Abstract
Cerebrospinal fluid (CSF) analysis is the most important tool for assessing central nervous system (CNS) disease. An elevated CSF leukocyte count rarely provides the final diagnosis, but is almost always an indicator of inflammation within the CNS.
The present study investigated the variety of diseases associated with CSF pleocytosis.
CSF analyses were identified through the biochemical database used in the capital region of Denmark in the period from 2003 to 2010. In patients >15 years, clinical diagnoses associated with the finding of a CSF leukocyte count >10 × 106 cells/L were obtained from discharge records and patient files.
A total of 1058 CSF samples from 1054 patients were included in the analysis. The median age was 50 (interquartile range: 36–67) and 53% were male. Eighty-one different diagnoses were identified in 1058 cases with an elevated CSF leukocyte count, besides unknown causes. Infections were the most common cause of CSF pleocytosis (61.4%) followed by miscellaneous causes (12.7%), vascular (9.7%), neurodegenerative (7%), neoplastic (5%), and inflammatory conditions (4.2%). Only infections presented with leukocyte counts >10,000 × 106/L. Infections represented 82.6% of all cases with a leukocyte count >100 × 106/L whereas 56.3% of cases with at leukocyte counts <100 × 106/L were dominated by disease not related to infection.
The present study may serve as a reminder to clinicians of what diseases and disease categories to suspect when patients present with CSF biochemistry indicating CNS inflammation.
Keywords: central nervous system infections, central nervous system pleocytosis, diagnostics
1. Introduction
Any means which will facilitate the difficult diagnosis of diseases of the central nervous system is of value, and the cerebrospinal fluid, which bathes its deepest recesses and washes the very nerve cells and fibers themselves, is in truth a mirror which reflects every change taking place in that system.[1]
The value of analyzing the cerebrospinal fluid (CSF) was recognized even before these words were written almost a hundred years ago, and has not diminished since then despite major advances in diagnostics. In particular, the presence and number of leukocytes in the CSF has been a major criterion in the diagnosis of diseases of the central nervous system (CNS).[2]
CSF analysis is a high-priority analysis upon suspicion of CNS disease. A variety of biomarkers indicating degenerative neurological disease are available, these are not specific and can merely serve as a guide to the clinical evaluation. Thus, clinician in the initial evaluation must rely on “basic” CSF biochemistry. An elevated CSF leukocyte count rarely provides the final diagnosis, but may indicate an inflammatory process within the CNS and is essential in guiding subsequent decision making and management. In addition to this, the level of CSF protein, CSF/blood albumin ratio, and glucose provide important information on indices of blood–brain barrier breakdown and the presence of glucose consuming cells/organisms.
Recruitment of leukocytes to the CSF is mediated by the release of cytokines and chemokines that attract leukocytes and open up the endothelial barrier, allowing leukocyte chemotaxis. The number of processes, cell types, and molecules identified as being involved in cellular recruitment is increasing and occurs in a broad spectrum of diseases covering infectious, inflammatory (autoimmune), metabolic, and malignant diseases.[3]
Insight into the biochemical profile of the CSF in different diseases should be useful knowledge to the clinical decision-making regarding treatment and supplemental diagnostics. To the best of our knowledge no such overview is available in the published literature. In this study, we therefore aimed to document the variety of conditions that may cause or be associated with CSF indices of neuroinflammation.
2. Methods and materials
2.1. Design, setting, and population
This retrospective study is based on the collection of all CSF cell counts and additional biochemistry from 2 hospitals from 2003 to 2010 and from an additional 3 hospitals from 2008 to 2010. All hospitals were located within the Capital Region of Denmark.
2.2. Inclusion criteria
CSF samples with a leukocyte count >10 × 106 cells/L from patients older than 15 years of age were included in the study.
2.3. Exclusion criteria
CSF samples with an elevated CSF count due to blood contamination; resampling in the same patient within 90 days of the primary sample; samples related to a neurosurgical procedure; and samples where patient records are missing were excluded from the study.
