Abstract
This study aimed to investigate the pulmonary function in patients with inflammatory bowel disease (IBD) and its clinical feature and risk factors.
One hundred fourteen patients with IBD and 120 healthy subjects were recruited. The medical information including general situation, biochemical examinations, lung function, and the treatment was recorded and analyzed.
In 107 patients (107/114, 93.86%), lung function testing showed the pulmonary ventilation, residual volume, and pulmonary diffusion in IBD patients significantly increased as compared to controls (P < .05). No significant differences were observed between ulcerative colitis (UC) patients and Crohn disease (CD) patients (P > .05). However, the vital capacity, forced vital capacity, MVV, forced expiratory volume in first second, peak expiratory flow rate, and maximum mid-expiratory flow in IBD patients significantly decreased when compared with controls (P < .01). There was no significant correlation between pulmonary function and severity and extent of IBD. The chronicity of inflammation might probably reduce the possibility of developing pulmonary dysfunction, while the erythrocyte sedimentation rate (ESR) was found as a harmful factor for developing pulmonary dysfunction.
The pulmonary function significantly decreases in IBD patients and is characterized by either simple restrictive/obstructive dysfunction or mixed. The pulmonary function of IBD patients has no relationship with the severity and extent of IBD. IBD combined with pulmonary dysfunction was imperceptible, and clinicians could consider performing pulmonary function testing for IBD patient as many as possible, especially for those who have high level of ESR or any respiratory symptoms like cough, in order to avoid severe pulmonary damage.
Keywords: Crohn disease, inflammatory bowel disease, lung function, ulcerative colitis
1. Introduction
The incidence of inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn disease (CD), is increasing worldwide over years. The incidence in developed countries is up to 24/100,000/year (for UC in Europe), with a prevalence as high as 505 and 322/100,000/year for UC and CD, respectively.[1] IBD has become one of the focuses in the field of digestive diseases. In the past 20 years, a variety of basic and clinical studies have been conducted to investigate IBD. It has not only a characteristic change of nonspecific chronic inflammation in the intestine, but may cause involvement of extraintestinal systems, such as bone and joint system, hepatobiliary system, respiratory system, and skin and mucous membranes. It has been regarded as a complicated and systemic disease.[2,3]
The pathogenesis of IBD is still unclear, and the cause of extraintestinal involvement is also poorly understood. The incidence of extraintestinal manifestations is approximately 6% to 47% in IBD patients,[4–6] and it was estimated that 40% to 60% of IBD patients had some degree of subclinical lung involvement evidenced through alterations in pulmonary function tests or high resolution tomographic imaging.[7,8]
From the point of view of embryonic development, the gut and the bronchial tree are derived from the same germ-layer: the foregut region of the endoderm.[9] This may partially explain why lung inflammation rapidly develops or exacerbates after the resection of involved intestine in UC patients. This phenomenon of inflammation shift provides evidence for the “gut-lung homology.” Kraft et al[10] for the first time proposed that the inflammation in IBD also affected the lung, based on the analysis of 6 IBD patients with productive cough. Thereafter, Basseri et al,[11] Mikhailova et al,[12] and Papanikolaou et al[13] reported IBD patients with unexplained pulmonary occult lesions and changes in lung function. By the end of 2000, the pulmonary dysfunction related to IBD has been reported in more than 100 cases worldwide, while rarely reported in China.
The pulmonary dysfunction in IBD patients can occur at any stage of the disease, from days to years after the diagnosis. Findings from available studies indicate that IBD may affect any part of the respiratory tract, but the symptoms and signs are nonspecific.[14] Some patients even have no respiratory manifestations, but the lung function test shows pulmonary dysfunction of varying degrees, which leads to misdiagnosis or missed diagnosis. Thus, the therapy by glucocorticoid is often delayed in them. Therefore, early detection of lung dysfunction, and comprehensive evaluation of IBD patients are crucial for the early treatment and interventions and may avoid or delay the deterioration of pulmonary function, and improve their prognosis.[15]
In the present study, medical information was collected from IBD patients who were recruited from our hospital between June 2013 and October 2014, the pulmonary function was analyzed and the correlation between IBD and lung dysfunction was evaluated.
