic intracranial hemorrhage outcome data for the desmoteplase subgroups and placebo group.
Figure 8.
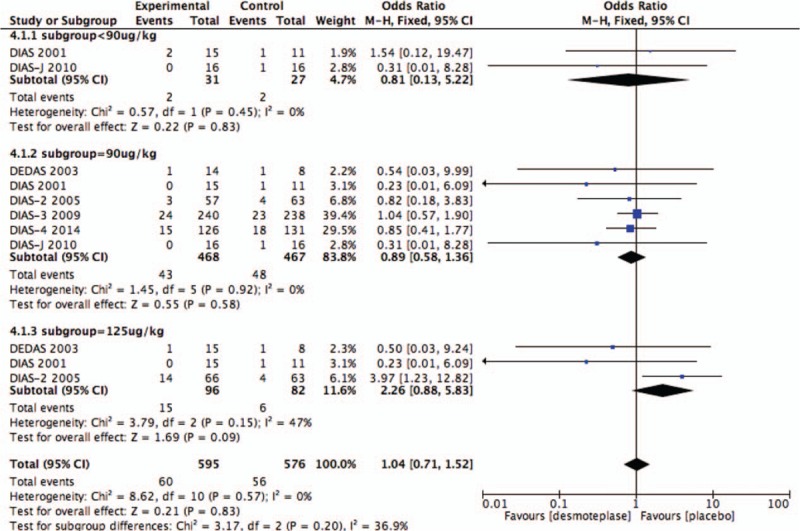
Forest plot of morality outcome data for the desmoteplase subgroups and placebo group.
Figure 10.
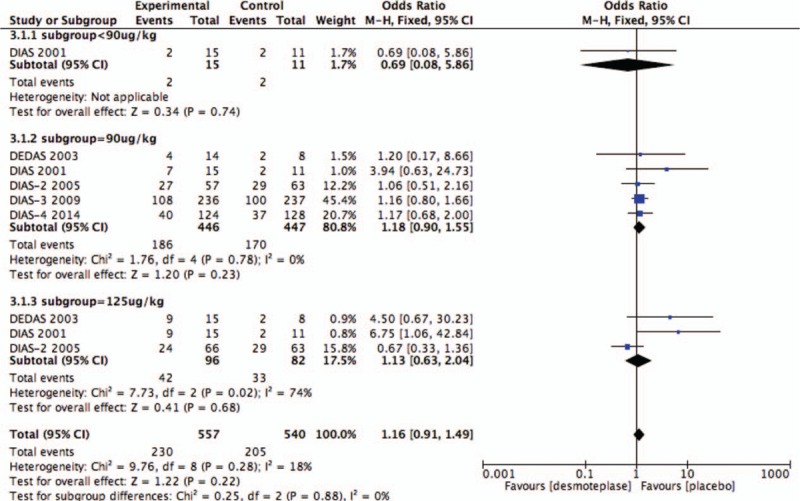
Forest plot of good clinical outcome at 90 days outcome data for the desmoteplase subgroup and placebo group.
4. Discussion
Thrombolysis with intravenous r-tPA is currently the only treatment that has proven efficacy and is approved for AIS patients. However, due to its narrow therapeutic time window, only 3.4% to 5.2% of all AIS patients in the United States receive r-tPA.[17] For this reason, desmoteplase, a new drug which may prolong the therapeutic time window, needs investigation. AICH is defined as an intracranial hemorrhage that does not meet the criteria for SICH or as computed tomography (CT)-documented hemorrhage without clinical evidence of neurological deterioration.[12,18] Study found that AICH is a marker of early successful recanalization that can rescue salvageable brain tissue.[19] However, others think that bleeding events after thrombolysis include either SICH or AICH.[12] In this meta-analysis, desmoteplase showed a tendency to increase AICH compared with placebo in total or subgroup analysis. Furthermore, analysis found an increased reperfusion rate in desmoteplase-treated AIS patients. Some studies also found that reperfusion was associated with good clinical outcome at 90 days.[11,20] However, from the result of this meat-analysis, the end point of good clinical outcome at 90 days was not significantly different between desmoteplase and placebo groups. A possible reason for this is the limited data available for meta-analysis. Thus, we believe that desmoteplase has efficacy for AIS patients 3 hours postsymptom onset.
