Abstract
This study aims to evaluate the discriminative and predictive capacity of the Fracture Risk Assessment Tool (FRAX) to determine the 10-year risk of osteoporotic fracture in Chinese rheumatoid arthritis (RA) patients.
This study included 168 RA patients and 168 healthy individuals as controls. The Chinese mainland FRAX model was applied to calculate the 10-year risk of osteoporotic fractures, defined as fracture of the spine, forearm, hip, or shoulder.
The incidence of osteoporosis was significantly increased in RA patients compared to controls (P < .05). Bone mineral density (BMD), lumbar vertebra T-score, and femoral neck T-score were significantly lower in RA patients compared to controls (P < .05). BMD, disease duration, DAS28, and glucocorticoid use were important risk factors for osteoporotic fractures in Chinese RA patients. Ten-year osteoporotic fracture risk in Chinese RA patients was higher when BMD was incorporated in FRAX.
There was a higher incidence of osteoporosis and reduced BMD in RA patients compared to controls. The FRAX model should integrate femoral neck BMD with other risk factors to evaluate osteoporotic fracture risk in RA patients, making it a valuable screening tool.
Keywords: Fracture Risk Assessment Tool, osteoporosis, rheumatoid arthritis
1. Introduction
Rheumatoid arthritis (RA) is a chronic autoimmune disorder that manifests as symmetrical, progressive, and erosive arthritis.[1] RA patients have an elevated incidence of reduced bone mass and osteoporosis due to active systemic inflammation and corticosteroid use. As a consequence, RA patients have a high risk of fracture, an outcome that can lead to impaired quality of life.[2,3]
In recent years, research has focused on the identification of risk factors associated with osteoporotic fractures in RA patients, including RA disease duration, lack of physical activity, smoking, and glucocorticoid use.[4] In particular, reduced bone mineral density (BMD) is a risk factor for osteoporosis in patients with RA, including those who do not receive oral glucocorticoids.[5,6] Currently, clinical diagnosis and assessment of osteoporosis is often made based on BMD evaluation using dual X-ray absorptiometry (DXA). BMD is commonly assessed by DXA in most RA patients with obvious clinical symptoms or secondary osteoporosis. However, other key risk factors for osteoporosis are not included when considering the probability of osteoporosis and osteoporotic fractures in this patient population.[4]
Since the Fracture Risk Assessment Tool (FRAX) was developed by the World Health Organization (WHO) in February 2008, it has been widely used for treatment decision making in osteoporosis. This Web-based algorithm has been incorporated into DXA reporting software to calculate the 10-year probability of major osteoporotic fracture of the vertebral column, hip, forearm, and humerus, and the 10-year probability of hip fracture in men and women based on easily obtained clinical risk factors and BMD of the femoral neck (optional). Algorithms that evaluate the probability of fractures and produce absolute risk estimates have been developed in various settings, and these are becoming widely available. FRAX is one of the most common FRAXs. FRAX incorporates demographic and clinical risk factors such as body mass index (BMI), and previous fracture to evaluate the 10-year risk of fractures and predict mortality.[7,8] FRAX has been used to assess the risk of fractures in postmenopausal women, and various country-specific FRAX models have been developed.[9] However, to the authors’ knowledge, FRAX has not been used to investigate risk factors or predict osteoporotic fracture risk in Chinese RA patients.
This study investigated the value of the Chinese-specific FRAX model as an FRAX in Chinese RA patients, and the role of osteoporotic-specific risk factors when evaluating the 10-year risk of osteoporotic fractures in this patient population.
2. Methods
2.1. Study population
This retrospective study included patients treated in our department for RA between 2012 and 2014. Patients who were ≥28 years old and diagnosed with RA were included. The diagnosis of RA was made in accordance with the 1987 American College of Rheumatology revised RA classification criteria. Exclusion criteria were: type I diabetes, osteogenesis imperfecta, untreated long-term hyperthyroidism, hypogonadism, premature menopause (≤45 years of age), chronic malnutrition, malabsorption, or chronic liver or kidney disease.
