Introduction
KEY TEACHING POINTS
|
Aconite is a potentially toxic root from which herbal preparations are used to treat common somatic ailments from headache to heartburn in the Southeast Asian population. Lack of awareness of aconite root use in the growing Hmong population in the United States (US) poses a potential for loss of life. We present a case of acute aconitine poisoning in a middle-aged Hmong man in whom (1) a broad spectrum of electrocardiographic changes and dysrhythmias manifested, and (2) critical illness and shock developed, requiring aggressive life support to prevent loss of life.
Case report
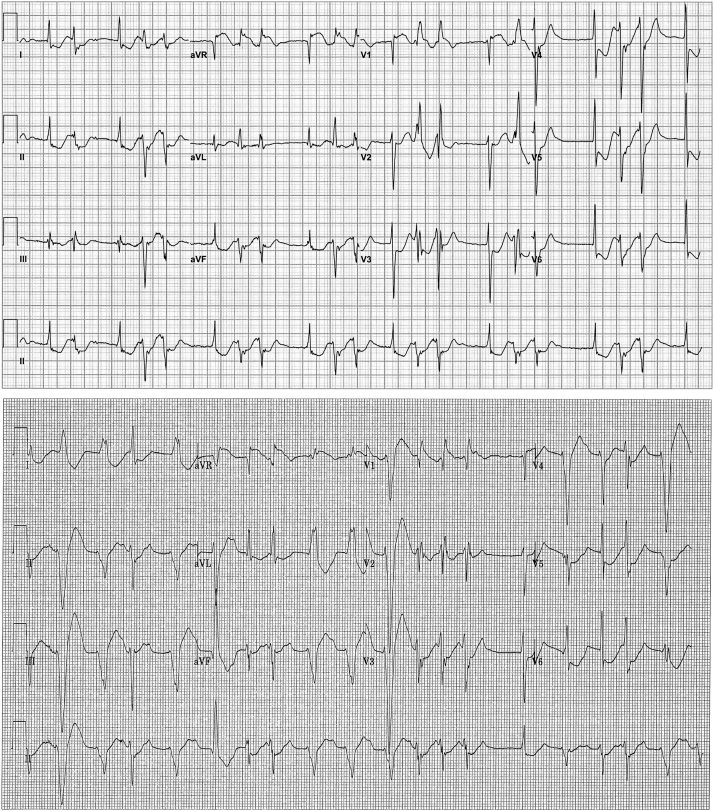
A 62-year-old Hmong man presented to the local emergency room with complaints of sudden-onset chest pain, dizziness, and palpitations. Little other history was obtainable owing to language barrier. The patient appeared in mild distress, exhibiting generalized weakness. Initial vital signs demonstrated hypotension and tachycardia with systolic blood pressure 60–80 mm Hg and heart rate 150–220 beats per minute. His initial electrocardiogram (ECG) (Figure 1, top) demonstrated sinus rhythm with low-amplitude P waves and junctional rhythm conducting with narrow QRS complex, followed by ventricular ectopics or couplets with right bundle branch block (RBBB) morphology and left axis deviation suggestive of left posterior fascicular origin. Diffuse ST-segment depression was present and the QT interval was prolonged. Ventricular ectopics occurred during the terminal phase of the T wave.
Figure 1.
Presenting electrocardiogram (ECG) (top) and ECG showing progressive changes in atrioventricular conduction (bottom). Both ECGs also demonstrate ventricular ectopy.
Initial blood tests revealed hypokalemia (2.9 mmol/L) and acidosis (pH 7.21) with increased anion gap (17) and elevated lactate levels (9.7 mmol/L). Troponin T (<0.01 ng/mL) and digoxin levels (0.1 ng/mL) were not elevated. Intravenous saline was administered. Bedside echocardiography demonstrated hyperdynamic left ventricular function with no regional wall motion abnormalities and no evidence of pericardial effusion. There were no findings of Takotsubo cardiomyopathy. Concern for coronary ischemia and lactic acidosis prompted urgent coronary angiography, which demonstrated nonobstructive coronary atherosclerosis with slow coronary flow. Ventriculography confirmed echocardiographic findings, demonstrating hyperdynamic left ventricular function with no wall motion abnormalities.
