Abstract
Isolated chloroplasts which have lost their envelopes and, in consequence, the soluble components which constitute the stroma, will nevertheless evolve O2 when supplied with an artificial oxidant (the Hill reaction). They will also evolve O2 with NADP as the Hill oxidant if supplemented with ferredoxin. With catalytic NADP, continuing O2 evolution can be maintained by the inclusion of a suitable reaction or reaction sequence which reoxidizes NADPH.
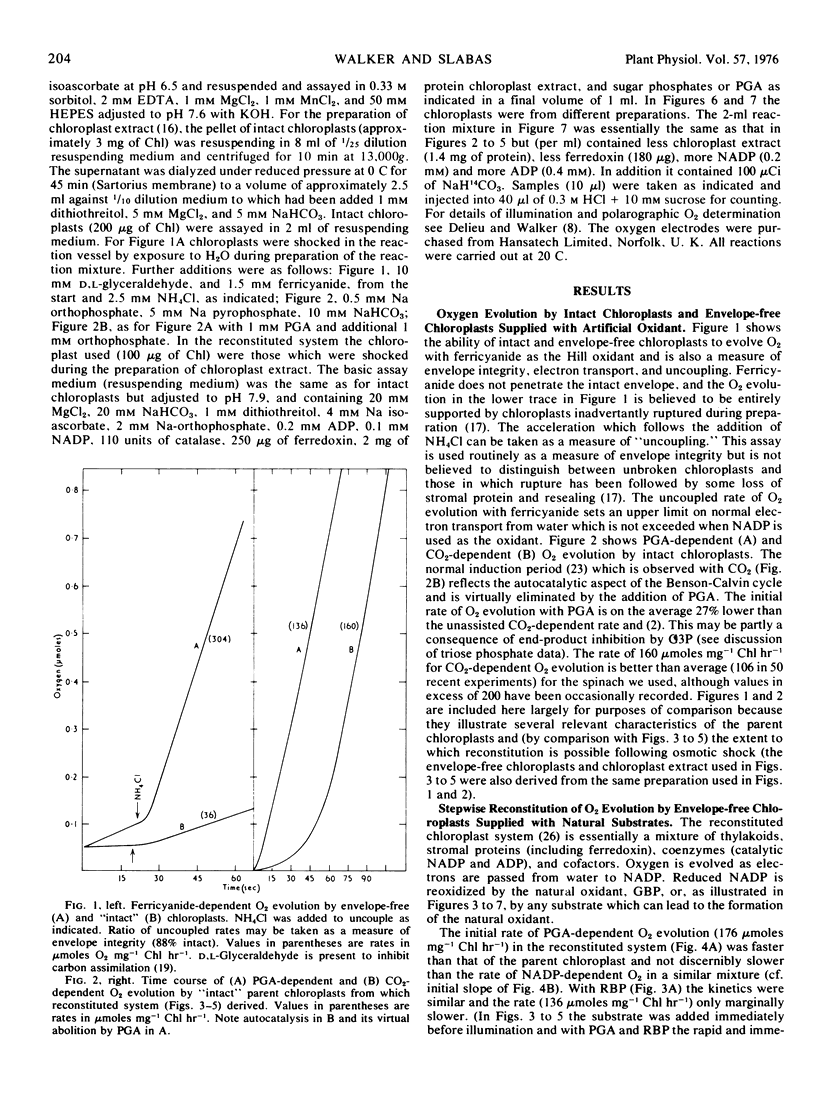
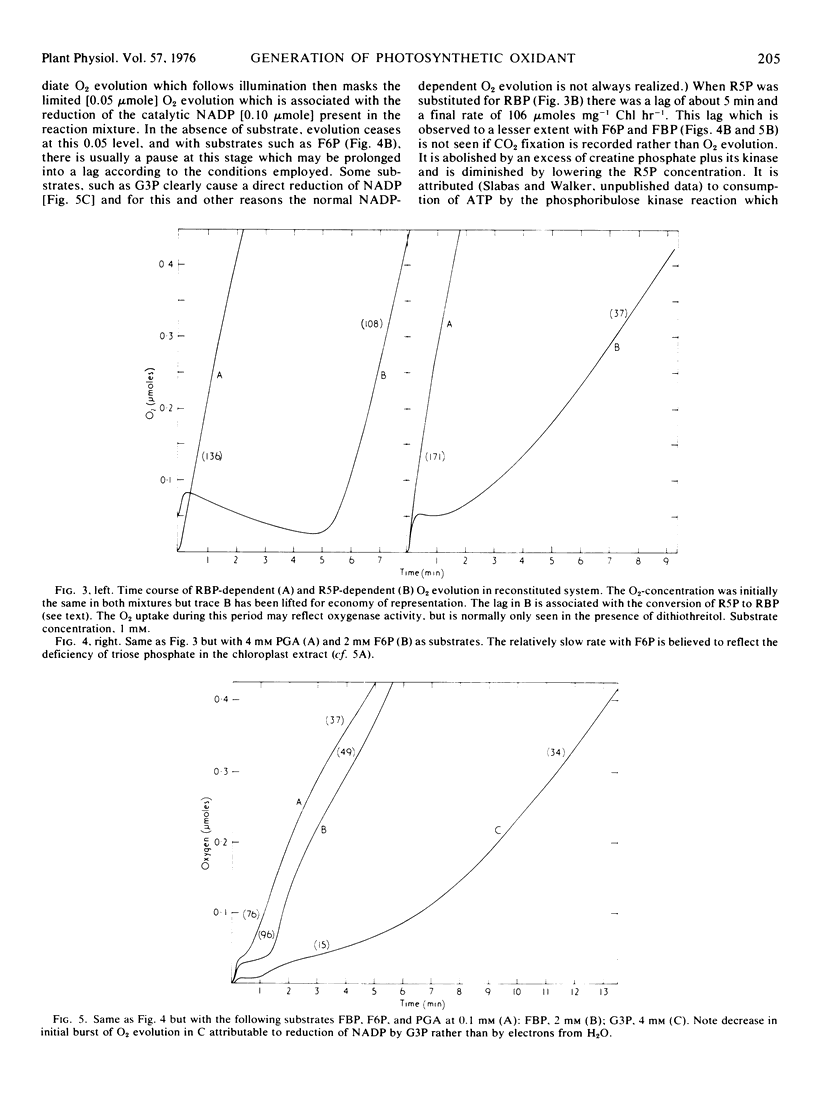
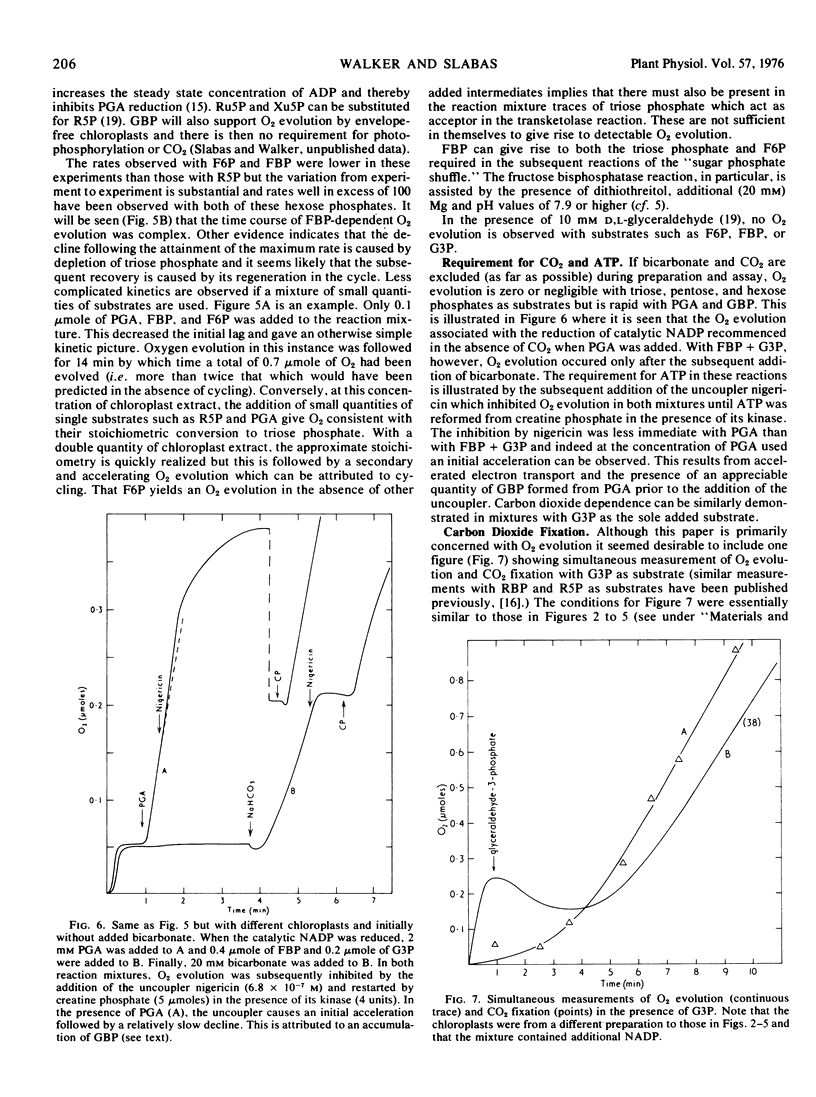
In the Benson-Calvin cycle the terminal oxidant is glycerate 1,3-bisphosphate which is generated by phosphorylation of 3-phosphoglycerate, its immediate precursor. Experiments with a reconstituted chloroplast system are described in which this reaction sequence is catalyzed by stromal protein and supported by photophosphorylation of catalytic ADP. In the presence of CO2, 3-phosphoglycerate can be progressively replaced by ribulose 1,5-bisphosphate, ribose 5-phosphate, fructose 6-phosphate, fructose 1,6-bisphosphate, and finally by glyceraldehyde 3-phosphate. In this last instance the natural oxidant is regenerated from its own reduction product (via the carboxylation step) and the reaction sequence therefore involves the entire photosynthetic carbon cycle.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., ALLEN M. B., WHATLEY F. R. Photosynthesis by isolated chloroplasts. Nature. 1954 Aug 28;174(4426):394–396. doi: 10.1038/174394a0. [DOI] [PubMed] [Google Scholar]