SICH is the major cause of early death in patients treated with intravenous thrombolysis.[2] Larger hematomas and SICH are important types of hemorrhagic transformation that will increase risk of early neurological deterioration or even mortality at 90 days.[21,22] SICH includes parenchymal hemorrhage, hemorrhagic infarction, or hemorrhagic transformation detected by CT that do not meet scoring criteria for SICH.[23] SICH and mortality, as two safety end points of this meta-analysis, were not significantly increased in the combined doses desmoteplase group or in the desmoteplase subgroups. Therefore, we can conclude that desmoteplase has a favorable safety profile.
Desmoteplase has many pharmacologic advantages over r-tPA,[24] so it was thought that desmoteplase might be associated with good clinical AIS outcomes and expand the therapeutic time window for patients. Although DIAS and DEDAS (randomized, placebo-controlled, and double-blind studies) demonstrated safety and potential efficacy for the use of desmoteplase in stroke patients, DIAS-2 and DIAS-3 did not find desmoteplase to be efficacious.
The results of this meta-analysis show that desmoteplase did not improve the 90-day clinical outcome in patients with AIS. The DIAS-3 study which had the largest diffusion mismatch volume found that the larger the mismatch volume, the more favorable the clinical outcome after recanalization[13]; however, the number of patients in DIAS-3 was almost a half of the total number in this meta-analysis, and the differences in arterial recanalization were modest. The fact that the mismatch volumes were small in this meta-analysis might explain the lack of improved 90-day outcome with desmoteplase. Another study found that patients with severe stenosis or proximal vessel occlusion were ideal candidates for thrombolytic therapy.[25] Also, a different study[26] found that larger core lesion volume was related to higher NIHSS scores, when the penumbral tissue was greater than 60 mL, the clinical effect of desmoteplase was significantly better compared with placebo. This suggests that desmoteplase is more beneficial in patients with greater volumes of penumbral tissue. DIAS-2 showed that there are several factors that affect the performance of desmoteplase, including NIHSS score, mismatch lesion volumes, study sample size, and the location and degree of vascular occlusion.[12]
The concept of penumbra varied among the included trials.[8–13] Past studies[8–10,12] selected participants with a diffusion–perfusion magnetic resonance imaging and then with a perfusion CT, with the evidence of perfusion CT mismatch can help guide thrombolysis decisions up to 6 hours after AIS onset.[27] However, the DIAS-3 and DIAS-4 studies,[11,13] which included more than a half of the patients in this meta-analysis, only used a simpler imaging selection method of CT or diffusion weighted imaging to define acute ischemic injury as hypodensity,[28] and then some patients with disappearance of penumbra would also be chosen.[20,29]
The results of DIAS-2, DIAS-3, and DIAS-4 studies were not consistent with those of DIAS and DEDAS, indicating that more RCTs are needed. Nevertheless, this meta-analysis does indicate that desmoteplase is a very promising drug that should be further studied, AIS patients with greater amounts of penumbral tissue are more likely to benefit from its use,[30] and the dose of desmoteplase between 90 and 125 μg/kg should be attempted.[20]
This meta-analysis has several limitations. First, the enrollment period of included studies ranged from 2001 to 2014, and the inclusion criteria and outcome standards were changed (Table 1). These changes may have resulted in different outcomes for intravenous thrombolysis and increased potential bias. Second, the doses of desmoteplase used in the included trials differed. We subgrouped patients based on these doses, but we do not know if we chose the best classification strategy. Third, the search terms and strategies were in English, which restricted the meta-analysis to English language trials. Therefore, although we did not find trials published online or elsewhere in other languages, there may be publication bias.
Strengths of our systematic review include the fact that in the included trials we are all double-blind, multicenter, parallel-group, randomized trials. This suggests that the results of this systematic review are reliable and accurate.
