Introduction
KEY TEACHING POINTS
|
Sudden cardiac death (SCD) in young athletes is always dramatic, because unexpected, and is sometimes a challenge to investigate. The prevalence is estimated at 0.6 per 100,000 in the United States.1 Coronary anomalies are the second most common cause of SCD in young athletes (16%) but are, unfortunately, difficult to recognize before a serious clinical event. Wolff-Parkinson-White (WPW) syndrome accounts for only 1.6% of all SCD.1
We present the case of a young athlete with aborted SCD and both WPW syndrome and an anomalous right coronary artery (RCA) origin.
Case report
A 19-year-old student with no prior cardiac history presented with aborted SCD while playing hockey at his university arena. He was resuscitated by his teammates using an external automated cardiac defibrillator. He reported palpitations and chest discomfort before passing out.
He had no family history of SCD or syncope. Two years before, he had a normal resting electrocardiogram (ECG), negative exercise testing, and a normal Holter monitoring after complaining of chest pain and palpitations.
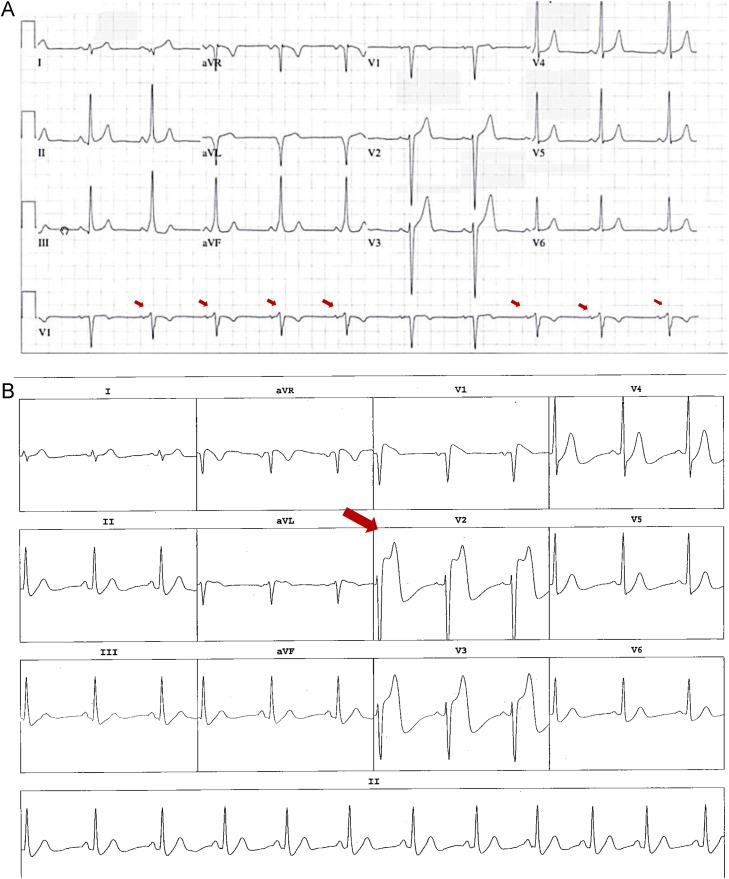
The physical examination was normal. The initial echocardiogram showed a structurally normal heart. The baseline ECG (Figure 1A) showed intermittent preexcitation with a positive delta wave in leads V1, DII, DIII, and aVF and negative delta wave in DI and AVL. This was consistent with a left anterolateral accessory pathway. Since the preexcitation was intermittent, the hypothesis that the WPW was responsible for his SCD had to be challenged. An electrophysiological study (EPS) was performed and confirmed the presence of an accessory pathway. The preexcitation was intermittent even on isoproterenol infusion, suggesting a low-risk accessory pathway, since the anterograde refractory period was 320 ms (Figure 2A). Orthodromic tachycardia was induced on isoproterenol infusion with a tachycardia cycle length of 315 ms (Figure 2B).
Figure 1.
A: Baseline electrocardiogram showing intermittent left anterolateral accessory pathway. Red arrows show intermittent delta waves. B: Anterior ST segment elevation on isoproterenol infusion. Red arrow shows marked ST elevation in the precordial leads.
Figure 2.
A: Accessory pathway refractory period determination (320 ms) followed by 2 echo beats. B: Orthodromic tachycardia triggered by 2 ventricular premature complexes.
