Abstract
Background:
The aim of this meta-analysis was to investigate the prenatal, perinatal, and postnatal risk factors for children autism.
Methods:
PubMed, Embase, Web of Science were used to search for studies that examined the prenatal, perinatal, and postnatal risk factors for children autism. A fixed-effects model or random-effects model was used to pool the overall effect estimates.
Results:
Data from 37,634 autistic children and 12,081,416 nonautistic children enrolled in 17 studies were collated. During the prenatal period, the factors associated with autism risk were maternal and paternal age≥35 years, mother's and father's race: White and Asian, gestational hypertension, gestational diabetes, maternal and paternal education college graduate+, threatened abortion, and antepartum hemorrhage. During perinatal period, the factors associated with autism risk were caesarian delivery, gestational age≤36 weeks, parity≥4, spontaneous labor, induced labor, no labor, breech presentation, preeclampsia, and fetal distress. During the postnatal period, the factors associated with autism risk were low birth weight, postpartum hemorrhage, male gender, and brain anomaly. Parity≥4 and female were associated with a decreased risk of autism. In addition, exposure to cigarette smoking, urinary infection, mother's and father's race: Black and Hispanic, mother's country of birth outside Europe and North America, umbilical cord around neck, premature membrane rupture, 5-minutes Apgar score<7, and respiratory infection were not associated with increased risk of autism.
Conclusion:
The present meta-analysis confirmed the relation between some prenatal, perinatal, and postnatal factors with autism. All these factors were examined individually, thus it was still unclear that whether these factors are causal or play a secondary role in the development of autism. Further studies are needed to verify our findings, and investigate the effects of multiple factors on autism, rather than the single factor.
Keywords: autism, children, perinatal, postnatal, prenatal, risk factors
1. Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition, which is characterized by cognitive, behavioral, and social dysfunction. Their onset occurs in early childhood and often results in severe lifelong impairments. ASD is currently regarded as one of the most common childhood morbidities, presenting in various degrees of severity.[1] According to the latest report, the global prevalence of autism has been estimated to be at 0.62%.[2]
The etiology of autism is poorly understood. ASD is multifactorial disease, and both genetic and environmental factors are believed to account for its development.[3] A recent study reported that 35% to 40% of autism could be explained by the genetic factors.[4,5] The remaining 60% to 65% are likely to be resulted from other factors, such as prenatal, perinatal, and postnatal environmental factors.[6,7]
Several studies have investigated the relationship between prenatal, perinatal and postnatal factors, and autism.[8] And their results showed that advanced maternal/paternal age, short gestation age, gestational hypertension, threatened abortion, caesarian delivery prematurity, low birth weight (LBW), and low Apgar score were associated with increased risk of autism in more than 1 study.[9–14] However, no single factor was consistently reported to be a positive factor for autism among these studies. These inconsistent results may be explained by the variations in methodologies, such as case definition, comparison groups, race and region, sample size, and exposure assessment methods. These variations have significant impact on the prevalence of autism, as well as the investigation of risk factors for autism.[15] Thus, a meta-analysis is needed to pool the inconsistent data from these studies and figure out which are the significant factors for autism.
Brasic et al[16] conducted a meta-analysis based on case-control studies to identify the obstetric factors for autism. However, only 2 studies of the 156 articles met the inclusion criteria, and these 2 studies had inconsistent results.[11,17] In addition, Kolevzon et al[8] also performed a similar meta-analysis with different sets of criteria. And 7 studies were included, 4 of which were prospective, population-based cohort studies, and the others were retrospective.[8] Three of the included studies had partially overlapping sample. The authors suggested that advanced parental age, maternal birth place outside of North America and Europe, LBW, and preterm delivery were significantly increased factors for autism.[8] Guinchat et al[18] published a systematic review and meta-analysis in 2011. Although the authors included studies with a full scope of prenatal, perinatal, and postnatal factors, they did not provide the magnitude of effect estimates for these factors.[18] Thus, we have reviewed the previously published studies, and conducted this meta-analysis to identify the relationship between prenatal, perinatal, and postnatal factors and autism, and figure out the magnitude of the effect estimates.
2. Materials and methods
Sine this study is a meta-analysis of previously published studies, the ethical approval and patient consent are not required.
This study was conducted and reported in adherence to Preferred Reporting Items for Systematic Reviews and Meta-analysis.[19]
2.1. Literature search
PubMed, Embase, and Web of Science were systematically searched for articles published up to October 12, 2016. No language or date restriction was imposed. The search algorithm was generated as follows: ((“child”[MeSH Terms] OR “child”[All Fields] OR “children”[All Fields]) AND (“autistic disorder”[MeSH Terms] OR (“autistic”[All Fields] AND “disorder”[All Fields]) OR “autistic disorder”[All Fields] OR “autism”[All Fields])) AND ((“prenatal care”[MeSH Terms] OR (“prenatal”[All Fields] AND “care”[All Fields]) OR “prenatal care”[All Fields] OR “prenatal”[All Fields]) OR perinatal [All Fields] OR postnatal [All Fields]). The last search was conducted on December 10, 2016.
Two investigators independently performed the initial search, deleted duplicate records, reviewed the title/abstracts, and determined as excluded or requiring further assessment. Then we screened the full-text articles for inclusion. We also searched the reference lists of those included studies to identify potential articles that may not be indexed in the common databases.