2. Methods
2.1. Patients
A total of 114 patients with active IBD, including UC in 50 cases and CD in 64 cases, were enrolled consecutively into this study from the Department of Gastroenterology, the Tenth People's Hospital of Tongji University between June 2013 and October 2014. IBD was diagnosed on the basis of clinical manifestations, colonoscopy, pathological examination of the mucosa or intestinal tissues collected during the surgery according to the World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD (2010).[16] The extent and activity of IBD were assessed endoscopically with Truelove and Witts score[17] for UC and CDAI for CD.[18] At the same time, 120 healthy subjects were collected as control from those who came to hospital for health examination. Chronic disease history of intestinal tract and respiratory system were excluded, but there may be long-term chronic smoking history.
Exclusion criteria of IBD patients were as follows: patients with a history of chronic respiratory disease, a history of smoking, exposure to harmful dust, and a history of upper respiratory tract infection within 1 month; pregnancy or breastfeeding; prior bowel resection leading to diarrhea, and/or pouch formation, toxic megacolon, hemorrhagic diathesis, present or past colorectal cancer; abnormal liver/kidney function tests.
Blood samples were collected for the measurements of hemoglobin, C-reaction protein (CRP) and erythrocyte sedimentation rate (ESR). This study was approved by the Ethics Committee of our hospital (No.: SHSY-IEC-pap-12-1), and signed informed consent was collected before study.
2.2. Methods
The age, sex, family history, duration of disease, biochemical parameters (CRP, ESR, blood routine test, etc.), lung function, chest X-ray, diagnoses, and treatments were collected. Patients and controls underwent standard pulmonary function testing. The vital capacity (VC), forced vital capacity (FVC), forced expiratory volume in first second (FEV1), peak expiratory flow rate (PEF), maximum mid-expiratory flow (MMEF), carbon monoxide diffusing capacity, and carbon monoxide diffusing capacity/lung volume were detected. The Master Screen spirometer was from Jaeger Company (Würzburg, Germany), and pulmonary function test was conducted by the same clinician. To avoid the impact of body surface on the lung function, results were normalized by the predicted value (%). As we known, lung ventilation is the process of gas exchange between the lungs and the outside environment while lung diffusion is the process of gas exchange within the lungs. The measurements of VC, FVC, and FEV1, MMEF are usually used to estimate ventilation function and PEF is able to reflect the severity of airway obstruction. In this study, either abnormality in ventilatory function, or diffuse function, or residual volume was defined as lung dysfunction.
2.3. Statistical analysis
PASW statistics (18.0, Polar Engineering and Consulting, Chicago, IL) was used for the statistical analysis. Comparisons of rates among multiple groups were done with Fisher exact test, continuous variables were compared with 1-way ANOVA, single factor correlation analysis was done by logistic regression and level data were analyzed with rank sum test. A value of P less than .05 was considered statistically significant.
3. Results
3.1. Patients’ characteristics
The characteristics of 114 IBD patients are shown in Table 1. Among them, UC was diagnosed in 50 patients (43.86%) and CD in 64 patients (56.14%). There were 52 males (45.95%) and 62 females (54.05%). The mean age was 44.05 ± 19.44 years (range: 17–81 years) in IBD patients and 50.93 ± 15.10 years (range: 14–75 years) in 120 healthy controls. Twenty-seven (22.5%) among the healthy controls had a history of smoking. There were no significant differences in the sex, age, and other parameters at baseline between IBD group and control group.
Table 1.
Medical characteristics of 114 patients with IBD.
