Introduction
KEY TEACHING POINTS
|
A vagal nerve stimulator (VNS) is a device designed to prevent seizures in patients with drug-refractory epilepsy by providing high-frequency (20–30 Hz) electrical stimulation of the left vagus nerve, activating vagal nerve afferent fibers that carry signals to the brain and reduce seizure activity. Similar to a cardiac pacemaker, the device generator is implanted in the left subpectoral area. A single lead is then attached to the left vagus nerve in the patient’s neck with a cuff, which provides pulsatile electrical stimulation. The device provides cyclical electrical stimulation every 30–300 seconds, and output can be increased by application of a magnet to the chest wall to abort a seizure for patients who experience premonitory symptoms.
Case Report
A 23-year-old male with refractory epilepsy from frequent complex partial seizures since the age of 4 years, which was well-managed with a VNS device (model number 103, Cyberonics, Houston, TX) that had been implanted 6 years earlier and stable doses of lacosamide, levetiracetam, and felbamate, presented to an outside hospital with chest pain, dizziness, and nausea of 1-day duration, with onset while doing manual work. On presentation, the patient was found to be hypotensive and in ventricular tachycardia at 230 beats per minute. Urgent electrical cardioversion was performed in the emergency room; a single 200-J shock restored normal sinus rhythm. Immediately thereafter, the patient began to experience cyclic episodes of complete atrioventricular (AV) block resulting in long pauses, which were associated with lightheadedness and cough. The patient was transferred to our facility for a higher level of care and management of these recurrent episodes of ventricular asystole.
On arrival, the patient appeared to be in distress, and telemetry showed periods of ventricular asystole resulting from sinus slowing with transient complete AV block (Figure 1) lasting 10–15 seconds and occurring every 78 seconds. The patient felt a prodrome to these episodes, followed by lightheadedness, chest discomfort, and urge to cough, and then resolution with a return to normal sinus rhythm with normal AV conduction. Given the patient’s VNS and recent cardioversion, and the apparent vagal mechanism of asystolic episodes, the VNS device was promptly interrogated, showing programming settings and parameters that were unchanged from the previous interrogation 15 months prior (1.5-mA output, 25-Hz signal output with pulse width 250 microseconds, 14-second signal ON, 66-second signal OFF, magnet output 1.75 mA, magnet ON time 60 seconds). Immediately following the deactivation of the device, no further asystolic episodes occurred. Of note, providers at the outside facility had attempted to disable the stimulator by application of a magnet, which does not result in inhibition of therapy as in implantable cardioverter-defibrillators but actually increased the vagal stimulation output of the device, which likely prolonged the episodes of asystole.
Figure 1.
A representative episode of asystole lasting 13 seconds, which was due to high-degree atrioventricular block following electrical cardioversion over the patient’s vagal nerve stimulator.
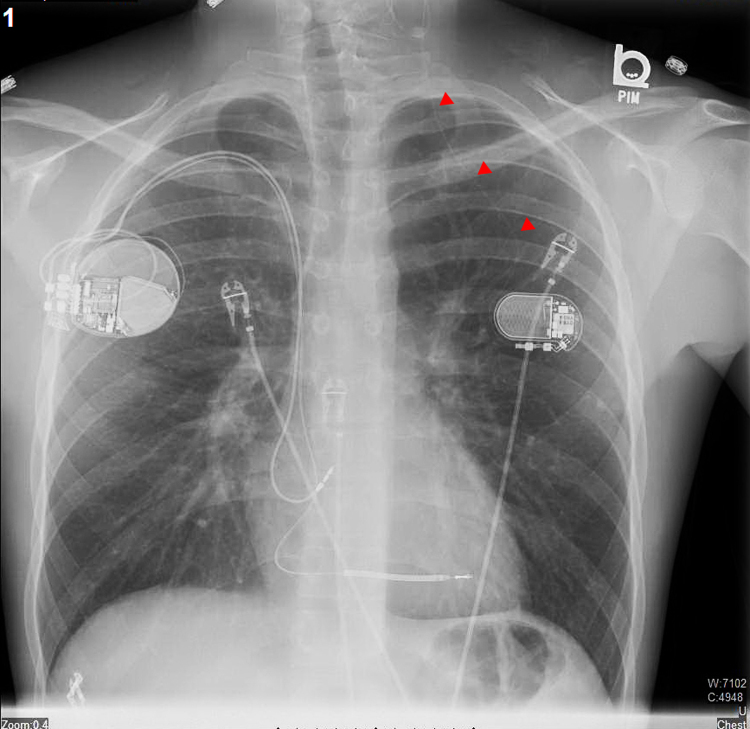
Further evaluation of the patient revealed a troponin level of 56 ng/mL and globally reduced left ventricular ejection fraction of 45%. Laboratory data reports from the outside hospital revealed a magnesium concentration of 0.9 mg/dL, which perhaps contributed to the initial presentation with ventricular tachycardia. During hospitalization at our institution, there were no further electrolyte imbalances. The results of an extensive workup including cardiac positron emission tomography imaging did not reveal structural cardiac abnormalities. Left ventricular function returned to normal by the time of discharge, and his initial reduction in function was attributed to cardiac stunning from prolonged ventricular tachycardia and external cardioversion. An implantable cardioverter-defibrillator for secondary prevention was implanted in the right chest wall because the VNS already occupied the left side (Figure 2). The patient’s VNS remained disabled, and his epilepsy was managed medically, with good control of his seizures.
Figure 2.
A posterioanterior chest radiograph that was obtained following the implantation of a dual-chamber implantable cardioverter-defibrillator in the right chest. The vagal nerve stimulator is seen in the left subpectoral area with a thin lead (red arrows) traversing up to the left vagal nerve in the neck to which is it is attached.