Furthermore, during the isoproterenol infusion, anteroseptal ST-segment elevation was documented (Figure 1B). The accessory pathway was successfully ablated without complication using the retrograde aortic approach. Because we already had arterial access for the ablation, we performed a coronary angiogram in the context of dynamic ECG changes during the isoproterenol infusion (supplemental material, available online). An abnormal RCA origin from the left sinus of Valsalva just above the left main artery was documented. Cardiac computed tomography (CT) confirmed a “slit-like” RCA coursing between the aortic root and the pulmonary trunk (Figure 3A). This was equivalent to a 70% stenosis at rest.
Figure 3.
Preoperative cardiac computed tomography with 3-D reconstruction (A, B) and postoperative reconstruction (C, D). LMA = left main artery; RCA = right coronary artery.
During cardiac surgery, the surgeon described a proximal aortic intramural course of the RCA treated by translocation of the ostium to the right sinus using an “unroofing technique” combined with an aortoplasty. Postoperative CT confirmed the complete correction (Figure 3B).
At 3 months follow-up, the patient did not report any recurrence of palpitation and had a normal ECG. He underwent a stress test myocardial perfusion scintigraphy with no evidence of residual ischemia. He resumed his normal activities, including hockey, with no events after 24 months follow-up.
Discussion
The overall risk of SCD in WPW syndrome is estimated at 0.1% per year2 in asymptomatic patients and 0.3% per year in symptomatic patients.3 Ventricular fibrillation is generally secondary to atrial fibrillation, leading to extremely rapid ventricular response in the presence of an accessory pathway with critically short anterograde refractory period (usually <250 ms). EPS has a central role in assessing the electrophysiological properties of the accessory pathway. In our patient, a previous noninvasive investigation found no evidence of preexcitation and the stress test was negative despite complaints of palpitations and chest pain. Post resuscitation, the ventricular preexcitation was intermittent and the echocardiogram showed no structural abnormality. We performed the EPS to confirm the presence of the accessory pathway and found it to be benign even on isoproterenol infusion. Because orthodromic tachycardia was inducible, the ablation was performed. With ST-T changes on isoproterenol infusion and history of aborted SCD during vigorous exercise, we completed the investigation with an angiogram to exclude an anomaly of the coronary arteries.
Intermittent loss of preexcitation at physiological heart rates has high specificity to identify patients at very low risk for SCD. However, Pauriah et al4 found that baseline EPS missed 16% of patients at risk of life-threatening arrhythmias when isoproterenol infusion was performed. Moreover, Gemma et al5 reported a patient with intermittent preexcitation and the shortest R-R interval in atrial fibrillation at 230 ms. In our patient, even on isoproterenol infusion, the accessory pathway anterograde refractory period remained long.
Both WPW and coronary anomalies are well-known causes of SCD. Their occurrence in the same patient is relatively novel. Yagita et al6 published a case of WPW syndrome associated with an anomalous RCA origin from the left sinus responsible for anterolateral ischemia on exercise scintigraphy. It was suspected that a left coronary steal phenomenon occurred during exercise to explain the ischemic territory, since the RCA origin is just above the left main artery. In our patient, during isoproterenol infusion, the ST-segment elevation was anteroseptal in location, probably related to the same phenomenon. It is, however, unknown if orthodromic tachycardia in our patient could have favored ischemia in the presence of his coronary anomaly.
The prevalence of athletic field SCD has been estimated at 0.6 per 100,000 per year1 in high school–age athletes in the United States registry. Maron et al1 reported 690 exertional cardiovascular-related SCDs in young athletes from 1980 to 2006. The main causes of death were hypertrophic cardiomyopathy (251/690; 36%) and coronary anomalies (119/690; 17%). Anomalous left main coronary artery from the right sinus of Valsalva was found in 65 patients and an anomalous RCA from the left sinus in 16 patients. In comparison, WPW syndrome was involved in only 11 of 690 patients (1.6%).
These coronary artery anomalies are difficult to diagnose before a catastrophic event. Indeed, Basso et al7 reported 27 sudden deaths in young athletes identified at autopsy with left main coronary artery from the right sinus (n = 23) or RCA from the left sinus (n = 4). In this review, it was proposed in case of strong clinical suspicion and normal screening tests (resting ECG, stress test) to explore those anomalies with noninvasive tests such as transesophageal echocardiography focused on coronary artery anatomy, cardiac CT, or magnetic resonance imaging.