2.2. Study selection
Studies meeting the following inclusion criteria were included: study design: case-control or cohort study; population: children diagnosed with autism; autism diagnostic criteria: Diagnostic Statistical Manual of Mental Disorders (DSM)-Fourth, or Fifth edition; International Classification of Diseases (ICD), Ninth, or Tenth Revision; CARS, Child Autistic Rating Scale; comparison group description: matching criteria, sibling control subjects, healthy versus abnormal control subjects; model of reporting: parental report or medical record review, or study physician assessment; outcomes: risk factors for autism (including prenatal, perinatal, postnatal factors), and the exposures among case and control subjects. Studies were excluded from the final analysis if their content was limited to reviews, letters, case reports, conference abstracts, health education, or they focused on adult autism, or they did not provide data of our interest.
2.3. Data extraction and quality assessment
Two investigators independently performed the data extraction. A standardized data collection form was built to extract the following information: name of the first author, year of publication, country, study design, number of participants, the risk factors for autism. When multiple publications were from the same population, we only included the article with the latest or most information. The disagreements between 2 investigators were resolved by discussion and consensus.
We used the modified Newcastle–Ottawa scale to assess the quality of observational studies.[20] The scale consists of 3 items in reporting of participants selection, comparability of the autistic and nonautistic children, and outcome assessment.[20] The total quality scale was 9 points. Articles with ≥6 points were considered to be of high quality.
2.4. Statistical analysis
All the outcomes were regarded as dichotomous variables; thus they were expressed as risk ratio (RR) with 95% confidence intervals (95% CIs). Before the data were synthesized, we used the Cochrane Q χ2 test and I2 statistic to test the heterogeneity across studies, in which P < .1 or I2 >50% was considered to have significant heterogeneity.[21] The DerSimonian–Laird method with random-effects model[22] was used to calculate the pooled RRs with 95% CIs when the heterogeneity was identified; otherwise, a fixed-effects model (Mantel–Haenszel method) was used to pool the estimates.[23] We also conducted subgroup analysis according to case definition, study design, and country. Publications bias was evaluated by the Begg[24] and Egger[25] test. A P value less than.05 was judged as statistically significant. All analyses were performed by using STATA version 12.0 (Stata Corporation, College Station, TX).
3. Results
3.1. Study identification and selection
The initial search yielded 2849 publications, and 1537 were excluded for duplicate records. After screening the tile/abstracts and full-text information, 1268 and 27 were excluded, respectively. Finally, 17 studies that met the inclusion criteria were included in this meta-analysis.[26–42] The selection flow chart is shown in Fig. 1.
Figure 1.
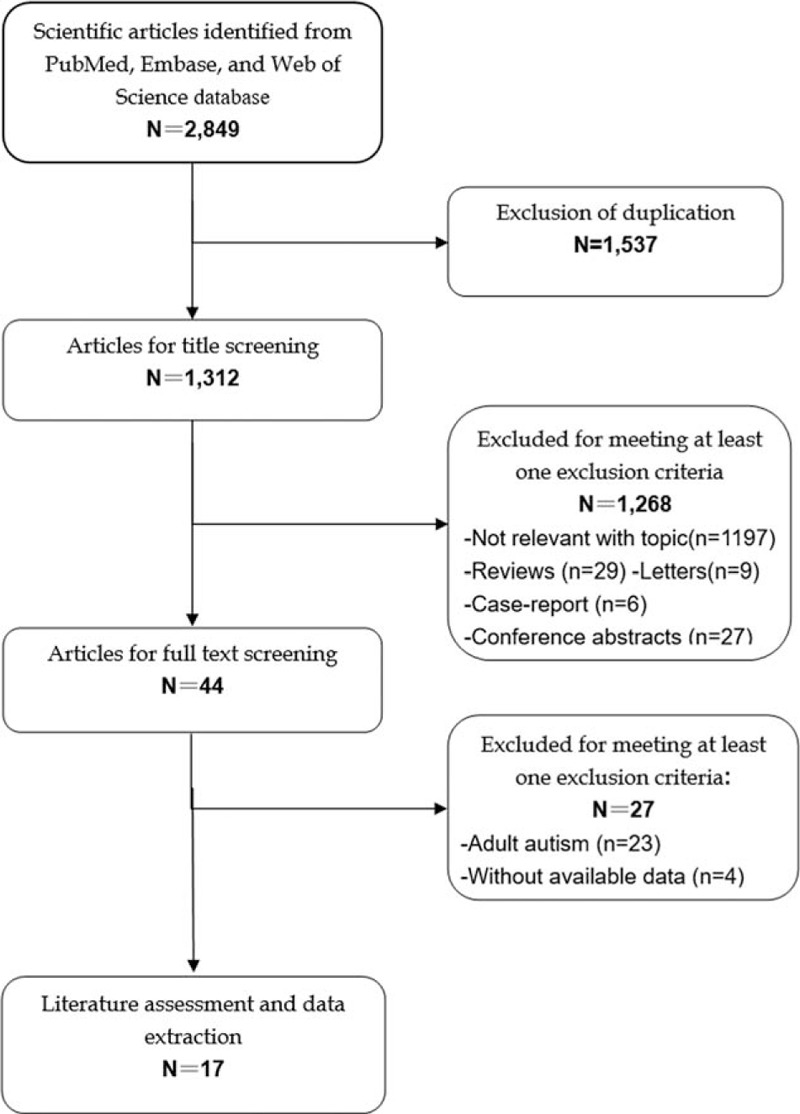
Flowchart of the literature search and selection.
