Abstract
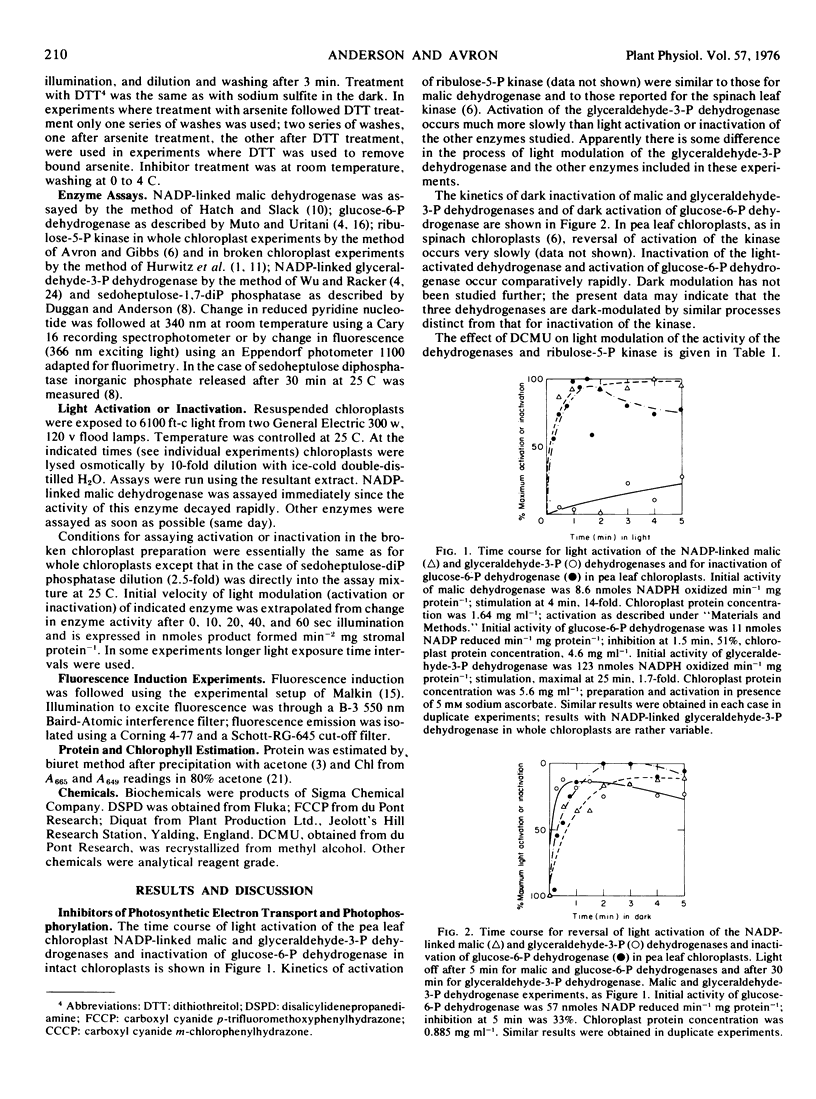
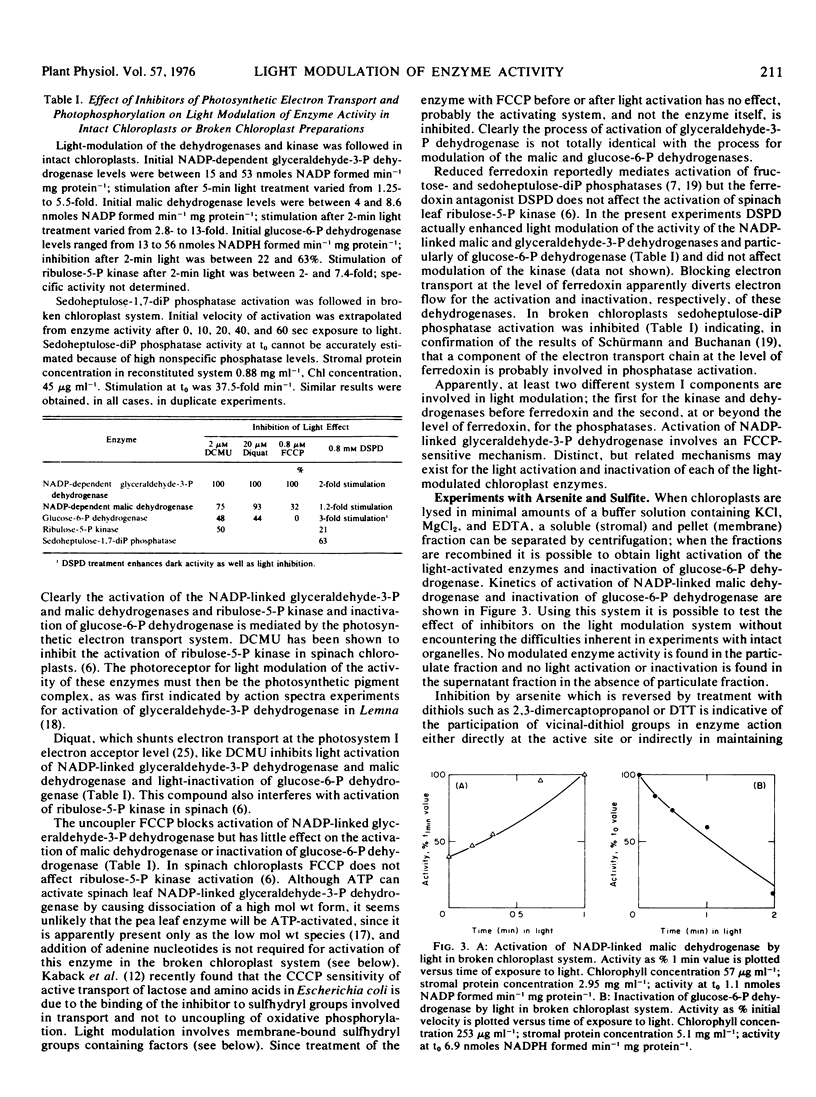
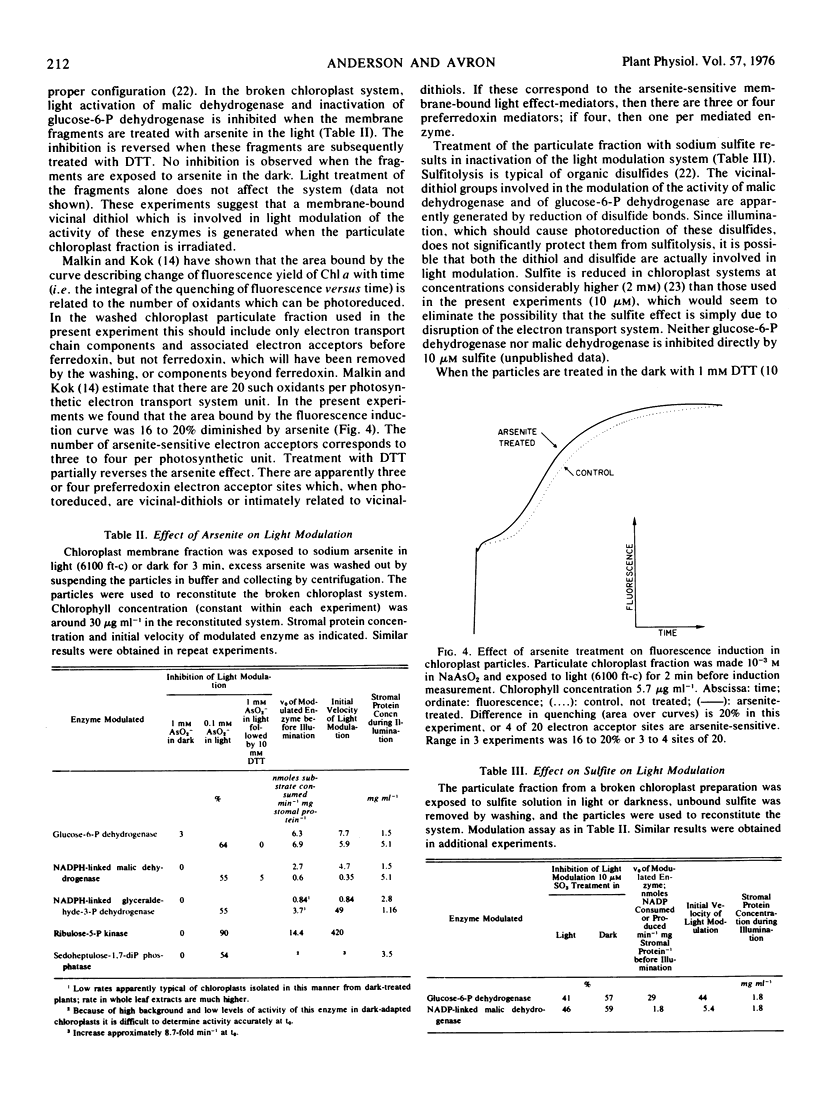
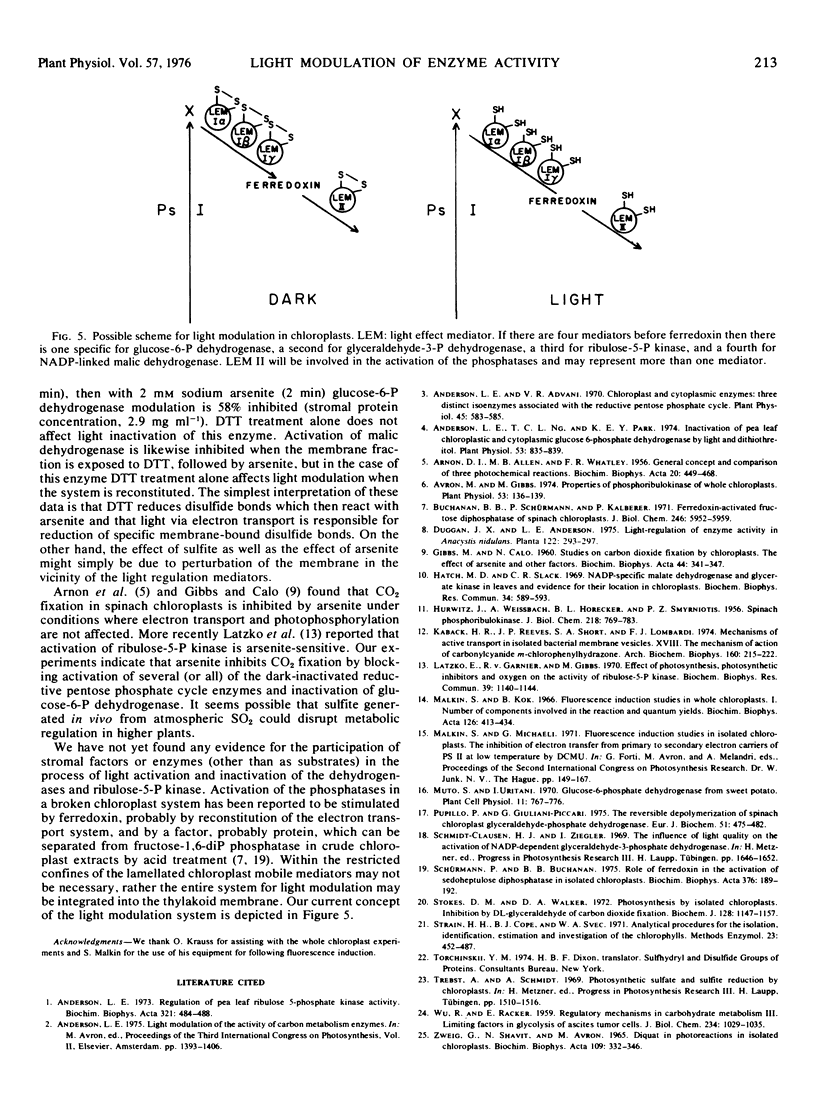
Inhibitor experiments indicate that photosynthetic electron transport is required for light activation of the pea (Pisum sativum) leaf chloroplast enzymes NADP-linked glyceraldehyde-3-phosphate dehydrogenase, NADP-linked malic dehydrogenase, ribulose-5-phosphate kinase and sedoheptulose-1,7-diphosphate phosphatase, and for inactivation of glucose-6-phosphate dehydrogenase. Modulation of the activity of the dehydrogenases and kinase apparently involves a component preceding ferredoxin in the photosynthetic electron transport chain; activation of the phosphatase involves an electron transport component at the level of ferredoxin. Modulation of enzyme activity can be obtained in a broken chloroplast system consisting of membrane fragments and stromal extract. The capacity for light regulation in this system is reduced or eliminated when the membrane fraction is exposed to arsenite in the light or to sulfite in light or dark. Light-generated vicinal-dithiols seem therefore to be involved in modulation of the activity of the enzymes included in this study.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARNON D. I., ALLEN M. B., WHATLEY F. R. Photosynthesis by isolated chloroplasts. IV. General concept and comparison of three photochemical reactions. Biochim Biophys Acta. 1956 Jun;20(3):449–461. doi: 10.1016/0006-3002(56)90339-0. [DOI] [PubMed] [Google Scholar]