The optimal surgical management for these anomalies (bypass surgery, reimplantation, or unroofing techniques) remains controversial. Bypass surgery is associated with a higher rate of graft thrombosis8 because of the competitive flow from the native vessels. This flow competition can be controlled by ligating the proximal portion of the bypassed vessel, but then the distal flow will rely on a conduit that might eventually suffer from attrition. Reimplantation surgery requires extensive dissection and does not address an intramural course. In the unroofing procedure, the ostium of the RCA is modified at the aorta by excising (“unroofing”) the common wall located between the aorta and the anomalous coronary artery. This procedure is associated with excellent results. In a single center experience, Sharma et al9 reported a low morbidity and mortality with no aortic valve incompetency in 75 patients. Two of them needed right internal mammary artery–to-RCA grafting owing to flow acceleration at the RCA ostium. All patients remained free of cardiac symptoms at a mean 18 months follow-up. There was 1 noncardiac death.
Conclusion
This case report invites us to remain vigilant and critical when assessing an aborted SCD. Even in the presence of a WPW pattern on the resting ECG, a benign accessory pathway cannot lead to ventricular fibrillation and other differential diagnoses must be considered. It also reminds us that congenital coronary artery anomalies are the second most common cause of SCD in young athletes, and symptoms at exertion, if present, should be appropriately explored by transesophageal echocardiography, cardiac CT, coronary angiography, or magnetic resonance imaging if routine tests are negative.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.hrcr.2016.05.004.
Appendix. Supplementary data
Video 1. This video was recorded during coronary angiography at the time of catheter ablation. The right coronary artery originates from the left sinus of Valsalva.
References
- 1.Maron B.J., Doerer J.J., Haas T.S., Tierney D.M., Mueller F.O. Sudden deaths in young competitive athletes: analysis of 1866 deaths in the United States, 1980-2006. Circulation. 2009;119:1085–1092. doi: 10.1161/CIRCULATIONAHA.108.804617. [DOI] [PubMed] [Google Scholar]
- 2.Obeyesekere M.N., Leong-Sit P., Massel D., Manlucu J., Modi S., Krahn A.D., Skanes A.C., Yee R., Gula L.J., Klein G.J. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: a meta-analysis. Circulation. 2012;125:2308–2315. doi: 10.1161/CIRCULATIONAHA.111.055350. [DOI] [PubMed] [Google Scholar]
- 3.Pappone C., Vicedomini G., Manguso F. Risk of malignant arrhythmias in initially symptomatic patients with Wolff-Parkinson-White syndrome: results of a prospective long-term electrophysiological follow-up study. Circulation. 2012;125:661–668. doi: 10.1161/CIRCULATIONAHA.111.065722. [DOI] [PubMed] [Google Scholar]
- 4.Pauriah M., Cismaru G., Sellal J.M., De Chillou C., Brembilla-Perrot B. Is isoproterenol really required during electrophysiological study in patients with Wolff-Parkinson-White syndrome? J Electrocardiol. 2013;46:686–692. doi: 10.1016/j.jelectrocard.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Gemma L.W., Steinberg L.A., Prystowsky E.N., Padanilam B.J. Development of rapid preexcited ventricular response to atrial fibrillation in a patient with intermittent preexcitation. J Cardiovasc Electrophysiol. 2013;24:347–350. doi: 10.1111/j.1540-8167.2012.02398.x. [DOI] [PubMed] [Google Scholar]
- 6.Yagita M., Senda Y., Nakashima Y., Kuroiwa A., Nakayama C. A case of anomalous origin of the right coronary artery from the left sinus of Valsalva exhibiting the Wolff-Parkinson-White syndrome. Eur Heart J. 1986;7:262–267. doi: 10.1093/oxfordjournals.eurheartj.a062061. [DOI] [PubMed] [Google Scholar]
- 7.Basso C., Maron B.J., Corrado D., Thiene G. Clinical profile of congenital coronary artery anomalies with origin from the wrong aortic sinus leading to sudden death in young competitive athletes. J Am Coll Cardiol. 2000;35:1493–1501. doi: 10.1016/s0735-1097(00)00566-0. [DOI] [PubMed] [Google Scholar]
- 8.Tavaf-Motamen H., Bannister S.P., Corcoran P.C., Stewart R.W., Mulligan C.R., DeVries W.C. Repair of anomalous origin of right coronary artery from the left sinus of Valsalva. Ann Thorac Surg. 2008;85:2135–2136. doi: 10.1016/j.athoracsur.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 9.Sharma V., Burkhart H.M., Dearani J.A., Suri R.M., Daly R.C., Park S.J., Horner J.M., Phillips S.D., Schaff H.V. Surgical unroofing of anomalous aortic origin of a coronary artery: a single-center experience. Ann Thorac Surg. 2014;98:941–945. doi: 10.1016/j.athoracsur.2014.04.114. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video 1. This video was recorded during coronary angiography at the time of catheter ablation. The right coronary artery originates from the left sinus of Valsalva.