3.2. Study characteristics
The main characteristics of included studies are presented in Table 1. These studies were published between 2002 and 2016. The sample size of these studies ranged from 101 to 5,661,883 with a total of 12,116,501 participants. All these participants were children. Among these studies, 12 were case-control studies, and 5 were cohort studies.
Table 1.
Summary of characteristics of included studies.
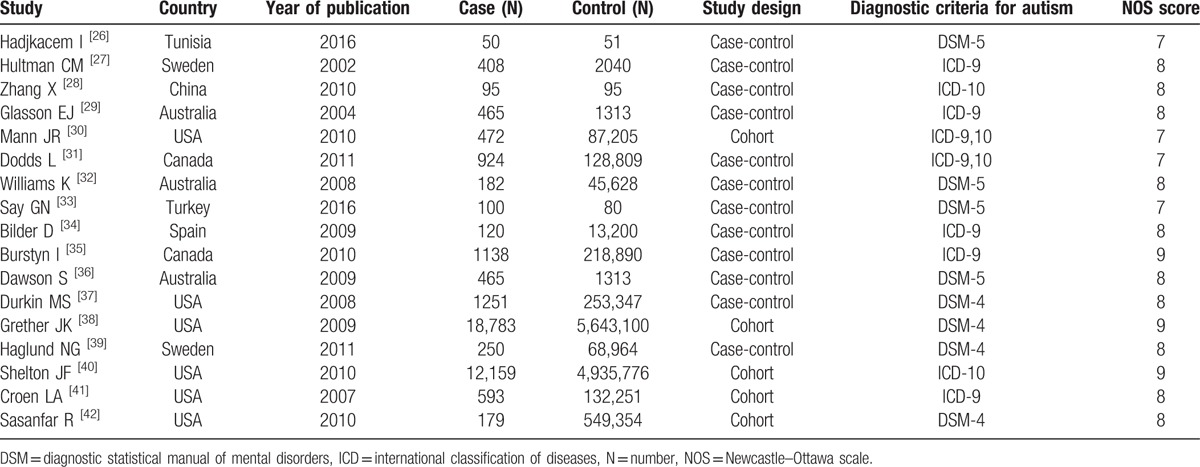
The median NOS score of the included studies was 8 (ranged from 7 to 9).
3.3. Prenatal risk factors
Table 2 lists the prenatal, perinatal, and postnatal risk factors that are included in this meta-analysis, as well as the summary effects estimate, 95% CIs, and the P values for the test of RR = 1. For some factors, such as difficult labor, auditory deficit, edema, delayed crying, apnoea, and anesthesia used, there were less than 2 studies reporting these outcomes, thus they were not analyzed in this meta-analysis.
Table 2.
Meta-analysis of prenatal, perinatal, and postnatal risk factors for autism.
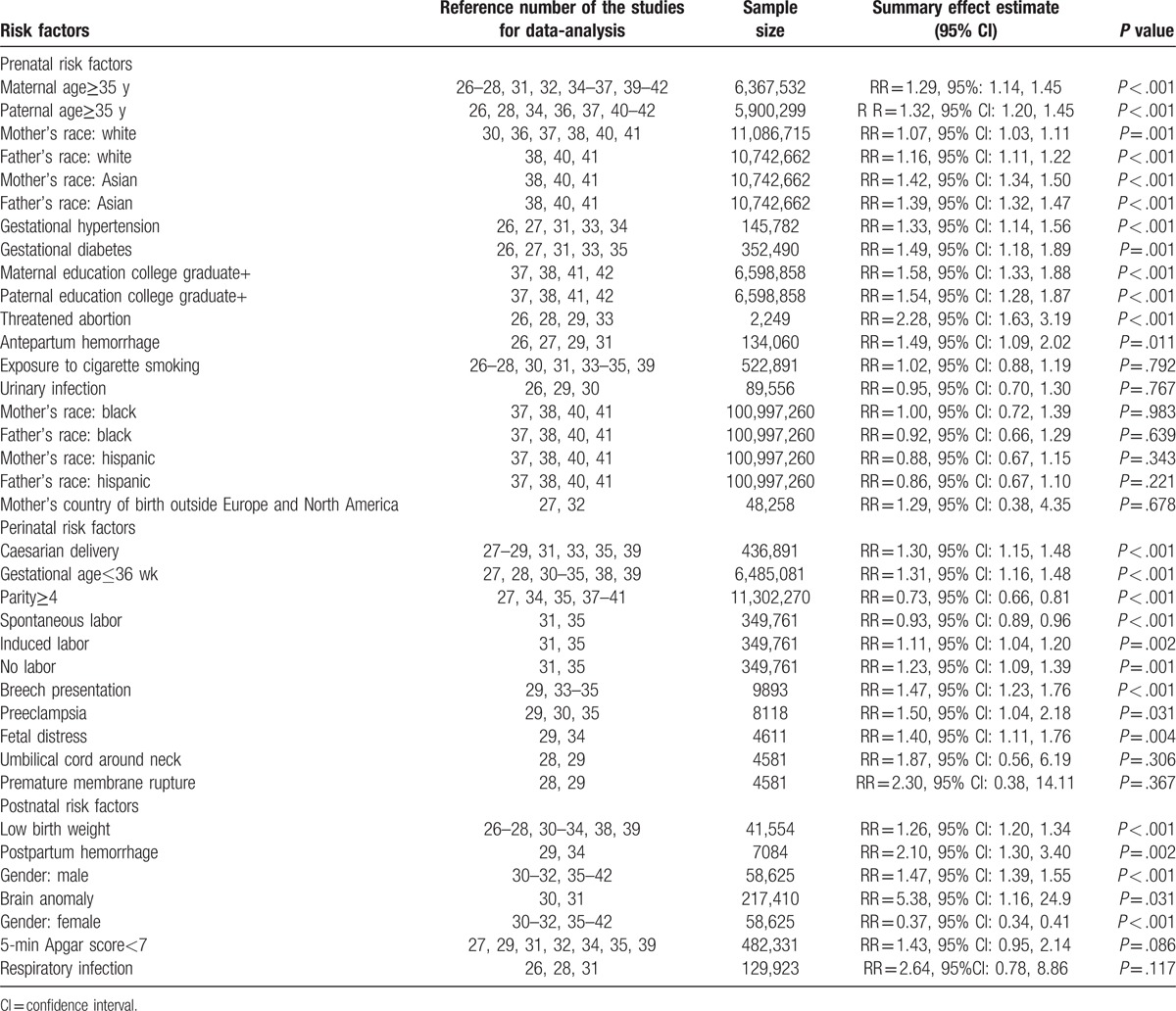
Among the studies with prenatal factors, meta-analysis using random-effects model showed that several factors were associated with increased risk for autism. These factors included: maternal age≥35 years (RR = 1.29, 95%: 1.14, 1.45; P < .001), paternal age≥35 years (RR = 1.32, 95% CI: 1.20, 1.45; P < .001), mother's race: White (RR = 1.07, 95% CI: 1.03, 1.11; P = .001), father's race: White (RR = 1.16, 95% CI: 1.11, 1.22; P < .001), mother's race: Asian (RR = 1.42, 95% CI: 1.34, 1.50; P < .001), father's race: Asian (RR = 1.39, 95% CI: 1.32, 1.47; P < .001), gestational hypertension (RR = 1.33, 95% CI: 1.14, 1.56; P < .001), gestational diabetes (RR = 1.49, 95% CI: 1.18, 