- Anderson L. E., Advani V. R. Chloroplast and cytoplasmic enzymes: three distinct isoenzymes associated with the reductive pentose phosphate cycle. Plant Physiol. 1970 May;45(5):583–585. doi: 10.1104/pp.45.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E., Ng T. C., Park K. E. Inactivation of pea leaf chloroplastic and cytoplasmic glucose 6-phosphate dehydrogenases by light and dithiothreitol. Plant Physiol. 1974 Jun;53(6):835–839. doi: 10.1104/pp.53.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. E. Regulation of pea leaf ribulose-5-phosphate kinase activity. Biochim Biophys Acta. 1973 Oct 10;321(2):484–488. doi: 10.1016/0005-2744(73)90190-3. [DOI] [PubMed] [Google Scholar]
- Avron M., Gibbs M. Properties of phosphoribulokinase of whole chloroplasts. Plant Physiol. 1974 Feb;53(2):136–139. doi: 10.1104/pp.53.2.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Schürmann P., Kalberer P. P. Ferredoxin-activated fructose diphosphatase of spinach chloroplasts. Resolution of the system, properties of the alkaline fructose diphosphatase component, and physiological significance of the ferredoxin-linked activation. J Biol Chem. 1971 Oct 10;246(19):5952–5959. [PubMed] [Google Scholar]