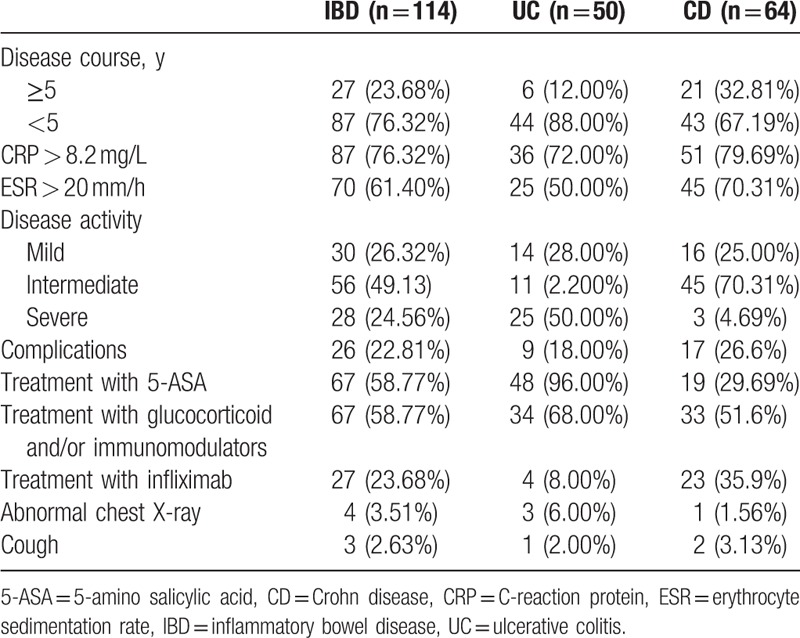
3.2. Prevalence of pulmonary function in both groups
Among IBD patients, lung dysfunction was found in 107 patients (93.86%), including 46 (92.00%) UC patients and 61 (95.31%) CD patients. However, abnormal lung function was found in 27 (22.5%) controls. Table 2 shows the counts of ventilatory dysfunction, diffusion dysfunction, and residual volume abnormality in each group. The proportion of patients with abnormal residual volume (RV), ventilatory dysfunction, and diffusion dysfunction in IBD patients was significantly higher than in control group (P < .05), but no difference was found between UC group and CD group (P > .05).
Table 2.
Incidences of pulmonary dysfunction [n/(%)].

3.3. Changes in pulmonary function of IBD patients
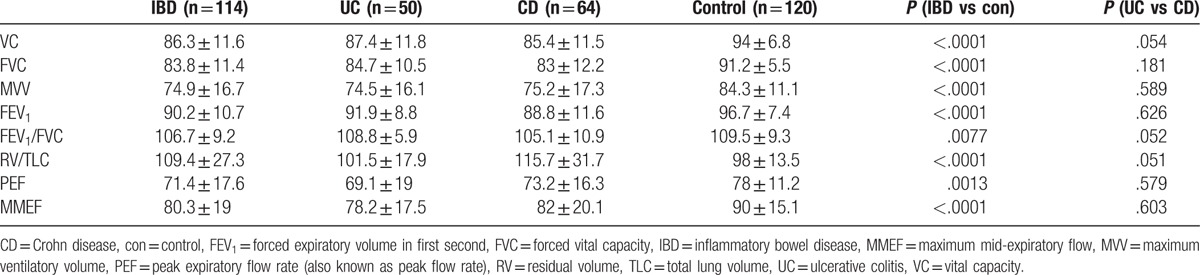
All 114 IBD patients and 120 controls underwent standard lung function testing. When compared with controls, VC, FVC, maximum ventilatory volume (MVV), FEV1, MMEF (P < .0001), and PEF (P < .001) reduced significantly in IBD patients, but FEV1/FVC was comparable between IBD patients and controls (P > .01) (Table 3). In addition, no difference was found in these parameters between UC group and CD group.
Table 3.
Pulmonary function parameters in controls and IBD patients (normalized to predicted value, %).
3.4. Correlation between pulmonary function and clinical characteristics
The correlation of pulmonary function with clinical manifestations including duration of disease, CDAI or UCAI, CRP, ESR, hemoglobin, use of glucocorticoid or infliximab, and complications, were evaluated with logistic regression analysis. As shown in Table 1, some probable factors which may cause or aggravate lung dysfunction in IBD patients were set out. So we put them into logistic regression and found that, the duration of disease and raised ESR were risk factors for IBD patients to suffer from pulmonary dysfunction (OR: 0.824, 95% CI: 0.687–0.987 and 1.093, 95% CI: 0.994–1.202, respectively). It is suggested that the duration of disease is a protective factor, while raised ESR a harmful factor.