1.89; P = .001), maternal education college graduate+ (RR = 1.58, 95% CI: 1.33, 1.88; P < .001), paternal education college graduate+ (RR = 1.54, 95% CI: 1.28, 1.87; P < .001), threatened abortion(RR = 2.28, 95% CI: 1.63, 3.19; P < .001), and antepartum hemorrhage (RR = 1.49, 95% CI: 1.09, 2.02; P = .011).
Moreover, these following factors were not associated with increased risk of autism: exposure to cigarette smoking (RR = 1.02, 95% CI: 0.88, 1.19; P = .792), urinary infection (RR = 0.95, 95% CI: 0.70, 1.30; P = .767), mother's race: Black (RR = 1.00, 95% CI: 0.72, 1.39; P = .983), father's race: Black (RR = 0.92, 95% CI: 0.66, 1.29; P = .639), mother's race: Hispanic (RR = 0.88, 95% CI: 0.67, 1.15; P = .343), father's race: Hispanic (RR = 0.86, 95% CI: 0.67, 1.10; P = .221), and mother's country of birth outside Europe and North America (RR = 1.29, 95% CI: 0.38, 4.35; P = .678).
3.4. Perinatal risk factors
As shown in Table 2, there were several factors that increased the risk of autism. These factors included: caesarian delivery (RR = 1.30, 95% CI: 1.15, 1.48; P < .001), gestational age≤36 weeks (RR = 1.31, 95% CI: 1.16, 1.48; P < .001), spontaneous labor (RR = 0.93, 95% CI: 0.89, 0.96; P < .001), induced labor (RR = 1.11, 95% CI: 1.04, 1.20; P = .002), no labor (RR = 1.23, 95% CI: 1.09, 1.39; P = .001), breech presentation (RR = 1.47, 95% CI: 1.23, 1.76; P < .001), preeclampsia (RR = 1.50, 95% CI: 1.04, 2.18; P = .031), and fetal distress (RR = 1.40, 95% CI: 1.11, 1.76; P = .004). Parity≥4 was a protective factor that decreased the risk of autism (RR = 0.73, 95% CI: 0.66, 0.81; P < .001). Umbilical cord around neck (RR = 1.87, 95% CI: 0.56, 6.19; P = .306) and premature membrane rupture (RR = 2.30, 95% CI: 0.38, 14.11; P = .367) were not risk for autism.