Vasodilatory shock was diagnosed. Poor response to fluids necessitated hemodynamic support with intravenous pressors and intraaortic balloon pump. The patient was intubated for impending respiratory failure. Blood cultures were drawn and empiric antibiotics initiated.
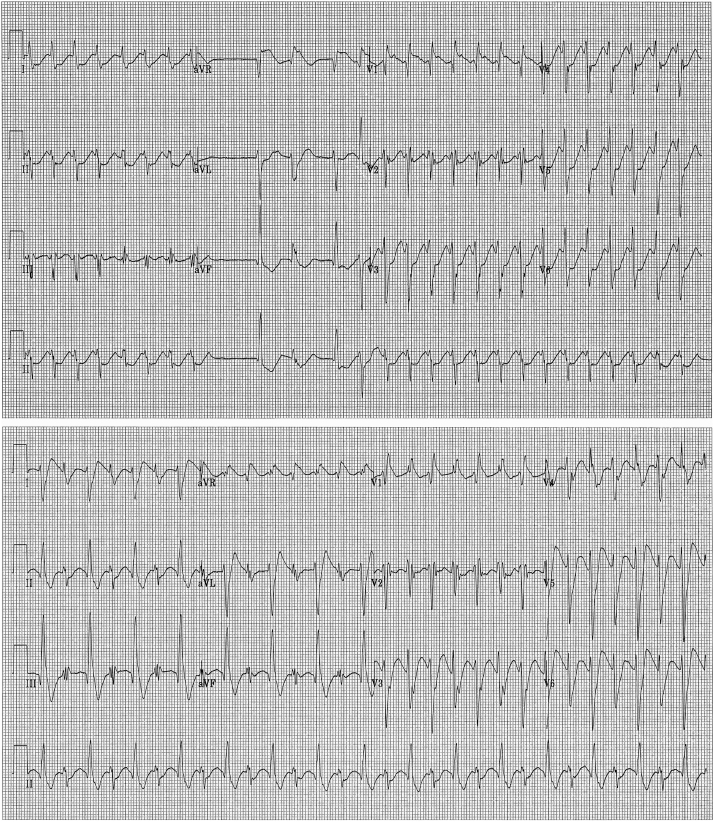
Incessant dysrhythmias continued, including progressive atrioventricular (AV) conduction delay, left axis deviation in beats conducting with variable QRS morphologies including left bundle branch block (LBBB), RBBB ventricular ectopy, nonsustained monomorphic ventricular tachycardia (VT) (Figure 1, bottom), sustained monomorphic VT (Figure 2, top), and bidirectional VT characterized by RBBB morphology and alternating QRS axis (Figure 2, bottom). Ventricular ectopy and sustained arrhythmias persisted despite treatment with intravenous amiodarone. Cardioversion restored sinus rhythm but with generally little impact on hemodynamics and with continued recurrence of dysrhythmias. Hence, further cardioversion was avoided. Empiric use of hemodynamic dialysis was considered but was deferred because of ongoing hemodynamic instability.
Figure 2.
Development of sustained monomorphic (top) and bidirectional (bottom) ventricular tachycardia.
English-speaking family members were eventually found and presented to provide additional history. The patient has a history of “heartburn” but was not taking any prescription medications. He had been in his usual health on the day of presentation. Later he developed his usual heartburn symptom and ingested an herbal decoction made from boiling various herbs and roots. His symptoms did not improve over an hour and he repeated the elixir 3–4 times. Over the next half-hour, he developed chest pain, dizziness, and generalized weakness with severe nausea, vomiting, and abdominal pain. The family confirmed use of aconite root. Serum aconitine level was not obtained, owing to the late discovery of this information.