5. Conclusion
Our study suggests that treatment with desmoteplase in patients beyond 3 hours after symptom onset has a favorable safety and encouraging efficacy profile compared with placebo. This statement is based on the findings that desmoteplase did not increase SICH or mortality but increased the rate of reperfusion and showed a tendency to increase AICH. However, due to the small number of patients and the different definitions of penumbra in the included patient cohorts, our conclusions should be confirmed by more RCTs.
Supplementary Material
Footnotes
Abbreviations: AICH = asymptomatic intracranial hemorrhage, AIS = acute ischemic stroke, BI = Barthel index, CT = computed tomography, DWI = diffusion weighted imaging, MRA = magnetic resonance angiography, MRI = magnetic resonance imaging, mRS = the Modified Rankin Scale, NIHSS = National Institutes of Health stroke scale, r-tPA = recombinant tissue-type plasminogen activator, SICH = symptomatic intracranial hemorrhage.
Ethics approval: none.
Funding/support: This research was supported by the National Natural Science Foundation of China (no. 81100898) and the Natural Science Foundation of Guangdong Province (no. 2014A030313681).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1]. GBD, 2015 Mortality, Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016;388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014;384:1929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bishop BM. Endovascular interventions for acute ischemic stroke: a review of recent trials. Ann Pharmacother 2016;50:219–28. [DOI] [PubMed] [Google Scholar]
- [4].Medcalf RL. Desmoteplase: discovery, insights and opportunities for ischaemic stroke. Br J Pharmacol 2012;165:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ellis V, Daniels M, Misra R, et al. Plasminogen activation is stimulated by prion protein and regulated in a copper-dependent manner. Biochemistry 2002;41:6891–6. [DOI] [PubMed] [Google Scholar]
- [6].Kingston IB, Castro MJ, Anderson S. In vitro stimulation of tissue-type plasminogen activator by Alzheimer amyloid beta-peptide analogues. Nat Med 1995;1:138–42. [DOI] [PubMed] [Google Scholar]
- [7].Liberatore GT, Samson A, Bladin C, et al. Vampire bat salivary plasminogen activator (desmoteplase): a unique fibrinolytic enzyme that does not promote neurodegeneration. Stroke 2003;34:537–43. [DOI] [PubMed] [Google Scholar]
- [8].Hacke W, Albers G, Al-Rawi Y, et al. The Desmoteplase in Acute Ischemic Stroke Trial (DIAS): a phase II MRI-based 9-hour window acute stroke thrombolysis trial with intravenous desmoteplase. Stroke 2005;36:66–73. [DOI] [PubMed] [Google Scholar]
- [9].Furlan AJ, Eyding D, Albers GW, et al. Dose Escalation of Desmoteplase for Acute Ischemic Stroke (DEDAS): evidence of safety and efficacy 3 to 9 hours after stroke onset. Stroke 2006;37:1227–31. [DOI] [PubMed] [Google Scholar]
- [10].Mori E, Minematsu K, Nakagawara J, et al. Safety and tolerability of desmoteplase within 3 to 9 hours after symptoms onset in Japanese patients with ischemic stroke. Stroke 2015;46:2549–54. [DOI] [PubMed] [Google Scholar]
- [11].von Kummer R, Mori E, Truelsen T, et al. Desmoteplase 3 to 9 hours after major artery occlusion stroke: the DIAS-4 trial (efficacy and safety study of desmoteplase to treat acute ischemic stroke). Stroke 2016;47:2880–7. [DOI] [PubMed] [Google Scholar]
- [12].Hacke W, Furlan AJ, Al-Rawi Y, et al. Intravenous desmoteplase in patients with acute ischaemic stroke selected by MRI perfusion-diffusion weighted imaging or perfusion CT (DIAS-2): a prospective, randomised, double-blind, placebo-controlled study. Lancet Neurol 2009;8:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Albers GW, von Kummer R, Truelsen T, et al. Safety and efficacy of desmoteplase given 3–9 h after ischaemic stroke in patients with occlusion or high-grade stenosis in major cerebral arteries (DIAS-3): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet Neurol 2015;14:575–84. [DOI] [PubMed] [Google Scholar]