A control group consisted of healthy individuals who visited the outpatient department of our hospital medical center. Patients with serious liver and kidney diseases, metabolic bone disease, and those on medication were excluded. The Ethics Committee of our hospital approved this study, and all patients gave their written informed consent.
2.2. Data collection
Demographic and clinical characteristics, including age, height, weight, BMI, smoking history, alcohol use, history of fragility fracture, family history of fragility fracture, BMD, lumbar vertebra (L1-L4) T-score, femoral neck T-score, DAS28, and oral glucocorticoid use (time and dosage), were recorded for all study participants.
Blood samples were collected from all study participants in the morning and analyzed for erythrocyte sedimentation rate (ESR), rheumatoid factor (RF), C-reactive protein (CRP), and anticyclic citrullinated peptide (anti-CCP).
2.3. Bone densitometry
The BMD of L1-L4 and the femoral neck were measured utilizing DXA (GE Lunar Prodigy, Madison, WI). BMD (g/cm2) and T-scores were calculated. Outcomes were categorized according to WHO diagnostic criteria,[5] where: T > −1.0 is considered normal; T > −2.5−≤ −1.0 is considered reduced bone mass; and T ≤ −2.5 is considered osteoporosis.
2.4. Chinese-specific FRAX model
The Chinese mainland FRAX model (http://www.shef.ac.uk/FRAX/) was used in this study. The model uses clinical data to calculate the 10-year risk of fractures in Chinese individuals.
2.5. Statistical analysis
Data were analyzed by 2 independent investigators using SPSS v19.0 software (SPSS Inc., USA). All data are expressed as mean ± SD. Students t test or chi-square test was used to evaluate differences between groups. Correlations between variables were analyzed using Spearman correlation analysis. Receiver-operating characteristic curve and area under the curve were used to evaluate the 10-year risk of osteoporotic fractures in RA patients according to FRAX with or without BMD as a risk factor. P < .05 was considered statistically significant.
3. Results
3.1. Baseline characteristics
This study included 168 RA patients, of which 23.2% (39) were male and 76.8% (129) were female. Average age was 54.3 ± 8.7 years (range, 28–80 years), and mean disease duration was 12.2 ± 9.5 years (range, 2–23 years). The control group included 168 participants, of which 27.38% (48) were male and 72.62% (122) were female. There were no significant differences in the baseline demographic and clinical characteristics between RA patients and controls (Table 1).
Table 1.
Baseline demographic and clinical characteristics in RA patients and participants in the control group.
3.2. BMD and T-score
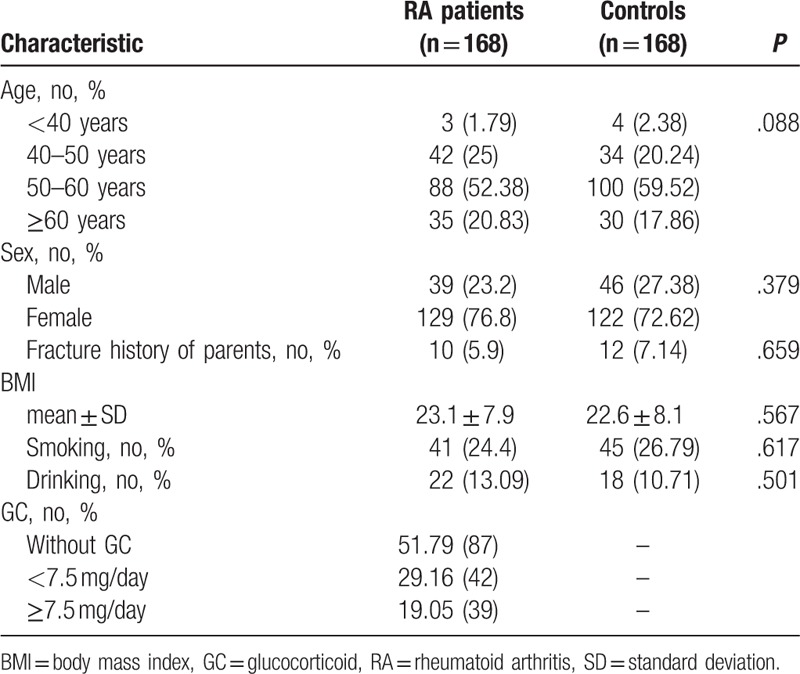
In RA patients, the incidence of osteoporosis was 41.1%; 43 cases were classified as normal, 56 cases were classified as reduced bone mass, and 69 cases were classified as osteoporosis.