3.5. Postnatal risk factors
As shown in Table 2, several factors were associated with the increased risk of autism. These factors included: low birth weight (RR = 1.26, 95% CI: 1.20, 1.34; P < .001), postpartum hemorrhage (RR = 2.10, 95% CI: 1.30, 3.40; P = .002), male gender (RR = 1.47, 95% CI: 1.39, 1.55; P < .001), and brain anomaly (RR = 5.38, 95% CI: 1.16, 24.9; P = .031). Female was associated with a decreased risk of autism (RR = 0.37, 95% CI: 0.34, 0.41; P < .001). And the following factors were not associated with increased risk for autism: 5-minutes Apgar score < 7 (RR = 1.43, 95% CI: 0.95, 2.14; P = .086), and respiratory infection (RR = 2.64, 95% CI: 0.78, 8.86; P = .117).
3.6. Subgroup analysis
Significant heterogeneity was identified in many factors in the data summarization. These factors included: 5-minutes Apgar score, caesarian delivery, exposure to cigarette smoking, gestational age≤36 weeks, gender: male/female, maternal age≥35 years, mother's country of birth outside Europe and North America, maternal and paternal education college graduate+, mother's and father's race: Asian, Black, White, and Hispanic, parity≥4, paternal age≥35 years, respiratory infection, premature membrane rupture, preeclampsia, umbilical cord around neck, and brain anomaly. Therefore, we conducted subgroup analysis based on inclusion criteria for case, study design, and country to explore the potential sources of heterogeneity that may have influenced this association. However, significant heterogeneity was still observed for most of those risk factors that showed heterogeneity in effect estimates among the studies, which indicated that heterogeneity could not be explained by this between-study variability in these methodological characteristics examined. These results are presented in Tables 3 and 4.
Table 3.
Subgroup analysis of risk factors for autism based on case definition and study design.
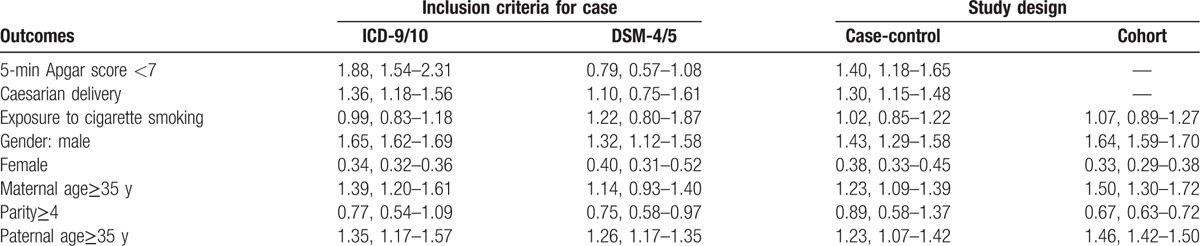
Table 4.
Subgroup analysis of risk factors for autism based on country.
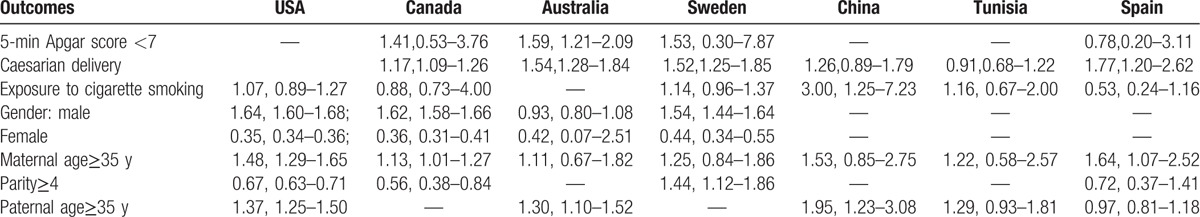
Subgroup analysis based on inclusion criteria for case (Table 3) suggested that the relationship between maternal age≥35 years and autism was only observed in studies using ICD-9/10 as the case inclusion criteria, but not in studies using DSM-4/5 as case inclusion criteria. A significant 27% decreased risk of autism in relationship to parity≥4 was observed in the overall effect estimates, but not found in the studies using ICD-9/10 as the case inclusion criteria. Although 5-minutes Apgar score < 7 was not observed as a risk for autism in the overall studies, it increased the risk of autism by 88% in the studies that identified the autism patients according to DSM-4/5.
Subgroup analysis based on study design had consistent results with the overall effect estimates except the finding of parity≥4, which showed no association with autism in case-control studies (Table 3).
Subgroup analysis based on country had inconsistent results with the overall effect estimates for most of the outcomes (Table 4). A significantly increased risk of autism was observed in relation to 5-minutes Apgar score<7 among the studies conducted in Australia, with no association observed in studies conducted in Sweden, Canada, and Spain. Caesarian delivery was associated with an increased risk for autism. However, it was not an increased risk for autism in studies conducted in China and Turkey. There was no significant relationship between exposure to cigarette smoking and autism. However, it increased the risk of autism in Chinese children. Gestational age≤36 weeks was a risk factor for autism. However, this relationship was not observed in Australia, Sweden, China, and Tunisia. Parity≥4 was associated with a decreased risk of autism. However, it was found to be an increased risk in Sweden. There was significant relationship between maternal age≥35 years and autism. However, this relationship was not found in studies conducted in Australia, Sweden, China, and Tunisia. Paternal age≥35 years was associated with an increased risk for autism. However, this association was not observed in Tunisia and Spain.