2.4. Data collection and variables
All CNS analyses in the study period were retrieved from the biochemistry databases LABKA I and LABKA II (CSC Scandihealth, Aarhus, Denmark). Data on CSF leukocyte counts were extracted automatically from the biochemistry databases. Samples with a CSF leukocyte count <10 × 106 leukocytes/L were excluded. Three medical doctors (GBE, KLK, and CTB) reviewed all patient files for a discharge diagnosis according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision from the World Health Organization. The 3 medical doctors (GBE, KLK, and CTB) evaluated all cases to ensure that the diagnosis was in agreement with information from the patient files and discharge records. All data were then reviewed and verified by a fourth medical doctor (GE). Cases of CNS infections diagnosed with encephalitis or viral meningitis but with positive bacterial CSF culture and CSF biochemistry in accordance with the diagnosis were corrected and vice versa. If a diagnosis related to the finding of CSF leukocytosis was not registered, and no cause for this was available from the patient records we registered the diagnosis as “unknown.”
CSF cell count was determined automatically. For each CSF sample proportion of neutrophils (%), protein (g/L), and glucose (mmol/L) level as well as the patients’ age and sex were recorded. Data on biochemical, microbiological, and radiological data were collected manually by the authors.
2.5. Diagnosis categories
The diagnoses were a priori divided into 6 groups based on the proposed pathogenesis of the disease: infectious, inflammatory, neurodegenerative, neoplastic, vascular, and miscellaneous. Since infections were the most common cause of CSF leukocytosis, a subgroup analysis was performed dividing infectious causes into the following groups: acute bacterial, chronic bacterial, fungal, and viral CNS infections and inflammation secondary to an infectious focus outside the CNS (extra-CNS).
2.6. Statistics
Descriptive data are reported as counts and percentage, and as medians and range. Information on missing data is provided in the tables. Predictive associations between CSF leukocyte count and a diagnosis of bacterial meningitis (BM) were analyzed using receiver operating characteristics (ROC). Youden J statistic/index (J = Sensitivity + (Specificity − 1)) was calculated in order to select a cut-off for maximizing classification accuracy.[4] Data were analyzed using SAS Enterprse Guide version 7.1 (SAS Institute A/S, Carey, NC) and we used SigmaPlot version 12.5 (Systat Software, San Jose, CA) for plotting the ROC curve.
2.7. Ethics and consent
The study was approved by the Danish Health and Medicines Authority (record no. 3-3013-135/1/) and by the Danish Data Protection Agency (record no. 01706 hvh-2012-016). Danish legislation does not require informed consent for register-based studies or ethical approval.
3. Results
A total of 8878 CSF analyses were reviewed. Of these, 7819 were excluded; 6248 due to fewer than 10 × 106 leukocytes/L, 697 due to age <16 years: 451 due to resampling within 90 days, 205 due to lack of leukocyte count analysis, 94 of samples was related to a neurosurgical procedure, 67 sampled were duplicates; and 28 with unavailable patient records: 22 due to blood contamination and 8 due to mislabeling.
A total of 1058 CSF samples from 1054 patients were included in the analysis. The median age was 50 (interquartile range: 36–67) and 53% were male. Eighty-one different diagnoses were related to an elevated CSF leukocyte count, apart from unknown causes (Table 1). Viral meningitis was the most prevalent cause (18.6%), followed by BM (16.7%), Lyme neuroborreliosis (8.5%), demyelinating disease (6.3%), and stroke (4.9%).
Table 1.
Causes of cerebrospinal fluid pleocytosis and associated cell counts.

3.1. Demographic data and supplemental CSF biochemistry
Data on patient age, sex, CSF leukocyte differential counts, protein levels, and glucose levels are presented in Table 2.
Table 2.
Cerebrospinal fluid analysis characteristics according to disease categories.