In controls, the incidence of osteoporosis was 21.43%; 103 cases were classified as normal, 29 cases were classified as reduced bone mass, and 36 cases were classified as osteoporosis.
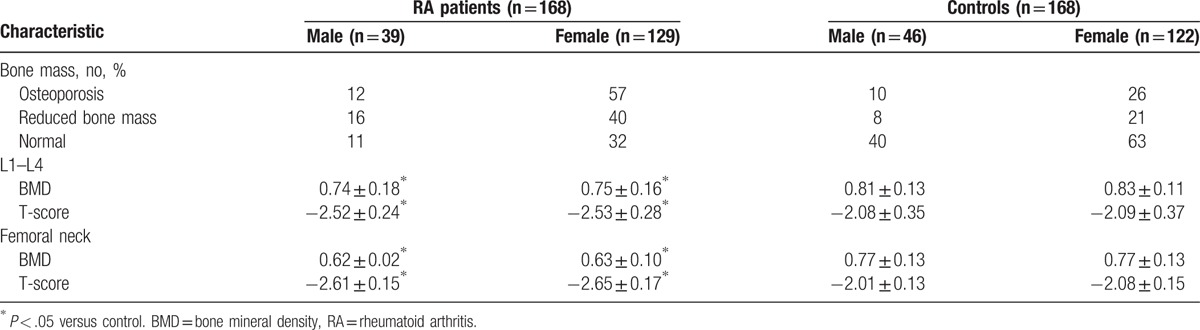
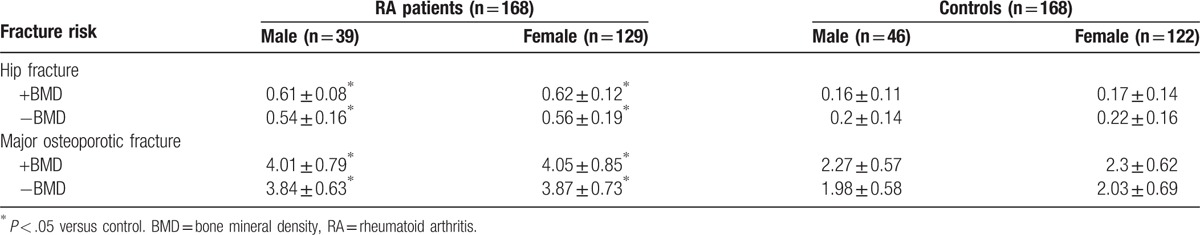
The incidence of osteoporosis was significantly higher in RA patients compared to controls (P < .05). The BMD and T-score of L1–L4 and the femoral neck were significantly lower in RA patients compared to controls (P < .05) (Table 2). Analysis of patients stratified by gender showed a higher incidence of osteoporosis and a lower BMD and T-score of L1–L4 in male and female RA patients compared to controls (P < .05, Table 3).
Table 2.
BMD and T-score of L1–L4 and the femoral neck in RA patients and controls.
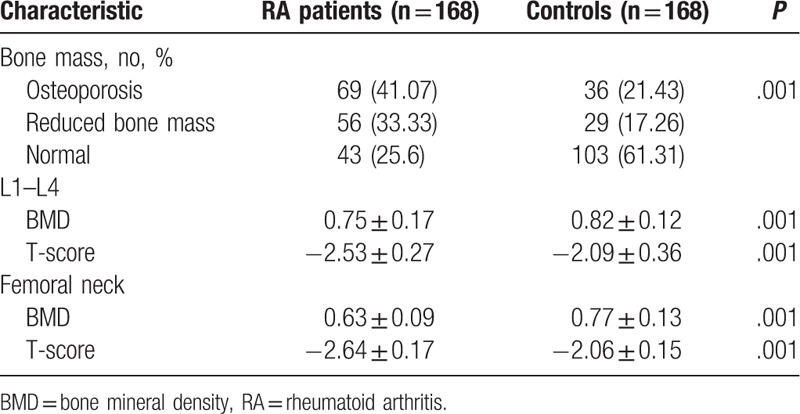
Table 3.