3.7. Publication bias
Assessment of publication bias was performed for all the risk factors that were investigated in more than 3 studies. The results showed that no significant publication bias was observed between all the risk factors and autism with the Begg test; whereas using Egger test, significant publication bias was identified for gestational age≤36 weeks, gestational diabetes, and gestational hypertension. We then used the trim-and-fill method to estimate missing studies and recalculated the overall effect estimates. The recalculated estimates did not change substantially, which still indicated a significant relationship between these risk factors and autism (data not shown).
4. Discussion
The current study was a meta-analysis with the objective to investigate the relationship between prenatal, perinatal, and postnatal factors and autism. In this meta-analysis, we assessed about 40 factors, and our results demonstrated that most of the factors we examined were associated with an increased risk of autism. These factors included maternal and paternal age≥35 years, mother's, and father's race: White and Asian gestational hypertension, gestational diabetes, maternal and paternal education college graduate+, threatened abortion, antepartum hemorrhage, caesarian delivery, gestational age≤36 weeks, spontaneous labor, induced labor, no labor, breech presentation, preeclampsia, and fetal distress, low birth weight, postpartum hemorrhage, male gender, and brain anomaly; whereas, for parity≥4 and female gender, they were found to be protective factors for autism. We also conducted subgroup analysis according to case definition criteria, study design, and country, but the relationship in several factors has been changed.
There has been 1 published meta-analysis in 2011 which investigated the association between perinatal and neonatal factors and autism risk.[6] For the factors that both of the 2 studies examined, our results were in line with the findings of the previous meta-analysis.[6] However, our study has several strengths. First, the present study included several recently published studies, which were not included in the previous study. As we know that, over the past decades, the distribution of children with autism has been probably changed by factors such as birthweight, the parental age, and delivery type. Thus, these changes may alter the distribution of risk factors among autistic children, and increase the heterogeneity of results between older and recent studies. Second, there were available data for us to conduct subgroup analysis across these included studies. Third, significant publication bias was identified for gestational age≤36 weeks, gestational diabetes, and gestational hypertension in this meta-analysis. In order to deal with publication bias, we used the trim-and-fill method. And the recalculated data did not change substantially, which indicated that publication bias may not have impact on our results. However, in the previous study, publication bias was found for high birth weight (>4000 g), meconium aspiration, and October to December birth.[6] The authors suggested that the publication bias may influence the summary effect estimates, and the significant results would be explained by the chance alone.[6]
In the present meta-analysis, advanced maternal or paternal age (≥35 years) was associated with an increased risk of autism. Our result was consistent with the data of a recently published meta-analysis,[43] which suggested an independent relation between higher maternal age and autism. The chosen 35 years as the age cutoff for both parents was based on the recommendations of many authors.[43,44] The theories supporting the positive relation lay in the facts that: the gametes of older fathers and mothers may have more possibility of genetic mutations; there was less favorable utero environment in older mothers, which would result in more obstetrical complications, such as LBW, prematurity, and cerebral hypoxia.[44] Furthermore, older mothers had a higher prevalence of chronic diseases, which also would lead to an increased risk of adverse birth outcomes.[43,45] These have been observed in serval previous studies, which demonstrated a high risk of obstetric complications among older mothers.[43–45]
Exposure to cigarette smoking was not considered an increased risk factor of autism in this study. This result was observed in most of the included studies. However, in the study conducted by Zhang et al,[28] they found that maternal second-hand smoking during pregnancy was associated with an increased risk of autism. The authors argued that there were several chemicals with adverse health effects in the second-hand smoke, such as polycyclic aromatic hydrocarbons, and metals, which would lead to fetal hypoxia and influence brain development.[28] Although these theories could support the positive relationship between exposure to cigarette smoking and autism, the authors thought that their study may have no adequate power to address this issue since the sample size of maternal smoking was very small.[28]
Despite the relation between cigarette smoking and autism remaining controversial among the studies,[27,46,47] some authors suggested that maternal cigarette smoking during pregnancy may have a commutative impact on the lineage of her reproductive cells.[27,47] Maternal cigarette smoking may increase the risk of spontaneous abortions, preterm delivery, reduced birth weight, and others.[48]
Gestational diabetes was considered a risk factor in this meta-analysis, which was in line with the previous studies.[39,40,42] A prospective study showed that gestational diabetes would adversely affect the fetal growth, and increase the rates of pregnancy complications.[49] Moreover, it also had impact on the fine and gross motor development, and lead to the learning difficulties and attention deficit hyperactivity disorder.[49] These adverse effects of maternal diabetes on brain may arise from the intrauterine increased fetal oxidative stress, as well as the epigenetic changes in the expression of several genes.[26] Moreover, the observed risk in maternal diabetes might be related to the pregnancy complications rather than the hyperglycemia complications. Whether control of diabetes would reduce this association still remains unknown.[49]
In the subgroup analysis we found that the association between several risk factors and autism had been changed. Maternal age≥35 years was regarded as an increased risk for autism in the previous studies and the current meta-analysis, but it was not found in the studies using DSM-4/5 as case inclusion criteria. Similar to the parity≥4, it was found to be a protective factor for autism in the overall effect estimate, but it was not observed in studies using ICD-9/10 as the case inclusion criteria. In the subgroup analysis based on country, studies conducted in Australia and Tunisia demonstrated adverse results with the studies in other countries. The male gender and maternal age≥35 years were found to be associated with autism in most of the studies and this study; however, this was not observed in studies conducted in Australia. Caesarian delivery, advanced maternal, and paternal age were associated with increased risk of autism. But these positive relationships were not found in Tunisia. The reasons for these conflict results still remained unknown, and further studies are needed to address these issues.