3.2. Disease categories
The distribution between the 6 disease categories is shown in Table 3. Infections were the most common cause of CSF pleocytosis (61.3%) followed by miscellaneous causes (12.7%), vascular (9.7%), neurodegenerative (7%), neoplastic (5%), and inflammatory causes (4.2%). Only infections presented with CSF leukocyte counts >10,000 × 106/L and infections were also the most frequent cause of disease associated with a CSF leukocyte count >100 × 106/L, representing 82.6%. Among cases with CSF leukocyte counts <100 × 106/L infections constituted 43.3% of the diagnoses.
Table 3.
Distribution leukocyte count levels in cerebrospinal fluid according to disease categories.
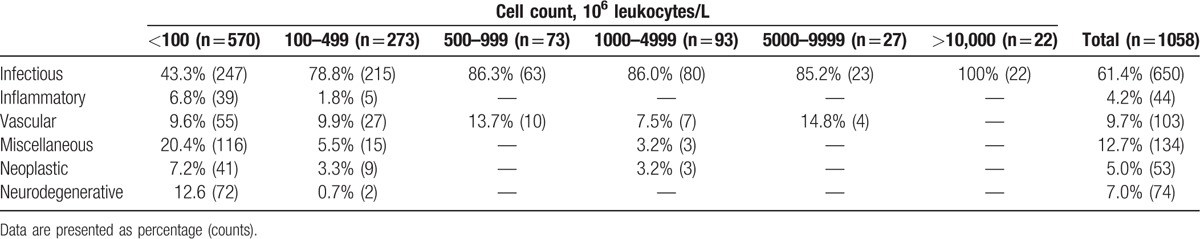
Eighty-one cases (7.7%) presented with CSF pleocytosis due to infections with a focus outside the CNS such as pneumonia, endocarditis, and septicemia. No microorganisms were detected and the samples assumed to represent sterile inflammation secondary to other infection.
In 32 cases (3%), no diagnosis was established. These cases were labeled “unknown.”
3.3. Infections
Infectious causes of CSF pleocytosis were further divided into the following groups: acute bacterial, chronic bacterial, fungal, and viral CNS infections and inflammation secondary to an infectious focus outside the CNS (extra-CNS). Only 2 cases of parasitic CNS infections were identified and they were not included in this context. Data on CSF biochemistry, age, and sex for these groups are presented in Table 4.
Table 4.
Cerebrospinal fluid analysis characteristics according to infectious categories.

Since BM is arguably the most urgently treatable cause of CNS inflammation, we evaluated cut-offs for CSF leukocyte count to discriminate between BM and other CNS disease. We performed an ROC analysis estimating the probability of BM versus all other causes (Fig. 1) and versus other infectious causes only (Fig. 2).
Figure 1.
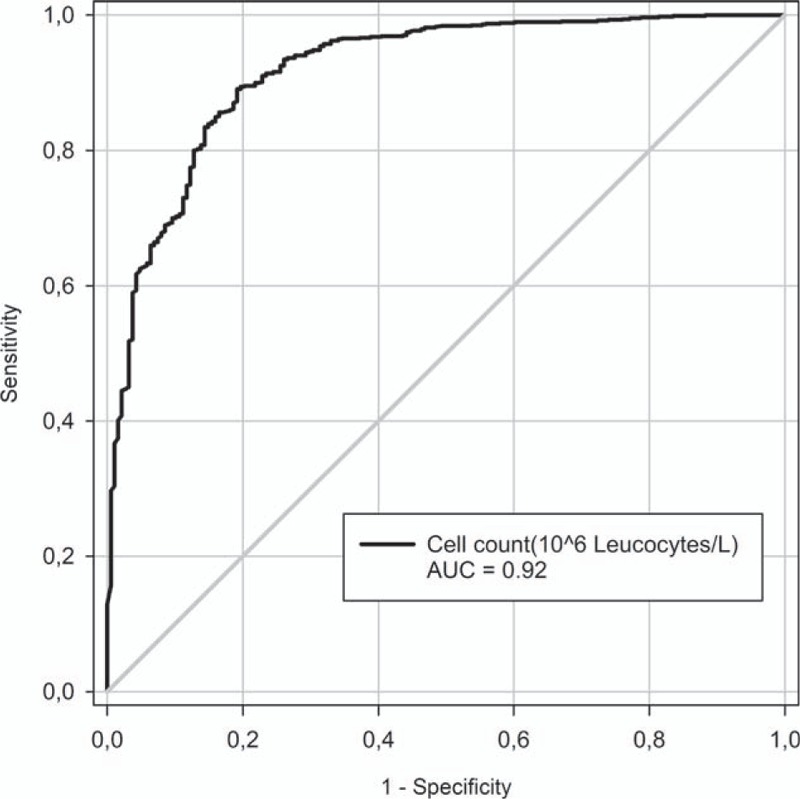
Probability of bacterial meningitis versus all other diagnoses by receiver operating curve.