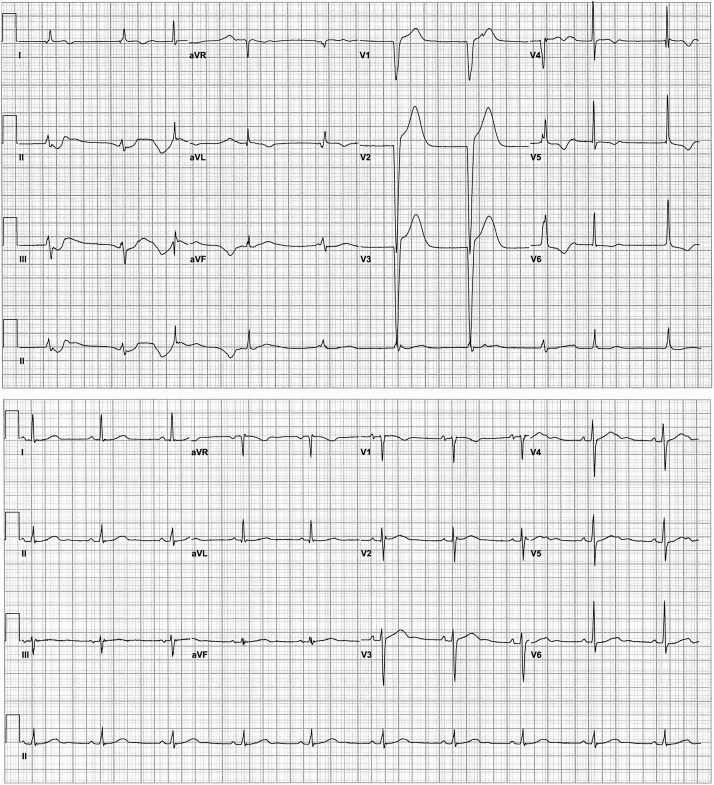
The patient improved both hemodynamically and electrically, and supportive care was weaned successfully over the next 48 hours. Ventricular ectopy and VT resolved and the rhythm transitioned through AV block with junctional broad and narrow escape rhythm (Figure 3, top) back to sinus rhythm with intact conduction and normal PR and QRS intervals with nonspecific T-wave abnormalities (Figure 3, bottom). The patient was discharged uneventfully and counseled against aconite root use. In follow-up, the patient has not had a recurrence of dizziness or weakness. He does still use aconite root, but only in limited amounts.
Figure 3.
Resolution of ventricular tachycardia and ventricular ectopy with persistent but improving conduction abnormalities (top) and near-complete resolution (bottom).
Discussion
Aconitine is a toxin found in the Aconitum plant, also known as “devil’s helmet” or “monkshood” (Aconitum napellus) and wolfsbane (Aconitum vulparia), known for its toxic properties. The use of aconite root for medicinal purposes is common in the Hmong, Asian, and Indian populations to treat various ailments, including heartburn and headache.1 Raw aconite roots are extremely toxic and must be processed to reduce the alkaloid content before use. Inadequate processing or ingestion of higher doses of the decoction of aconite roots increases the risk of poisoning.2
Pure aconitine has low bioavailability with little variability in the pharmacokinetic behavior after single or multiple administrations. In contrast, multiple administrations of processed Fuzi extract, the processed lateral roots of Aconitum, could increase the bioavailability of aconitine, which results in toxicity.3
Cellular mechanisms
Aconitine interacts with the voltage-dependent sodium channel present on cell membranes of excitable tissues, including myocardium, striated and smooth muscle, and neurons, altering membrane depolarization and repolarization. Aconitine binds with high affinity to the voltage-sensitive sodium channel in its open state and suppresses the conformational change to the inactive state,4 delaying repolarization by prolonging sodium influx and membrane depolarization. Conformational changes to the channel induced by aconitine binding shift the activation threshold of the channel to a more negative potential,2 while the inactivation curve is shifted toward a more positive potential, resulting in prolonged opening of the channel that predisposes to the development and persistence of triggered activity.1
At lower concentrations, aconite increases the strength of muscle fiber contraction by increasing acetylcholine release from nerve endings owing to depolarization. In synaptic clefts, the released acetylcholine binds to postsynaptic cholinergic receptors to activate sodium channels, generating action potential and muscle contraction. At higher concentrations, persistence of sodium channels in the open state results in suppression of action potential transmission and reduced axonal end-terminus acetylcholine release and depression of muscle contraction.2
In addition, the powerful increase in late sodium channel current owing to delayed sodium channel inactivation promotes conditions for early afterdepolarization development by prolonging depolarization, as well as activation of the sodium-calcium exchanger generating a transient inward current and development of delayed afterdepolarization;1 thus, aconitine-induced triggered activity is related to increased late INa.