There were several potential limitations in this meta-analysis. First, our study was conducted based on 17 studies. Although most of the risk factors were analyzed based on several studies (n≥8), some risk factors were based on 4 or 5 studies, which had potential impact on the overall effect estimates. Second, there was moderate heterogeneity in the present study. Several factors may contribute to this heterogeneity, such as case definition, inclusion criteria, sample size, study design, and country. We therefore conducted subgroup analysis to investigate the sources of heterogeneity. However, there was still significant heterogeneity existing in most of the subgroups. Third, publication bias was detected in this meta-analysis. Although the reanalyzed estimates using the trim-and-fill method did not change significantly, we could not exclude the possibility that the missing data from the gray literature would influence the overall effect estimates. Fourth, this meta-analysis was conducted based on published studies rather than individual data, which may limit our ability to explore more possible factors, and gain a better understanding of the sources of heterogeneity.
In conclusion, this study identified about 40 prenatal, perinatal, and postnatal factors that may be increased risk for autism. These factors could interact or contribute in combination with other cofactors to play a role in the development of autism. Further studies are needed to confirm our results and investigate the single or combination factors for autism.
Footnotes
Abbreviations: ASD = autism spectrum disorder, CIs = confidence intervals, DSM = diagnostic statistical manual of mental disorders, ICD = international classification of diseases, LBW = low birth weight, RR = risk ratio.
CW and HG contributed equally to this work.
The authors have no conflicts of interest to disclose.
References
- [1].Association AP. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Washington, DC. American Psychiatric Association 1994. [Google Scholar]
- [2].Elsabbagh M, Divan G, Koh YJ, et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res 2012;5:160–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bertrand J, Mars A, Boyle C, et al. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics 2001;108:1155–61. [DOI] [PubMed] [Google Scholar]
- [4].Hallmayer J, Cleveland S, Torres A, et al. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 2011;68:1095–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Froehlich-Santino W, Londono Tobon A, Cleveland S, et al. Prenatal and perinatal risk factors in a twin study of autism spectrum disorders. J Psychiatr Res 2014;54:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gardener H, Spiegelman D, Buka SL. Perinatal and neonatal risk factors for autism: a comprehensive meta-analysis. Pediatrics 2011;128:344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tchaconas A, Adesman A. Autism spectrum disorders: a pediatric overview and update. Curr Opin Pediatr 2013;25:130–44. [DOI] [PubMed] [Google Scholar]
- [8].Kolevzon A, Gross R, Reichenberg A. Prenatal and perinatal risk factors for autism: a review and integration of findings. Arch Pediatr Adolesc Med 2007;161:326–33. [DOI] [PubMed] [Google Scholar]
- [9].Reichenberg A, Gross R, Weiser M, et al. Advancing paternal age and autism. Arch General Psychiatry 2006;63:1026–32. [DOI] [PubMed] [Google Scholar]
- [10].Burd L, Severud R, Kerbeshian J, et al. Prenatal and perinatal risk factors for autism. J Perinat Med 1999;27:441–50. [DOI] [PubMed] [Google Scholar]
- [11].Gillberg C, Gillberg IC. Infantile autism: a total population study of reduced optimality in the pre-, peri-, and neonatal period. J Autism Dev Disord 1983;13:153–66. [DOI] [PubMed] [Google Scholar]
- [12].Lord C, Mulloy C, Wendelboe M, et al. Pre- and perinatal factors in high-functioning females and males with autism. J Autism Dev Disord 1991;21:197–209. [DOI] [PubMed] [Google Scholar]
- [13].Bryson SE, Smith IM, Eastwood D. Obstetrical suboptimality in autistic children. J Am Acad Child Adolesc Psychiatry 1988;27:418–22. [DOI] [PubMed] [Google Scholar]
- [14].Finegan JA, Quarrington B. Pre-, peri-, and neonatal factors and infantile autism. J Child Psychol Psychiatry 1979;20:119–28. [DOI] [PubMed] [Google Scholar]
- [15].Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry 2005;66(suppl 10):3–8. [PubMed] [Google Scholar]
- [16].Brasic JR, Holland JA. A qualitative and quantitative review of obstetric complications and autistic disorder. J Dev Phys Disabil 2007;19:337–64. [Google Scholar]
- [17].Cryan E, Byrne M, O’Donovan A, et al. A case-control study of obstetric complications and later autistic disorder. J Autism Dev Disord 1996;26:453–60. [DOI] [PubMed] [Google Scholar]
- [18].Guinchat V, Thorsen P, Laurent C, et al. Pre-, peri- and neonatal risk factors for autism. Acta Obstet Gynecol Scand 2012;91:287–300. [DOI] [PubMed] [Google Scholar]
- [19].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [20].Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. 3rd Symposium on Systematic Reviews: Beyond the Basics 2000:3–5. [Google Scholar]