BMD and T-score of L1–L4 and the femoral neck in male and female patients and controls.
3.3. Ten-year risk of osteoporotic fractures
The 10-year risk of hip fracture was (0.62 ± 0.11)% in RA patients and (0.17 ± 0.13)% in controls when BMD of the femoral neck was incorporated in FRAX. The 10-year risk of hip fracture was (0.56 ± 0.18)% in RA patients and (0.21 ± 0.61)% in controls when BMD of the femoral neck was not incorporated in FRAX. The 10-year risk of hip fracture in RA patients was significantly higher when BMD of the femoral neck was incorporated in FRAX compared with and without BMD (Table 4). Similar findings were found in male and female RA patients (Table 5).
Table 4.
The 10-year risk of osteoporotic fracture in RA patients and controls.
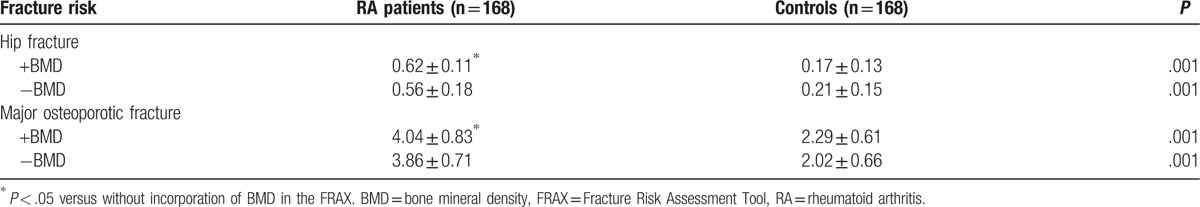
Table 5.
The 10-year risk of osteoporotic fracture in male and female RA patients and controls.
The 10-year risk of major osteoporotic fracture was (4.04 ± 0.83)% in RA patients and (2.29 ± 0.61)% in controls when BMD was incorporated in FRAX. The 10-year risk of major osteoporotic fracture was (3.86 ± 0.71)% in RA patients and (2.02 ± 0.66)% in controls when BMD was not incorporated in FRAX. The 10-year risk of major osteroporosis in RA patients was significantly higher when BMD of the femoral neck was incorporated in FRAX compared with and without BMD (Table 4). Similar findings were found in male and female RA patients (Table 5).
Receiver-operating characteristic curve analysis showed that the area under the curve for 10-year risk of major osteoporotic fracture was 70.2% in RA patients when BMD was incorporated in FRAX and 68.2% when BMD was not incorporated in FRAX (Fig. 1).
Figure 1.
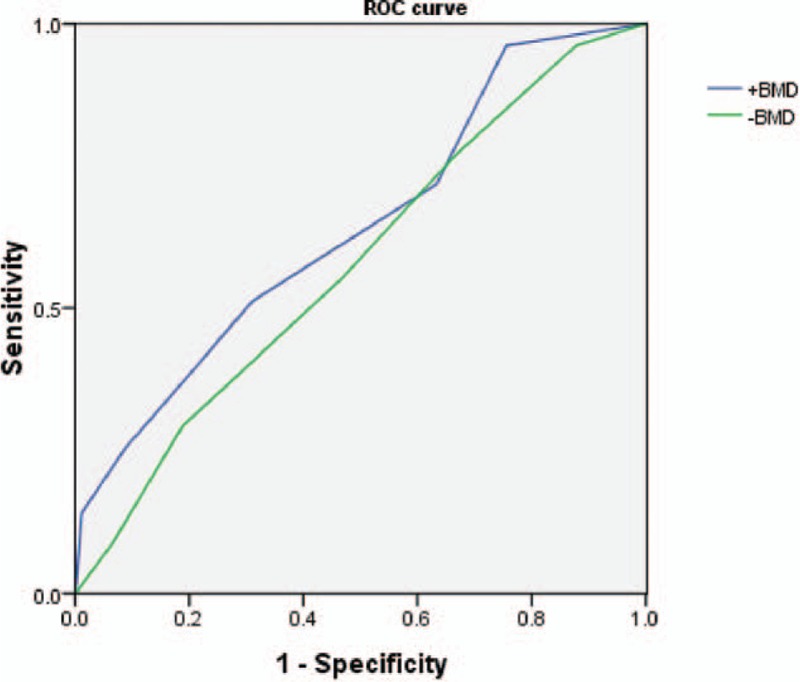
Receiver-operating characteristic (ROC) curve for 10-year risk of major osteoporotic fracture.