Electrocardiographic manifestations and possible underlying mechanisms
Important ECG manifestations observed in this case were supraventricular abnormalities, including low-amplitude P waves and sinus bradycardia; AV conduction abnormalities with conduction prolongation and fascicular and bundle branch block; repolarization abnormalities with QT prolongation; and ventricular ectopy and nonsustained and sustained VT with monomorphism, as well as bidirectionality that exhibited sharp, rapid QRS onset with RBBB morphology suggesting left fascicular origin.
The binding of aconitine to the sodium channel promoting persistence of the activated state can explain the ECG findings in this patient. The AV interval was observed to prolong with LBBB, suggesting conduction delay within and/or below the His bundle.5 Preferential block in the left system fascicles or proximal block in the left bundle system may contribute to left fascicular and bundle branch blocks, respectively, which could be attributable to prolonged depolarization of the His-Purkinje system owing to persistence of sodium channel activation.
The persistence of the activated states occurs at a more polarized transmembrane voltage than normal, thereby facilitating development of triggered activity from early afterdepolarization and increasing the risk of triggered ventricular arrhythmias.2, 6 Salvos of monomorphic VT and bidirectional VT were observed, presumably related to this enhanced triggered activity.
The mechanism of QT prolongation related to persistent sodium influx, also seen in long QT syndrome 3, has been described by many investigators, in which delayed inactivation of the late sodium channel results in prolonged sodium influx during the action potential plateau, prolonging action potential duration and QT interval.6 This mechanism likely explains the QT interval prolongation seen in our patient during conducted and junctional beats.
The sinus P waves were difficult to identify and were nonexistent to low-amplitude at presentation. P-wave amplitude fluctuated on successive ECGs. In addition, conducted beats developed a broad LBBB morphology with delayed and prolonged intrinsicoid deflection. After discontinuation of aconitine and clinical improvement over time, the P waves appeared, albeit at a slow sinus rate, permitting an escape rhythm, possibly junctional or fascicular/ventricular origin with narrower QRS than during LBBB morphology–conducted beats, manifesting as AV dissociation. With complete clinical resolution, sinus P waves assumed their normal amplitude and morphology with intact AV conduction and normal ECG intervals. Atrial and ventricular myocardium is rich in sodium channels like the His-Purkinje system, and aconitine-induced abnormalities in membrane sodium regulation can explain the intramyocardial atrial and ventricular conduction abnormalities observed.
Other cardiovascular effects of aconite include hypotension, chest pain, palpitations, bradycardia, and sinus tachycardia. The hypotensive and bradycardic actions of aconitine are attributable to central activation of the ventromedial nucleus of the hypothalamus.2 The latter plays an important role in controlling autonomic nervous system activity and is associated with depression of the circulatory system, as seen in our patient presenting with vasodilatory shock. In addition, aconitine has a positive inotropic effect, also observed in our patient during ventriculography, related to prolonged sodium influx during the action potential owing to persistence of the open state.2
Peripheral nerve manifestations include numbness and motor weakness.7 Aconitine, mesaconitine, and hypaconitine can all induce strong contractions of the ileum through acetylcholine release from the postganglionic cholinergic nerves and cause gastrointestinal symptoms such as nausea, vomiting, and diarrhea.