- [21].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [23].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [24].Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088–101. [PubMed] [Google Scholar]
- [25].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hadjkacem I, Ayadi H, Turki M, et al. Prenatal, perinatal and postnatal factors associated with autism spectrum disorder. J Pediatr (Rio J) 2016;92:595–601. [DOI] [PubMed] [Google Scholar]
- [27].Hultman CM, Sparen P, Cnattingius S. Perinatal risk factors for infantile autism. Epidemiology 2002;13:417–23. [DOI] [PubMed] [Google Scholar]
- [28].Zhang X, Lv CC, Tian J, et al. Prenatal and perinatal risk factors for autism in China. J Autism Dev Disord 2010;40:1311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Glasson EJ, Bower C, Petterson B, et al. Perinatal factors and the development of autism: a population study. Arch Gen Psychiatry 2004;61:618–27. [DOI] [PubMed] [Google Scholar]
- [30].Mann JR, McDermott S, Bao H, et al. Pre-eclampsia, birth weight, and autism spectrum disorders. J Autism Dev Disord 2010;40:548–54. [DOI] [PubMed] [Google Scholar]
- [31].Dodds L, Fell DB, Shea S, et al. The role of prenatal, obstetric and neonatal factors in the development of autism. J Autism Dev Disord 2011;41:891–902. [DOI] [PubMed] [Google Scholar]
- [32].Williams K, Helmer M, Duncan GW, et al. Perinatal and maternal risk factors for autism spectrum disorders in New South Wales, Australia. Child Care Health Dev 2008;34:249–56. [DOI] [PubMed] [Google Scholar]
- [33].Say GN, Karabekiroglu K, Babadagi Z, et al. Maternal stress and perinatal features in autism and attention deficit/hyperactivity disorder. Pediatr Int 2016;58:265–9. [DOI] [PubMed] [Google Scholar]
- [34].Bilder D, Pinborough-Zimmerman J, Miller J, et al. Prenatal, perinatal, and neonatal factors associated with autism spectrum disorders. Pediatrics 2009;123:1293–300. [DOI] [PubMed] [Google Scholar]
- [35].Burstyn I, Sithole F, Zwaigenbaum L. Autism spectrum disorders, maternal characteristics and obstetric complications among singletons born in Alberta, Canada. Chronic Dis Can 2010;30:125–34. [PubMed] [Google Scholar]
- [36].Dawson S, Glasson EJ, Dixon G, et al. Birth defects in children with autism spectrum disorders: a population-based, nested case-control study. Am J Epidemiol 2009;169:1296–303. [DOI] [PubMed] [Google Scholar]
- [37].Durkin MS, Maenner MJ, Newschaffer CJ, et al. Advanced parental age and the risk of autism spectrum disorder. Am J Epidemiol 2008;168:1268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Grether JK, Anderson MC, Croen LA, et al. Risk of autism and increasing maternal and paternal age in a large north American population. Am J Epidemiol 2009;170:1118–26. [DOI] [PubMed] [Google Scholar]
- [39].Haglund NG, Kallen KB. Risk factors for autism and Asperger syndrome. Perinatal factors and migration. Autism 2011;15:163–83. [DOI] [PubMed] [Google Scholar]
- [40].Shelton JF, Tancredi DJ, Hertz-Picciotto I. Independent and dependent contributions of advanced maternal and paternal ages to autism risk. Autism Res 2010;3:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Croen LA, Najjar DV, Fireman B, et al. Maternal and paternal age and risk of autism spectrum disorders. Arch Pediatr Adolesc Med 2007;161:334–40. [DOI] [PubMed] [Google Scholar]
- [42].Sasanfar R, Haddad SA, Tolouei A, et al. Paternal age increases the risk for autism in an Iranian population sample. Mol Autism 2010;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sandin S, Hultman CM, Kolevzon A, et al. Advancing maternal age is associated with increasing risk for autism: a review and meta-analysis. J Am Acad Child Adolesc Psychiatry 2012;51:477–86. [DOI] [PubMed] [Google Scholar]
- [44].Parner ET, Baron-Cohen S, Lauritsen MB, et al. Parental age and autism spectrum disorders. Ann Epidemiol 2012;22:143–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Polo-Kantola P, Lampi KM, Hinkka-Yli-Salomaki S, et al. Obstetric risk factors and autism spectrum disorders in Finland. J Pediatr 2014;164:358–65. [DOI] [PubMed] [Google Scholar]
- [46].Larsson M, Weiss B, Janson S, et al. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6-8 years of age. Neurotoxicology 2009;30:822–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Lee BK, Gardner RM, Dal H, et al. Brief report: maternal smoking during pregnancy and autism spectrum disorders. J Autism Dev Disord 2012;42:2000–5. [DOI] [PubMed] [Google Scholar]
- [48].Kiechl-Kohlendorfer U, Ralser E, Pupp Peglow U, et al. Smoking in pregnancy: a risk factor for adverse neurodevelopmental outcome in preterm infants? Acta Paediatr 2010;99:1016–9. [DOI] [PubMed] [Google Scholar]
- [49].Ornoy A, Ratzon N, Greenbaum C, et al. School-age children born to diabetic mothers and to mothers with gestational diabetes exhibit a high rate of inattention and fine and gross motor impairment. J Pediatr Endocrinol Metab 2001;14 suppl 1:681–689. [DOI] [PubMed] [Google Scholar]