- [14].Lundh A, Gøtzsche PC. Recommendations by Cochrane Review Groups for assessment of the risk of bias in studies. BMC Med Res Methodol 2008;8:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mishra NK, Albers GW, Davis SM, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke 2010;41:e25–33. [DOI] [PubMed] [Google Scholar]
- [16].Mazighi M, Meseguer E, Labreuche J, et al. Bridging therapy in acute ischemic stroke: a systematic review and meta-analysis. Stroke 2012;43:1302–8. [DOI] [PubMed] [Google Scholar]
- [17].Islam MN, Kuddus R, Chowdhury NS, et al. Radiologic evaluation of hyperacute brain infarction: a review. Mymensingh Med J 2014;23:621–35. [PubMed] [Google Scholar]
- [18].Tanne D, Kasner SE, Demchuk AM, et al. Markers of increased risk of intracerebral hemorrhage after intravenous recombinant tissue plasminogen activator therapy for acute ischemic stroke in clinical practice: the Multicenter rt-PA Stroke Survey. Circulation 2002;105:1679–85. [DOI] [PubMed] [Google Scholar]
- [19].Molina CA, Alvarez-Sabín J, Montaner J, et al. Thrombolysis-related hemorrhagic infarction: a marker of early reperfusion, reduced infarct size, and improved outcome in patients with proximal middle cerebral artery occlusion. Stroke 2002;33:1551–6. [DOI] [PubMed] [Google Scholar]
- [20].Elmaraezy A, Abushouk AI, Saad S, et al. Desmoteplase for acute ischemic stroke: a systematic review and meta-analysis of RCTs. CNS Neurol Disord Drug Targets 2016;Dec 13. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [21].Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999;30:2280–4. [DOI] [PubMed] [Google Scholar]
- [22].Marsh EB, Llinas RH, Schneider ALC, et al. Predicting hemorrhagic transformation of acute ischemic stroke: prospective validation of the HeRS Score. Medicine 2016;95:e2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].LaMonte MP, Nash ML, Wang DZ, et al. Argatroban anticoagulation in patients with acute ischemic stroke (ARGIS-1): a randomized, placebo-controlled safety study. Stroke 2004;35:1677–82. [DOI] [PubMed] [Google Scholar]
- [24].Dafer RM, Biller J. Desmoteplase in the treatment of acute ischemic stroke. Expert Rev Neurother 2007;7:333–7. [DOI] [PubMed] [Google Scholar]
- [25].Fiebach JB, Al-Rawi Y, Wintermark M, et al. Vascular occlusion enables selecting acute ischemic stroke patients for treatment with desmoteplase. Stroke 2012;43:1561–6. [DOI] [PubMed] [Google Scholar]
- [26].Warach S, Al-Rawi Y, Furlan AJ, et al. Refinement of the magnetic resonance diffusion-perfusion mismatch concept for thrombolytic patient selection: insights from the desmoteplase in acute stroke trials. Stroke 2012;43:2313–8. [DOI] [PubMed] [Google Scholar]
- [27].Sztriha LK, Manawadu D, Jarosz J, et al. Safety and clinical outcome of thrombolysis in ischaemic stroke using a perfusion CT mismatch between 3 and 6 hours. PLoS One 2011;6:e25796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hill MD, Menon BK. Desmoteplase for late treatment of stroke: still in the dark. Lancet Neurol 2015;14:560–1. [DOI] [PubMed] [Google Scholar]
- [29].Shi L, Liang F, Li Y, et al. Desmoteplase for acute ischemic stroke within 3 to 9 hours after symptom onset: evidence from randomized controlled trials. Sci Rep 2016;6:33989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].De Georgia M. Extending the time window for stroke treatment: advanced brain imaging ± bat saliva. Int J Stroke Off J Int Stroke Soc 2009;4:94–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