Discussion
We present a case of cyclic asystole characterized by high-degree AV block due to overstimulation of the vagus nerve by malfunction of a VNS, likely from inadvertent external cardioversion over the device. This is the first case of its nature, and this was reported to the US Food and Drug Administration for review.
Malfunction of a VNS by external cardioversion or defibrillation has been cited by the manufacturer as a potential complication, and it is recommended in the physician’s manual that external cardioversion/defibrillation be performed at the lowest energy possible, with defibrillation pads placed perpendicular to the generator and lead system, and device function tested after defibrillation is applied.1 However, when patients present with unstable ventricular arrhythmias or in cardiac arrest and little is known about the medical history, inadvertent cardioversion/defibrillation over the device, which lies within the shock vector of usual defibrillation pad placement, can occur as it did in this case. Whereas no apparent changes to the programmed settings were noted, the causal relationship between VNS malfunction and the episodes of asystole was evident; evidence included cyclic episodes lasting the approximate length of signal ON time (14 seconds), cycles occurring at regular intervals approximately equal to that of the combined signal ON and OFF times (66 and 14 seconds, respectively), and the cessation of asystole episodes immediately on disabling VNS output.
Contrary to the use of a magnet in deactivating tachytherapies of an implantable cardioverter-defibrillator, external magnet application over the VNS pulse generator in the chest wall results in an increase in output and stimulation of the vagal nerve. Depending on a patient’s VNS model, programming, and seizure history, the application of a magnet may augment output such as to provide 30–60 seconds of continuous vagal nerve stimulation in an effort to abort a seizure (in our patient’s case, 60 seconds at 1.75 mA compared with the baseline 1.5-mA output). On implantation of the VNS, the patient is provided with a magnet specific to the device, which is often worn around the wrist similar to how a watch is worn. The patient is instructed to “wave” the magnet over the chest wall at the prodrome of a seizure. Similarly, a family member may be instructed to use this magnet to abort a grand mal seizure in a patient.
The device manufacturer Cyberonics supplies programming wands (Figure 3) to physicians authorized to care for and manage these devices. These wands function similarly to the programmers used for implantable cardiac devices: when placed over the generator, these wands are able to wirelessly gather information about the device settings and transmit them to a computer. Reprogramming of settings, including disabling device function (by changing the stimulation output to zero), can be performed through this programming wand. Short of emergently removing the VNS surgically, there are no other temporizing measures that can be undertaken to halt or deactivate these devices in case of a malfunction without access to a device wand.
Figure 3.
A programming wand for the Cyberonics vagal nerve stimulator. This tool connects to a tablet computer and is placed over the vagal nerve stimulator to interrogate and program device settings.
VNS is a valuable treatment option in refractory epilepsy, and it has been applied to other clinical entities, such as depression. Given the proven benefit of heart-rate-lowering therapies such as beta blockers2 and ivadrabine3 in cardiomyopathy patients, there is also significant interest in modulating the parasympathetic outflow to the heart and its applications in heart failure and arrhythmia prevention; so far, however, clinical trials have not demonstrated significant benefit.4, 5
These devices have been shown to be relatively safe, especially from a cardiovascular perspective, with only a few case reports of severe bradycardia or asystole reported in the literature.6, 7 Both cases of late presentation of asystole, the first occurring 2 years and 4 months and the other 9 years following implantation, were successfully managed by discontinuing VNS therapies, as was done in this case. No case of ventricular arrhythmia has been associated with VNS therapy, and we believe these are unlikely to be associated in this patient’s case. Generally, vagal stimulation provides a protective effect on ventricular arrhythmias, though theoretically torsade de pointes may arise from excessive vagal stimulation and resultant bradycardia; this was not the presenting rhythm in our patient’s case.
Conclusion
Malfunction of a VNS, though rare, can occur after external cardioversion/defibrillation and may result in potentially life-threatening episodes of asystole that can be managed by discontinuing VNS therapies.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Funding: No sources of financial support.
References
- 1.VNS therapy systems physician’s manual for healthcare professionals. Cyberonics [LivaNova], 2015. 〈www.VNStherapy.com〉.
- 2.Packer M., Bristow M.R., Cohn J.N., Colucci W.S., Fowler M.B., Gilbert E.M., Shusterman N.H. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med. 1996;334(21):1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 3.Swedberg K., Komajda M., Bohm M., Borer J.S., Ford I., Dubost-Brama A., Lerebours G., Tavazzi L. SHIFT Investigators. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomized placebo-controlled study. Lancet. 2010;376(9744):875–885. doi: 10.1016/S0140-6736(10)61259-7. [DOI] [PubMed] [Google Scholar]
- 4.Zannad F., De Ferrari G.M., Tuinenburg A.E. Chronic vagal stimulation for the therapy of low ejection fraction heart failure: results of the neural cardiac therapy for heart failure (NECTAR-HF) randomized controlled trial. Euro Heart J. 2015;36(7):425–433. doi: 10.1093/eurheartj/ehu345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauptman P., Schwartz P., Gold M., Borggrefe M., Van Veldhuisen D., Starling R., Mann D.-H.F. Am Heart J. 2012;163(6):954–962. doi: 10.1016/j.ahj.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Amark P., Stodberg T., Wallstedt L. Late onset bradyarrhythmia during vagus nerve stimulation. Epilepsia. 2007;48(5):1023–1025. doi: 10.1111/j.1528-1167.2007.01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Iriarte J., Urrestarazu E., Alegre M., Macias A., Gomez A., Amaro P., Artieda J., Viteri C. Late-onset periodic asystolia during vagus nerve stimulation. Epilepsia. 2008;50(4):928–932. doi: 10.1111/j.1528-1167.2008.01918.x. [DOI] [PubMed] [Google Scholar]