- GIBBS M., CALO N. Studies on carbon dioxide fixation by chloroplasts, the effect of arsenite and other factors. Biochim Biophys Acta. 1960 Nov 4;44:341–347. doi: 10.1016/0006-3002(60)91570-5. [DOI] [PubMed] [Google Scholar]
- HURWITZ J., WEISSBACH A., HORECKER B. L., SMYRNIOTIS P. Z. Spinach phosphoribulokinase. J Biol Chem. 1956 Feb;218(2):769–783. [PubMed] [Google Scholar]
- Hatch M. D., Slack C. R. NADP-specific malate dehydrogenase and glycerate kinase in leaves and evidence for their location in chloroplasts. Biochem Biophys Res Commun. 1969 Mar 10;34(5):589–593. doi: 10.1016/0006-291x(69)90778-5. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Reeves J. P., Short S. A., Lombardi F. J. Mechanisms of active transport in isolated bacterial membrane vesicles. 18. The mechanism of action of carbonylcyanide m-chlorophenylhydrazone. Arch Biochem Biophys. 1974 Jan;160(1):215–222. doi: 10.1016/s0003-9861(74)80028-7. [DOI] [PubMed] [Google Scholar]
- Latzko E., von Garnier R., Gibbs M. Effect of photosynthesis, photosynthetic inhibitors and oxygen on the activity of ribulose 5-phosphate kinase. Biochem Biophys Res Commun. 1970;39(6):1140–1144. doi: 10.1016/0006-291x(70)90678-9. [DOI] [PubMed] [Google Scholar]