3.4. Risk factor analysis
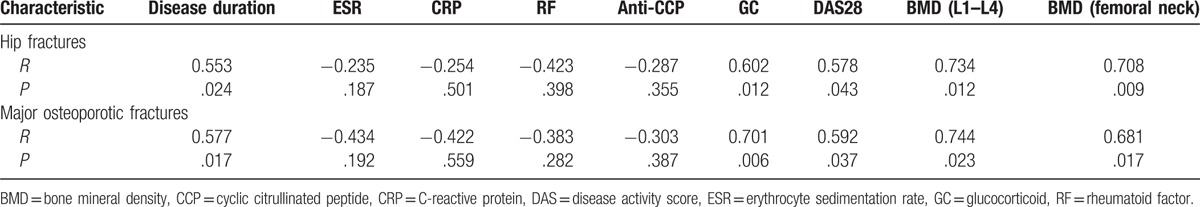
The risk of hip fractures was negatively correlated with the BMD of L1–L4 (r = 0.734, P < .05) and femoral neck (r = 0.708, P < .01), and positively correlated with disease duration (r = 0.553, P < .05), DAS28 (r = 0.578, P < .05), and GC (r = 0.602, P < .05). No obvious correlation was found between ESR, CRP, RF, anti-CCP, and hip fractures. Similar results were identified in osteoporotic fractures (Table 6).
Table 6.
Risk factor analysis.
4. Discussion
This study evaluated the 10-year risk of osteoporotic fractures in Chinese RA patients and healthy individuals using the Chinese mainland FRAX model. Results showed that the BMD and T-score were lower and the 10-year risk of osteoporotic fractures was higher in Chinese RA patients compared with healthy individuals. BMD, disease duration, DAS28, and glucocorticoid use were important risk factors for hip and major osteoporotic fractures in Chinese RA patients, and the 10-year risk of osteoporotic fractures in Chinese RA patients was higher when BMD was incorporated in the FRAX model. In accordance with our findings, previous studies show that the rate of osteoporosis and the incidence of osteoporotic fractures are significantly increased in RA patients compared to the general population.[3,6,10–14]
The systemic effects of rheumatoid inflammation, immobility, nutritional problems, and weight loss are reported to increase bone resorption and contribute to osteoporosis in RA patients.[15,16] We used FRAX, which provides an easily applied and clinically relevant method for estimating the individual risk of fractures in RA patients. FRAX includes data routinely recorded in medical records, thus providing general practitioners the potential to use FRAX to evaluate the risk of fractures in RA patients in primary care. Currently, BMD is a major index for osteoporosis diagnosis. However, secondary osteoporosis in RA patients is considered a multifactorial disease. Therefore, BMD in combination with other risk factors should be used to predict the risk of fractures in patients with secondary osteoporosis. It is widely believed that treatments for osteoporosis are only effective in patients with reduced BMD. Thus, the use of FRAX without BMD to identify individuals is the subject of some debate and is a research topic of considerable interest. In fact, Kanis et al[17] found that including BMD of the femoral neck in the FRAX model when calculating the probability of fracture facilitates the identification of patients who are most likely to require clinical intervention. In this study, we found that the Chinese mainland FRAX model that incorporated BMD of the femoral neck was superior for evaluating 10-year risk of osteoporotic fractures in Chinese patients with RA compared to the model without BMD. Similarly, Fraser et al[18] reported that the Canadian FRAX model that incorporated BMD of the femoral neck was superior for predicting osteroporotic fracture in a Canadian population compared to the model without BMD; the authors concluded that the Canadian FRAX model that includes BMD of the femoral neck can be used to identify osteoporotic patients who require treatment. Furthermore, evidence suggests that the Spanish FRAX model that does not include BMD underestimates the risk of osteroporotic fracture in Spanish populations.[19] Although several studies have reported that the use of FRAX for evaluating the risk of fracture in patients with inflammatory arthritis,[20,21] to our knowledge, this is the first study to use the Chinese-mainland FRAX model to evaluate fracture risk in Chinese patients with RA. In accordance with previous findings, our study showed that the risk of osteoporotic fractures in Chinese RA patients was highest with the FRAX model that included BMD.