Figure 2.
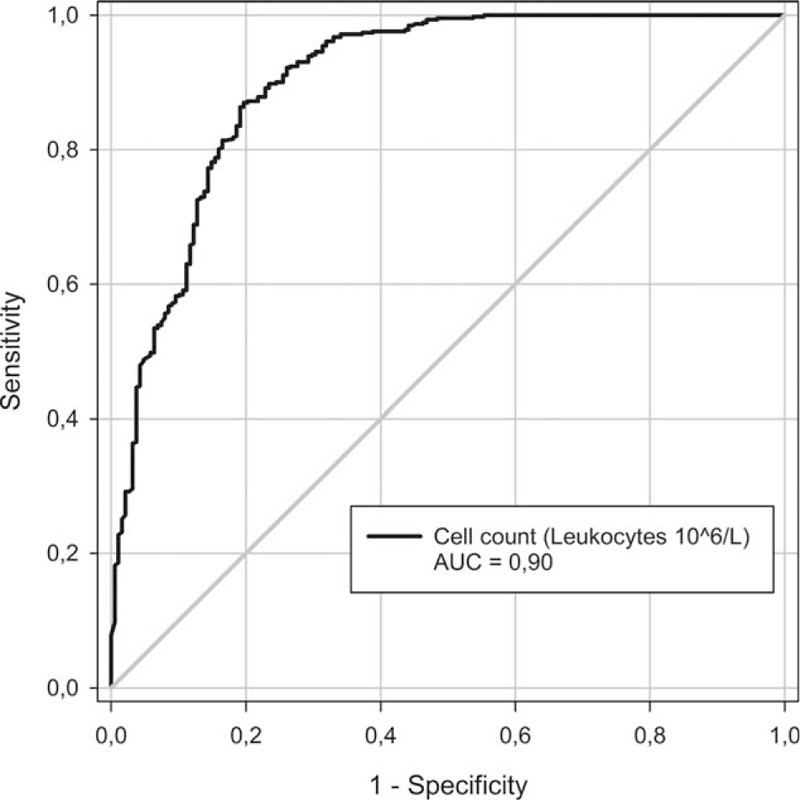
Probability of bacterial meningitis versus other infectious diagnoses by receiver operating curve.
The first ROC curve (Fig. 1) yielded an area under the curve (AUC) of 0.92. Calculating the Youden J index suggested a leukocyte count of 414 as cut-off for diagnosing bacterial infection versus all other causes and it resulted in a sensitivity of 81%, a specificity of 89%, a positive predictive value off 62%, and a negative predictive value off 96% (Table 5).
Table 5.
Probability of bacterial meningitis.

The second ROC curve (Fig. 2) yielded an AUC of 0.90. Calculating the Youden J index suggested a leukocyte count of 421 as cut-off for diagnosing bacterial infection versus other infectious causes and it resulted in a sensitivity of 80%, a specificity of 86%, a positive predictive value off 71%, and a negative predictive value off 92% (Table 5).