4. Discussion
The incidence of IBD is increasing universally over years. Its extraintestinal manifestations (such as fever, anemia, arthritis, oral ulcers, and iritis) are complex and frequently found in clinical practice.[19,20] However, few studies have been conducted to report the respiratory abnormalities related to IBD.
As early as in 1976, Kraft et al[10] described 6 patients in whom chronic bronchial suppuration appeared between 3 and 13 years after the onset of UC, including 4 chronic bronchitis and bronchiectasis in 4 cases and lung damage after bowel resection in 2 cases, and five were associated with obstructive lung dysfunction. This was the first report on the lung dysfunction secondary to IBD. Thereafter, increasing studies report the pulmonary manifestations in IBD patients. Emerging evidence shows that intestinal inflammation is related to the lung dysfunction, and may cause diseases such as bronchitis, bronchiectasis, asthma, pulmonary parenchymal disease, pulmonary vascular disease, and so on.[11,12,21]
In a study on UC patients, small airway obstruction characterized by 25% to 75% diminished FEF in 57.6% of patients, 30.7% with restrictive dysfunction in and 11.5% with obstructive dysfunction. The proportion of patients with pulmonary dysfunction significantly increased in active UC patients when compared with controls.[7] Desai et al[22] also found small airway dysfunction in IBD patients despite of their normal baseline spirometric values, but there was no significant difference between active IBD patients and nonactive IBD patients. In this study, among all patients, the most common abnormalities in lung dysfunction were decreases in VC, FVC, FEV1, MVV, PEF, MMEF, and FEV1/FVC. The decreases in VC, FVC, and FEV1 suggest restrictive ventilatory dysfunction, while reduced FEV1, FEV1/FVC, PEF, MVV, and RV suggest obstructive ventilatory dysfunction, and the coexistence of these changes suggests mixed ventilatory dysfunction. However, the MVV%/VC% of <1 in both UC group and CD group indicates that obstructive dysfunction is the major feature of mixed ventilatory dysfunction.[23] All these findings reveal pulmonary function damages in IBD: it may be expressed as a simple restrictive or obstructive ventilatory dysfunction, or as mixed ventilatory dysfunction dominated by obstructive dysfunction. However, the pulmonary dysfunction has no relationship with the activity of IBD.
In our study, the incidence of pulmonary dysfunction was relatively high in patients with IBD (93.86%), but few of them showed abnormality in respiratory symptoms (3.51%) and X-ray findings (2.63%), respectively, which was consistent with previously reports.[22] In addition, the pulmonary function damages in IBD are insidious, and thus little attention is paid to the pulmonary complications in IBD patients.[24] The specific incidence of IBD complicated with pulmonary dysfunction is still unclear, but it is underestimated in present studies. On one hand, there are no obvious discomforts in most patients, and thus they may not visit hospital. On the other hand, clinicians occasionally neglect the respiratory symptoms appearing years after the diagnosis of intestinal diseases before the later stage of these diseases. Thus, clinicians should pay more attention to the relationship between IBD and pulmonary dysfunction.