The current study identified BMD, disease duration, DAS28, and glucocorticoid use as important risk factors for osteoporotic fractures in Chinese RA. These data are in accordance with previously published reports.[21–25] In particular, 3 months of oral glucocorticoids and glucocorticoid ≥7.5 mg/day is associated with an increase in fracture risk. Long-term use of glucocorticoids can inhibit the intestinal absorption of calcium and promote calcium excretion, leading to lower serum calcium and bone resorption. Glucocorticoids can inhibit collagen synthesis and mineralization by osteoblasts and increase the activity of osteoclasts.[26,27]
This study was associated with several limitations. First, the data were obtained retrospectively from a relative small patient population who were admitted to our department, which is located in a single-center. The evaluation of the small sample size and unmatched groups can introduce bias. Well-designed prospective studies are required to confirm our findings. Second, the follow-up duration was short. Third, the 10-year probability of fractures in RA patients was evaluated while pharmacological interventions were used. Bisphosphonates and selective estrogen receptor modulators reduce osteoporotic fractures. Therefore, the probability of fractures in RA patients receiving pharmacotherapies may differ from untreated patients.
5. Conclusion
In summary, this study found a higher incidence of osteoporosis and reduced BMD in RA patients compared to healthy individuals. The FRAX model should include risk factors for osteoporotic fractures such as BMD, disease duration and activity, and glucocorticoid use when assessing the probability of fractures in RA patients.
Footnotes
Abbreviations: BMD = bone mineral density, DXA = dual X-ray absorptiometry, FRAX = Fracture Risk Assessment Tool.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:1094–108. [DOI] [PubMed] [Google Scholar]
- [2].Hoes JN, Bultink IE, Lems WF. Management of osteoporosis in rheumatoid arthritis patients. Expert Opin Pharmacother 2015;16:559–71. [DOI] [PubMed] [Google Scholar]
- [3].Nampei A, Hashimoto J, Koyanagi J, et al. Characteristics of fracture and related factors in patients with rheumatoid arthritis. Mod Rheumatol 2008;18:170–6. [DOI] [PubMed] [Google Scholar]
- [4].Wen L, Kang JH, Yim YR, et al. Risk factors for treatment failure in osteoporotic patients with rheumatoid arthritis. Mod Rheumatol 2016;26:194–9. [DOI] [PubMed] [Google Scholar]
- [5].Kanis JA, Melton LJ, 3rd, Christiansen C, et al. The diagnosis of osteoporosis. J Bone Miner Res 1994;9:1137–41. [DOI] [PubMed] [Google Scholar]
- [6].van Staa TP, Geusens P, Bijlsma JW, et al. Clinical assessment of the long-term risk of fracture in patients with rheumatoid arthritis. Arthritis Rheum 2006;54:3104–12. [DOI] [PubMed] [Google Scholar]
- [7].Bauer DC. FRAX, falls, and fracture prediction: predicting the future. Arch Intern Med 2011;171:1661–2. [DOI] [PubMed] [Google Scholar]
- [8].O’Connor MB, Rathi J, Bond U, et al. The advent of FRAX. Ir Med J 2010;103:251. [PubMed] [Google Scholar]
- [9].Murariu RV, Macovei LA, Brujbu IC. Osteoporosis in rheumatoid arthritis. Rev Med Chir Soc Med Nat Iasi 2013;117:394–403. [PubMed] [Google Scholar]
- [10].Furuya T, Kotake S, Inoue E, et al. Risk factors associated with incident fractures in Japanese men with rheumatoid arthritis: a prospective observational cohort study. J Bone Miner Metab 2008;26:499–505. [DOI] [PubMed] [Google Scholar]