We further investigated 3 different cut-offs for CSF leukocyte count: ≥100, ≥500, and ≥1000, to discriminate BM from all other causes and from other infectious causes, see Table 5. We found that sensitivity decreased and specificity increased with increasing CSF leukocyte count, as expected.
4. Discussion
The presented data illustrate the variety of diseases that may be associated with an inflammatory process within the CNS. To our knowledge, no recent studies have presented data related to the causes of CNS inflammation on such a large number of cases.
Infections presented with the highest CSF leukocyte counts and were also the most frequent cause of CSF pleocytosis, representing 61.4% of cases. Among patients presenting with CSF leukocyte counts below 100 × 106/L, however, the causes were more heterogeneous and noninfectious causes represented 56.7% of cases.
Our finding of a considerable number of patients with sterile CSF inflammation secondary to systemic infections such as septicemia or pneumonia is not surprising since these infections may precipitate BM. Thus, these cases may represent a continuum toward the group of patients with culture negative meningitis although not treated as such. Among children, it is a well-described phenomenon that urinary tract infections may also present with sterile CSF pleocytosis.[5] Also a number of patients did not receive a diagnosis related to the presence of CSF inflammation. This group labeled “unknown” may represent a group of patient without CNS inflammatory disease albeit with CSF biochemistry differing from the normal population—thus normal variation.
Based on the CSF leukocyte count alone, which is often the most quickly obtainable result, we calculated that CSF leukocyte counts below 414 × 106/L had a high specificity (89%) and negative predictive value (96%) for BM in this population. These results were largely unaffected when performed against all included diagnoses compared to the selection of infectious conditions only. Determining the “optimal cut-off” for diagnosis BM requires weighting the pros and cons. BM is a serious disease with mortality up to 30%[6] and failing to make a rapid and correct diagnosis might have fatal consequences. The cut-off suggested by Youdens index results in an NPV of 96%. Most clinicians might find this inadequate, as 4% would be left undiagnosed. Lowering the cut-off to 100 would improve the NPV with 2%, but reduce the PPV with nearly 30%. The reduced PPV could lead to inappropriate antibiotic use, which in this case would be of little concern due to the limited number of cases. Although the high AUC of ≥0.90, the cell count cannot alone confirm or discharge the diagnosis of BM. As shown by Hasbun et al,[7] predictive scores defined to safely identify urgently treatable CNS disease need also to include the clinical situation, the presentation of the patient and further biochemical results.
Our cut-offs determined by Youden (414 and 421 × 106 leukocytes/L) are in agreement with previous studies in children, albeit our cut-offs were significantly lower, and we reached a similar specificity without including any other patient data.[8,9] The lower CSF leukocyte threshold is likely due to our adult population.
A number of limitations should be acknowledged due to the retrospective collection of data and diagnoses obtained from discharge records. We did, however, attempt to minimize these weaknesses by reviewing the records twice and drawing other clinical and diagnostic data into consideration where available. A recent Danish study including also our population specifically evaluated the validity of the diagnosis herpes simplex encephalitis in the Danish National Patient Registry and found that this diagnosis could only be confirmed in ∼51% of cases based on strict inclusion criteria and another ∼8% considered to be probable cases, meaning more than 40% were incorrectly diagnosed.[10] The diagnostic confusion primarily involves the diagnosis of viral meningitis and the continuum toward viral encephalitis, but also culture negative BM may be difficult to define.[6]
Many of the patients presenting with an elevated leukocyte count would most likely have received initial treatment for a suspected infection prior to receiving the final diagnosis. Thus, the number of infectious conditions may be underestimated. Also, it is well known that a few cases of severe CNS infections, that is, BM may present without CSF inflammation but often with positive microscopy. These patients presenting with <10 (×106/L) CSF leukocytes are not included in the presented data. Even though likely to be few, we cannot for certain exclude that they would affect our data.