- Andersen W. R., Gibbs M. Inhibition of CO2 fixation in intact spinach chloroplasts by 3-phosphoglyceric acid. Biochem Biophys Res Commun. 1975 Feb 17;62(4):953–956. doi: 10.1016/0006-291x(75)90415-5. [DOI] [PubMed] [Google Scholar]
- Arnon D. I., Tsujimoto H. Y., McSwain B. D. Ferredoxin and photosynthetic phosphorylation. Nature. 1967 May 6;214(5088):562–566. doi: 10.1038/214562a0. [DOI] [PubMed] [Google Scholar]
- Baier D., Latzko E. Properties and regulation of C-1-fructose-1,6-diphosphatase from spinach chloroplasts. Biochim Biophys Acta. 1975 Jul 8;396(1):141–148. doi: 10.1016/0005-2728(75)90197-8. [DOI] [PubMed] [Google Scholar]
- Cockburn W., Baldry C. W., Walker D. A. Some effects of inorganic phosphate on O2 evolution by isolated chloroplasts. Biochim Biophys Acta. 1967;143(3):614–624. doi: 10.1016/0005-2728(67)90067-9. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Hill R. The biochemists' green mansions: the photosynthetic electron-transport chain in plants. Essays Biochem. 1965;1:121–151. [PubMed] [Google Scholar]
- Hind G., Nakatani H. Y., Izawa S. Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1484–1488. doi: 10.1073/pnas.71.4.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalberer P. P., Buchanan B. B., Arnon D. I. Rates of photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1542–1549. doi: 10.1073/pnas.57.6.1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilley R. M., Schwenn J. D., Walker D. A. Inorganic pyrophosphatase and photosynthesis by isolated chloroplasts. II. The controlling influence of orthophosphate. Biochim Biophys Acta. 1973 Dec 14;325(3):596–604. doi: 10.1016/0005-2728(73)90219-3. [DOI] [PubMed] [Google Scholar]