The pathogenesis of pulmonary involvement in IBD is still poorly understood. One proposed hypothesis is the shared embryological derivation of the lung and gastrointestinal tract, and the similarity in the immune systems of pulmonary and intestinal mucosa.[25] A theory proposes that lymphocytes sensitized by the gastrointestinal tract may induce inflammation on the mucosal surfaces of other organs. A study revealed alveolar lymphocytosis in CD patients in spite of free clinical symptoms and normal lung function.[26] Another study indicated the CD4/CD8 ratio significantly increased in induced sputum from patients with active CD,[21] suggesting that there was a migration of intestinal lymphocytes through the general circulation to the intestinal mucosal immune system.[27,28] The gut mucosa with abnormal immunity is associated with increased nonspecific inflammatory mediators, including cytokines, chemokines, growth factors, arachidonic acid metabolites (prostaglandins and leukotrienes) and nitrous oxide. These mediators participate in and promote the mucosal injury. Previous studies also reported that, in the lung tissues of patients with colitis, the activity of oxidases (such as lipid peroxidase and myeloperoxidase) significantly increased, while that of antioxidases (such as superoxide dismutase, catalase, glutathione, and glutathione peroxidase) significantly reduced. This indicates that the production of oxygen-free radicals and oxidation intermediates secondary to oxidative stress leads to lung injury, which may be one of main causes of lung injury associated with IBD.[29] In our study, the duration of disease and ESR were closely related to the pulmonary dysfunction. On the basis of our findings, the duration of disease functions as a protective factor, suggesting that the pulmonary dysfunction might be related to the acute inflammation in the early stage. The severe inflammatory response in early stage may increase the possibility of developing pulmonary dysfunction in IBD patients. With the progression of IBD, the chronicity of inflammation might probably reduce the possibility of developing pulmonary dysfunction. ESR was found as a harmful factor, and IBD patients with higher ESR had a greater risk for developing pulmonary dysfunction. This might be concerned with the duration and severity of inflammation, the severity of the disease itself, and the decreased immune function of the patients. However, the confidence interval of ESR was from 0.994 to 1.202, which may be ascribed to the small sample size and the confounding factors. Further studies with large sample size are required to confirm our findings.
In summary, IBD is a systemic and complicated disease involving a lot of systems including the lung. Its incidence is increasing and there are great challenges in both clinical and basic studies. In clinical practice, IBD patients with a high percentage are caught by pulmonary dysfunction but only a small proportion are identified. Thus clinicians should pay attention to the recognition and diagnosis of lung dysfunction in IBD patients and luckily more and more clinicians have recognized this. Pulmonary function test might be one of the noninvasive means of determining the activity of disease and might contribute to the early detection of latent pulmonary involvement. As other noninvasive methods improved, such as detection of exhaled air (nitric oxide, carbon monoxide, and markers of oxidative stress) and induced sputum (cytological analysis), will allow more in-depth studies with large sample size on IBD patients, which may facilitate a deeper understanding of the pathogenesis responsible for the IBD-related pulmonary diseases.
Footnotes
Abbreviations: CD = Crohn disease, CRP = C-reaction protein, ESR = erythrocyte sedimentation rate, FEV1 = forced expiratory volume in first second, FVC = forced vital capacity, IBD = inflammatory bowel disease, MMEF = maximum mid-expiratory flow, MVV = maximum ventilatory volume, PEF = peak expiratory flow rate, UC = ulcerative colitis, VC = vital capacity.
Funding: This study was supported by the Fundamental Research Funds for the Central Universities (no. 1501219051).
YZ and JW contributed equally to this work and should be considered cofirst authors.
The authors have no conflicts of interest to disclose.
References
- [1].Ponder A, Long MD. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin Epidemiol 2013;5:237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bandyopadhyay D, Bandyopadhyay S, Ghosh P, et al. Extraintestinal manifestations in inflammatory bowel disease: prevalence and predictors in Indian patients. Indian J Gastroenterol 2015;34:387–94. [DOI] [PubMed] [Google Scholar]