New diagnostics and improved methodologies could increase the number of correct diagnoses. Interestingly, we did not find any cases receiving a final diagnosis of autoimmune encephalitis, although 2 cases were suspected as having a postinfectious autoimmune condition and paramalignant limbic encephalitis, respectively. These diagnoses were not confirmed. Although autoimmune brain disease does not necessarily present with pleocytosis, the lack of cases in our data set could imply that this disease has received focus only in the most recent years and our data may include misdiagnosed cases.
Even though this study is not a guideline to diagnostics of CNS diseases, we believe that our data may serve as a reminder to clinicians of what diseases and disease categories to suspect when patients present with CSF biochemistry indicating CNS inflammation.
Acknowledgment
The authors wish to thank Annette Farre for assisting in retrieving CSF analyses from the biochemistry databases LABKA I and LABKA II.
Footnotes
Abbreviations: AUC = area under the curve, BM = bacterial meningitis, CNS = central nervous system, CSF = cerebrospinal fluid, ROC = receiver operating characteristic.
Joined first authorship between Gertrud Baunbæk Egelund and Gideon Ertner.
CTB has received funding from the OTICON foundation for the studies of “cochlear damage in central nervous system infections.” This funding is not related to the present. No conflicts of interest and no other disclosures.
TLB is the Assistant Editor of the Danish Medical Journal and reports personal fees from Bristol Myers Squibb, personal fees from Gilead, personal fees from Bristol Myers Squibb, personal fees from Bristol Myers Squibb, personal fees from GSK, nonfinancial support from Bristol Myers Squibb, nonfinancial support from Gilead, personal fees from Abbvie, outside the submitted work. The remaining authors have no conflicts of interest to disclose.
References
- [1].Boyd W. Physiology and Pathology of the Cerebrospinal Fluid. New York, NY: The Macmillan Company; 1920. [Google Scholar]
- [2].Lundie A, Thomas DJ, Fleming S. Cerebro-spinal meningitis: diagnosis and prophylaxis is lumbar puncture justifiable? Br Med J 1915;1:628–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fishman R. Cerebrospinal Fluid in Diseases of the Nervous System. 2nd ed.Philadelphia, PA: WB Saunders; 1992. [Google Scholar]
- [4].Youden WJ. Index for rating diagnostic tests. Cancer 1950;3:32–5. [DOI] [PubMed] [Google Scholar]
- [5].Syrogiannopoulos GA, Grivea IN, Anastassiou ED, et al. Sterile cerebrospinal fluid pleocytosis in young infants with urinary tract infection. Pediatr Infect Dis J 2001;20:927–30. [DOI] [PubMed] [Google Scholar]
- [6].Baunbaek-Knudsen G, Solling M, Farre A, et al. Improved outcome of bacterial meningitis associated with use of corticosteroid treatment. Infect Dis (Lond) 2016;48:281–6. [DOI] [PubMed] [Google Scholar]
- [7].Hasbun R, Bijlsma M, Brouwer MC, et al. Risk score for identifying adults with CSF pleocytosis and negative CSF Gram stain at low risk for an urgent treatable cause. J Infect 2013;67:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nigrovic LE, Kuppermann N, Malley R. Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics 2002;110:712–9. [DOI] [PubMed] [Google Scholar]
- [9].Bonsu BK, Ortega HW, Marcon MJ, et al. A decision rule for predicting bacterial meningitis in children with cerebrospinal fluid pleocytosis when gram stain is negative or unavailable. Acad Emerg Med 2008;15:437–44. [DOI] [PubMed] [Google Scholar]
- [10].Jorgensen LK, Dalgaard LS, Ostergaard LJ, et al. Validity of the coding for herpes simplex encephalitis in the Danish National Patient Registry. Clin Epidemiol 2016;8:133–40. [DOI] [PMC free article] [PubMed] [Google Scholar]