- Malkin S., Kok B. Fluorescence induction studies in isolated chloroplasts. I. Number of components involved in the reaction and quantum yields. Biochim Biophys Acta. 1966 Nov 8;126(3):413–432. doi: 10.1016/0926-6585(66)90001-x. [DOI] [PubMed] [Google Scholar]
- Pupillo P., Giuliani Piccari G. The reversible depolymerization of spinach chloroplast glyceraldehyde-phosphate dehydrogenase. Interaction with nucleotides and dithiothreitol. Eur J Biochem. 1975 Feb 21;51(2):475–482. doi: 10.1111/j.1432-1033.1975.tb03947.x. [DOI] [PubMed] [Google Scholar]
- Schürmann P., Buchanan B. B. Role of ferredoxin in the activation of sedoheptulose diphosphatase in isolated chloroplasts. Biochim Biophys Acta. 1975 Jan 31;376(1):189–192. doi: 10.1016/0005-2728(75)90217-0. [DOI] [PubMed] [Google Scholar]
- Stokes D. M., Walker D. A. Photosynthesis by isolated chloroplasts. Inhibition by DL-glyceraldehyde of carbon dioxide assimilation. Biochem J. 1972 Aug;128(5):1147–1157. doi: 10.1042/bj1281147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WU R., RACKER E. Regulatory mechanisms in carbohydrate metabolism. III. Limiting factors in glycolysis of ascites tumor cells. J Biol Chem. 1959 May;234(5):1029–1035. [PubMed] [Google Scholar]
- Zweig G., Shavit N., Avron M. Diquat (I,I'-ethylene-2,2'-dipyridylium dibromide) in photo-reactions of isolated chloroplasts. Biochim Biophys Acta. 1965 Nov 29;109(2):332–346. [PubMed] [Google Scholar]