- [3].Danese S, Semeraro S, Papa A, et al. Extraintestinal manifestations in inflammatory bowel disease. World J Gastroenterol 2005;11:7227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Mendoza JL, Lana R, Taxonera C, et al. [Extraintestinal manifestations in inflammatory bowel disease: differences between Crohn's disease and ulcerative colitis]. Med Clin (Barc) 2005;125:297–300. [DOI] [PubMed] [Google Scholar]
- [5].Ott C, Takses A, Obermeier F, et al. Smoking increases the risk of extraintestinal manifestations in Crohn's disease. World J Gastroenterol 2014;20:12269–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ricart E, Panaccione R, Loftus EV, Jr, et al. Autoimmune disorders and extraintestinal manifestations in first-degree familial and sporadic inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2004;10:207–14. [DOI] [PubMed] [Google Scholar]
- [7].Mohamed-Hussein AA, Mohamed NA, Ibrahim ME. Changes in pulmonary function in patients with ulcerative colitis. Respir Med 2007;101:977–82. [DOI] [PubMed] [Google Scholar]
- [8].Yilmaz A, Yilmaz Demirci N, Hosgun D, et al. Pulmonary involvement in inflammatory bowel disease. World J Gastroenterol 2010;16:4952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sadler TW. Langman's Medical Embryology. 12th ed Philadelphia: Wolters Kluwer LWW; 2011. [Google Scholar]
- [10].Kraft SC, Earle RH, Roesler M, et al. Unexplained bronchopulmonary disease with inflammatory bowel disease. Arch Intern Med 1976;136:454–9. [PubMed] [Google Scholar]
- [11].Basseri B, Enayati P, Marchevsky A, et al. Pulmonary manifestations of inflammatory bowel disease: case presentations and review. J Crohns Colitis 2010;4:390–7. [DOI] [PubMed] [Google Scholar]
- [12].Mikhailova ZF, Lazebnik LB, Parfenov AI. [Bronchopulmonary lesions in chronic inflammatory bowel diseases]. Ter Arkh 2010;82:61–4. [PubMed] [Google Scholar]
- [13].Papanikolaou I, Kagouridis K, Papiris SA. Patterns of airway involvement in inflammatory bowel diseases. World J Gastrointest Pathophysiol 2014;5:560–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Majewski S, Piotrowski W. Pulmonary manifestations of inflammatory bowel disease. Arch Med Sci 2015;11:1179–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ji XQ, Ji YB, Wang SX, et al. Alterations of pulmonary function in patients with inflammatory bowel diseases. Ann Thorac Med 2016;11:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bernstein CN, Fried M, Krabshuis JH, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. [DOI] [PubMed] [Google Scholar]
- [17].Truelove SC, Witts LJ. Cortisone in ulcerative colitis; final report on a therapeutic trial. Br Med J 1955;2:1041–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Best WR, Becktel JM, Singleton JW, et al. Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology 1976;70:439–44. [PubMed] [Google Scholar]
- [19].Huang V, Mishra R, Thanabalan R, et al. Patient awareness of extraintestinal manifestations of inflammatory bowel disease. J Crohns Colitis 2013;7:e318–24. [DOI] [PubMed] [Google Scholar]
- [20].Peluso R, Manguso F, Vitiello M, et al. Management of arthropathy in inflammatory bowel diseases. Ther Adv Chronic Dis 2015;6:65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tzanakis NE, Tsiligianni IG, Siafakas NM. Pulmonary involvement and allergic disorders in inflammatory bowel disease. World J Gastroenterol 2010;16:299–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Desai D, Patil S, Udwadia Z, et al. Pulmonary manifestations in inflammatory bowel disease: a prospective study. Indian J Gastroenterol 2011;30:225–8. [DOI] [PubMed] [Google Scholar]
- [23].Lippincott Williams & Wilkins, Hyatt RE, Scanlon PD, Nakamura M. Interpretation of Pulmonary Function Tests: A Practical Guide. 4rd edition.2014. [Google Scholar]
- [24].Wang H, Liu JS, Peng SH, et al. Gut-lung crosstalk in pulmonary involvement with inflammatory bowel diseases. World J Gastroenterol 2013;19:6794–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Keely S, Talley NJ, Hansbro PM. Pulmonary-intestinal cross-talk in mucosal inflammatory disease. Mucosal Immunol 2012;5:7–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Raj AA, Birring SS, Green R, et al. Prevalence of inflammatory bowel disease in patients with airways disease. Respir Med 2008;102:780–5. [DOI] [PubMed] [Google Scholar]
- [27].D’Andrea N, Vigliarolo R, Sanguinetti CM. Respiratory involvement in inflammatory bowel diseases. Multidiscip Respir Med 2010;5:173–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fireman E, Masarwy F, Groisman G, et al. Induced sputum eosinophilia in ulcerative colitis patients: the lung as a mirror image of intestine? Respir Med 2009;103:1025–32. [DOI] [PubMed] [Google Scholar]
- [29].Ozyilmaz E, Yildirim B, Aydogdu M, et al. Is there any link between oxidative stress and lung involvement due to inflammatory bowel disease: an experimental study. Hepatogastroenterology 2011;58:1898–903. [DOI] [PubMed] [Google Scholar]