Diagnosis, management, and clinical course
Aconite poisoning usually appears approximately 20 minutes to 2 hours after oral intake and includes paraesthesia, sweating, and nausea, which is followed by vomiting, diarrhea, abdominal pain, and then skeletal muscle paralysis.
The diagnosis of aconitine poisoning is made by obtaining history compatible with persistence of the sodium channel–activated state (ie, cardiac, neuromuscular, and gastrointestinal manifestations) and suggestion or confirmation of aconite use. Two milligrams of pure aconite or 1 g of aconite plant may cause death.8 Serum aconitine levels may be measured as well, but little is known regarding nontoxic levels. Aconitine poisoning is managed with supportive care. Atropine can be used to counteract bradycardia. Vasopressor support and even cardiopulmonary bypass have been used to maintain perfusion in refractory shock.7, 9 Ventricular arrhythmias are frequently refractory to cardioversion owing to triggered activity that permits arrhythmia persistence and are often only minimally responsive to antiarrhythmics; hence aggressive supportive care is the mainstay.
Following the onset of life-threatening arrhythmia, including VT/ventricular fibrillation, death may occur as a result of respiratory paralysis or cardiac arrest. The overall in-hospital mortality with clinical aconite poisoning has been reported to be 5.5%.
Prior cases
There have been very few reported cases of aconite poisoning causing ventricular arrhythmias in the US and only 1 other reported case of bidirectional VT in the US.7 Some cases have been reported in Taiwan and Hong Kong,7, 10 with similar presentation of bidirectional VT after consuming aconite root. In 1 case the arrhythmia was susceptible to vagotonic maneuvers, cholinesterase inhibition, and adenosine triphosphate.10 Our case is unusual in that a full spectrum of bradyarrhythmic, tachyarrhythmic, and hemodynamic consequences manifested in a single patient.
The most common cause of mortality from aconite is ventricular arrhythmias. Intravenous antiarrhythmic agents with sodium channel–blocking properties, including amiodarone, flecainide, procainamide, and mexiletine,11, 12, 13 seem to be partially effective.4 The mechanism is felt to be related to suppressing persistence of the sodium channel active state.12 The use of percutaneous cardiopulmonary support7 and cardiopulmonary bypass9 for cardiogenic shock from refractory VT after aconite ingestion has been reported as well.
Patients usually stabilize within 24–48 hours with excretion of aconitine in the urine. Hence, careful attention must be paid to maintaining renal perfusion and function. Activated charcoal can be used acutely to reduce gastric absorption when patients present within 30–60 minutes of consumption.
The Hmong population in the US has more than quadrupled since 1980 and is now estimated at 250,000–300,000. As of 2010, more than 90,000 Hmong live in California, more than 60,000 live in Minnesota, and more than 50,000 Hmong live in Wisconsin,14 where our case occurred. While the large majority of Hmong have settled in these 3 states, the Hmong can be found in every state in the US. Awareness of the role of aconite as an herbal remedy in their culture, and in particular this unique constellation of cardiac, neurologic, and gastrointestinal manifestations with shock and arrhythmias, may aid in rapid recognition, diagnosis, and appropriate management of aconitine toxicity.
Acknowledgments
The authors acknowledge Susan Nord and Jennifer Pfaff for editorial preparation, and Brian J. Miller and Brian Schurrer for assistance with figures.
Footnotes
Dr. Jahangir’s research effort was, in part, supported by the National Heart, Lung, and Blood Institute grants RO1 HL101240 and R01 HL089542.