- Lilley R. M., Walker D. A. The reduction of 3-phosphoglycerate by reconstituted chloroplasts and by chloroplast extracts. Biochim Biophys Acta. 1974 Dec 19;368(3):269–278. doi: 10.1016/0005-2728(74)90174-1. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Phosphoglycerate as a hill oxidant in a reconstituted chloroplast system. Plant Physiol. 1971 Aug;48(2):163–165. doi: 10.1104/pp.48.2.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHATLEY F. R., ALLEN M. B., ROSENBERG L. L., CAPINDALE J. B., ARNON D. I. Photosynthesis by isolated chloroplasts. V. Phosphorylation and carbon dioxide fixation by broken chloroplasts. Biochim Biophys Acta. 1956 Jun;20(3):462–468. doi: 10.1016/0006-3002(56)90340-7. [DOI] [PubMed] [Google Scholar]
- Walker D. A. Correlation between Photosynthetic Activity and Membrane Integrity in Isolated Pea Chloroplasts. Plant Physiol. 1965 Nov;40(6):1157–1161. doi: 10.1104/pp.40.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A. Improved rates of carbon dioxide fixation by illuminated chloroplasts. Biochem J. 1964 Sep;92(3):22C–23C. doi: 10.1042/bj0920022c. [DOI] [PubMed] [Google Scholar]
- Walker D. A., Lilley R. M. Autocatalysis in a reconstituted chloroplast system. Plant Physiol. 1974 Dec;54(6):950–952. doi: 10.1104/pp.54.6.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. A., McCormick A. V., Stokes D. M. CO 2 -dependent oxygen evolution by envelope-free chloroplasts. Nature. 1971 Oct 1;233(5318):346–347. doi: 10.1038/233346a0. [DOI] [PubMed] [Google Scholar]