- [11].Gilboe IM, Kvien TK, Haugeberg G, et al. Bone mineral density in systemic lupus erythematosus: comparison with rheumatoid arthritis and healthy controls. Ann Rheum Dis 2000;59:110–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim SY, Schneeweiss S, Liu J, et al. Risk of osteoporotic fracture in a large population-based cohort of patients with rheumatoid arthritis. Arthritis Res Ther 2010;12:R154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McCloskey E, Kanis JA. FRAX updates 2012. Curr Opin Rheumatol 2012;24:554–60. [DOI] [PubMed] [Google Scholar]
- [14].Sinigaglia L, Varenna M, Girasole G, et al. Epidemiology of osteoporosis in rheumatic diseases. Rheum Dis Clin North Am 2006;32:631–58. [DOI] [PubMed] [Google Scholar]
- [15].Heidari B, Hassanjani Roushan MR. Rheumatoid arthritis and osteoporosis. Caspian J Intern Med 2012;3:445–6. [PMC free article] [PubMed] [Google Scholar]
- [16].Vosse D, de Vlam K. Osteoporosis in rheumatoid arthritis and ankylosing spondylitis. Clin Exp Rheumatol 2009;27(4 Suppl 55):S62–7. [PubMed] [Google Scholar]
- [17].Kanis JA, McCloskey E, Johansson H, et al. FRAX((R)) with and without bone mineral density. Calcif Tissue Int 2012;90:1–3. [DOI] [PubMed] [Google Scholar]
- [18].Fraser LA, Langsetmo L, Berger C, et al. CaMos Research Group. Fracture prediction and calibration of a Canadian FRAX® tool: a population-based report from CaMos. Osteoporos Int 2011;22:829–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].González-Macías J, Marin F, Vila J, et al. Probability of fractures predicted by FRAX® and observed incidence in the Spanish ECOSAP Study cohort. Bone 2012;50:373–7. [DOI] [PubMed] [Google Scholar]
- [20].Klop C, de Vries F, Bijlsma JW, et al. Predicting the 10-year risk of hip and major osteoporotic fracture in rheumatoid arthritis and in the general population: an independent validation and update of UK FRAX without bone mineral density. Ann Rheum Dis 2016;75:2095–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee JH, Suh YS, Koh JH, et al. The risk of osteoporotic fractures according to the FRAX model in Korean patients with rheumatoid arthritis. J Korean Med Sci 2014;29:1082–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sivas F, Barca N, Onder M, et al. The relation between joint erosion and generalized osteoporosis and disease activity in patients with rheumatoid arthritis. Rheumatol Int 2006;26:896–9. [DOI] [PubMed] [Google Scholar]
- [23].Celiker R, Gokce-Kutsal Y, Cindas A, et al. Osteoporosis in rheumatoid arthritis: effect of disease activity. Clin Rheumatol 1995;14:429–33. [DOI] [PubMed] [Google Scholar]
- [24].Haugeberg G, Strand A, Kvien TK, et al. Reduced loss of hand bone density with prednisolone in early rheumatoid arthritis: results from a randomized placebo-controlled trial. Arch Intern Med 2005;165:1293–7. [DOI] [PubMed] [Google Scholar]
- [25].Shibuya K, Hagino H, Morio Y, et al. Cross-sectional and longitudinal study of osteoporosis in patients with rheumatoid arthritis. Clin Rheumatol 2002;21:150–8. [DOI] [PubMed] [Google Scholar]
- [26].Rizzoli R, Adachi JD, Cooper C, et al. Management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2012;91:225–43. [DOI] [PubMed] [Google Scholar]
- [27].Pereira RM, Carvalho JF, Canalis E. Glucocorticoid-induced osteoporosis in rheumatic diseases. Clinics (Sao Paulo) 2010;65:1197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]