References
- 1.Dhesi P., Ng R., Shehata M.M., Shah P.K. Ventricular tachycardia after ingestion of ayurveda herbal antidiarrheal medication containing aconitum. Arch Intern Med. 2010;170:303–305. doi: 10.1001/archinternmed.2009.518. [DOI] [PubMed] [Google Scholar]
- 2.Chan T.Y. Aconite poisoning presenting as hypotension and bradycardia. Hum Exp Toxicol. 2009;28:795–797. doi: 10.1177/0960327109353056. [DOI] [PubMed] [Google Scholar]
- 3.Tang L., Gong Y., Lv C., Ye L., Liu L., Liu Z. Pharmacokinetics of aconitine as the targeted marker of Fuzi (Aconitum carmichaeli) following single and multiple oral administrations of Fuzi extracts in rat by UPLC/MS/MS. J Ethnopharmacol. 2012;141:736–741. doi: 10.1016/j.jep.2011.08.070. [DOI] [PubMed] [Google Scholar]
- 4.Yeih D.F., Chiang F.T., Huang S.K. Successful treatment of aconitine induced life threatening ventricular tachyarrhythmia with amiodarone. Heart. 2000;84:E8. doi: 10.1136/heart.84.4.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akhtar M., Gilbert C., Al-Nouri M., Denker S. Site of conduction delay during functional block in the His-Purkinje system in man. Circulation. 1980;61:1239–1248. doi: 10.1161/01.cir.61.6.1239. [DOI] [PubMed] [Google Scholar]
- 6.Clancy C.E., Tateyama M., Kass R.S. Insights into the molecular mechanisms of bradycardia-triggered arrhythmias in long QT-3 syndrome. J Clin Invest. 2002;110:1251–1262. doi: 10.1172/JCI15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fujita Y., Terui K., Fujita M., Kakizaki A., Sato N., Oikawa K., Aoki H., Takahashi K., Endo S. Five cases of aconite poisoning: toxicokinetics of aconitines. J Anal Toxicol. 2007;31:132–137. doi: 10.1093/jat/31.3.132. [DOI] [PubMed] [Google Scholar]
- 8.Singh S., Fadnis P.P., Sharma B.K. Aconite poisoning. J Assoc Physicians India. 1986;34:825–826. [PubMed] [Google Scholar]
- 9.Ohuchi S., Izumoto H., Kamata J., Kawase T., Ishibashi K., Eishi K., Kawazoe K. [A case of aconitine poisoning saved with cardiopulmonary bypass] Kyobu Geka. 2000;53:541–544. [PubMed] [Google Scholar]
- 10.Tai Y.T., Lau C.P., But P.P., Fong P.C., Li J.P. Bidirectional tachycardia induced by herbal aconite poisoning. Pacing Clin Electrophysiol. 1992;15:831–839. doi: 10.1111/j.1540-8159.1992.tb06849.x. [DOI] [PubMed] [Google Scholar]
- 11.Yan G.X., Antzelevitch C. Cellular basis for the Brugada syndrome and other mechanisms of arrhythmogenesis associated with ST-segment elevation. Circulation. 1999;100:1660–1666. doi: 10.1161/01.cir.100.15.1660. [DOI] [PubMed] [Google Scholar]
- 12.Lin C.C., Chan T.Y., Deng J.F. Clinical features and management of herb-induced aconitine poisoning. Ann Emerg Med. 2004;43:574–579. doi: 10.1016/j.annemergmed.2003.10.046. [DOI] [PubMed] [Google Scholar]
- 13.Tai Y., Lau C., Young K., But P.P. Cardiotoxicity after accidental herb-induced aconite poisoning. Lancet. 1992;340:1254–1256. doi: 10.1016/0140-6736(92)92951-b. [DOI] [PubMed] [Google Scholar]
- 14.Hmong.org. Hmong American Partnership; c2009. 2010 Census Hmong and Southeast Asian Americans Data > 2010 Census Hmong Populations by State. 2010. Available at: http://www.hmong.org/page33422626.aspx. Accessed July 2, 2014.