KEY TEACHING POINTS
|
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a genetically determined heart muscle disease with a wide spectrum of clinical manifestations, ranging from concealed myocardial abnormalities to overt clinical disease with life-threatening arrhythmias and/or ongoing heart failure.1 Owing to the heterogeneous nature of the disease and its clinical manifestations, there is not a single criterion that determines a definitive diagnosis; even histopathologic findings such as fibrofatty myocardial replacement, although suggestive of the disease, could be a final common pathway of several types of myocardial injury. For these reasons a multiparametric approach based on several criteria has been adopted.2 Since the publication of the original Task Force criteria in 1994, attempts have been made to improve their sensitivity, leading to the release of the modified Task Force criteria in 2010.3 In the last decade, advanced technologies have emerged and ongoing efforts are still directed toward improvement of diagnostic techniques, sensitivities, and specificities, especially in the setting of the early stages of the disease.4 We report a case of a patient with sustained ventricular tachycardia (VT) originating from the right ventricular outflow tract (RVOT) in whom electroanatomical mapping (EAM) was the only technique able to identify subtle myocardial structural abnormalities, leading to the hypothesis of ARVC, subsequently confirmed by genetic testing, whereas all the previous established diagnostic tools to identify criteria failed to diagnose the disease.
Case report
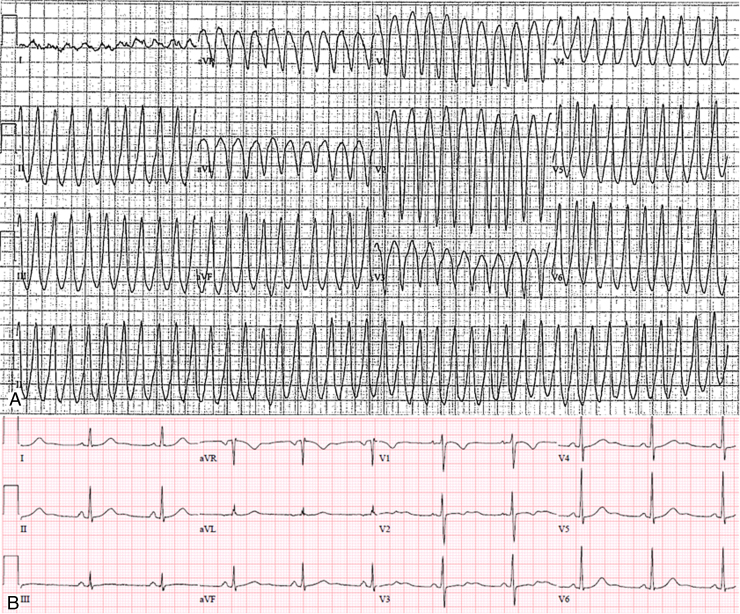
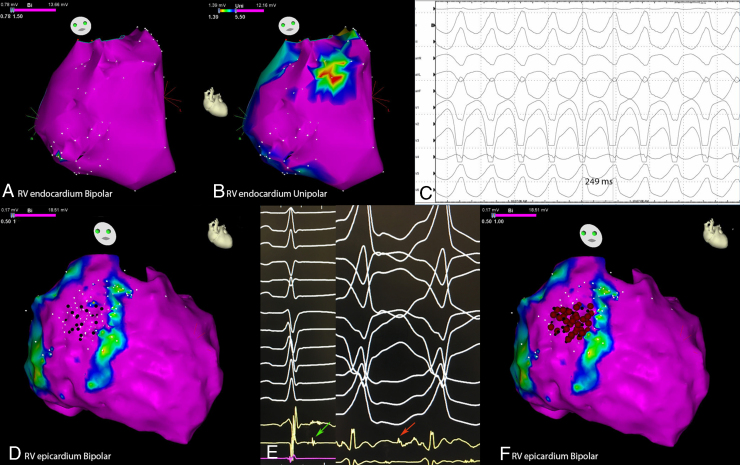
A 50-year-old woman with a family history of unspecified cardiomyopathy in a second-degree relative, and no other known cardiovascular risk factors, presented to the emergency room for sudden onset of palpitations associated with shortness of breath that occurred during mild physical activity. Surface 12-lead electrocardiogram (ECG) showed a monomorphic VT at 245 beats per minute with a left bundle branch block morphology, V4 transition, and left inferior axis (Figure 1A), consistent with an RVOT origin, which was effectively treated with external cardioversion. Sinus rhythm 12-lead ECG was normal (Figure 1B). Transthoracic echocardiography was unremarkable and coronary artery disease was ruled out by coronary angiography. A cardiac magnetic resonance imaging (cMRI) was performed showing normal biventricular thickness, volumes, and function. T1-weighted, T2-weighted, and contrast-enhanced imaging did not demonstrate any abnormalities. The patient refused treatment with antiarrhythmic drugs and underwent electrophysiology study and ablation for presumed “idiopathic VT.” A right ventricle (RV) endocardial bipolar voltage was created and showed normal voltage (>1.5 mV).5 However, a localized area of endocardial unipolar voltage abnormality (≤5.5 mV)6 was noted in the RVOT free wall, suggestive of a mid-myocardial/subepicardial disease process (Figure 2A and B). Programmed ventricular stimulation induced the clinical VT (Figure 2C). Entrainment could not be performed, as the VT would terminate with any pacing attempt. Endocardial activation mapping was earliest at the RVOT free wall; however, it was diffuse and not pre-QRS. At this stage, a decision for an epicardial approach was made owing to the high suspicion of an epicardial origin. An abnormal epicardial area with late potentials and fractionated signals was seen in the epicardium opposite the area of abnormal endocardial unipolar signals (Figure 2D). The overall voltage was normal. Catheter manipulation during mapping in this area induced clinical VT and mid-diastolic potentials were recorded. This area was targeted with radiofrequency ablation, which resulted in early termination of the VT (Figure 2E and F). Additional radiofrequency lesions were then applied in the adjacent area, eliminating the late potentials and fractionated signals (Figure 2E and F). After epicardial ablation, VT could no longer be induced with programmed electrical stimulation. Given the evidence of sustained VT in the setting of RV epicardial scar and unspecified familial history of cardiomyopathy, we decided to proceed with genetic testing for desmosomal gene abnormalities, looking for a major criterion because the patient fulfilled only 1 minor criterion (sustained VT with left bundle branch block morphology and inferior axis) for the diagnosis of ARVC according to the 2010 Task Force criteria.3 Genetic testing revealed a heterozygous pathogenic variant in the plakophilin-2 gene (variant p.Arg355Ter; c.1063C>T), leading to a definite diagnosis of ARVC. The genetic abnormality was also found in her brother, who has not experienced any arrhythmias and does not fit diagnostic criteria.7 After discussion with the patient, an implantation of a subcutaneous defibrillator was undertaken.
Figure 1.
A: Twelve-lead electrocardiogram (ECG) of the clinical ventricular tachycardia showing a left bundle branch block morphology, left inferior axis, and V4 precordial transition consistent with a right ventricular outflow tract/anterior right ventricular free wall origin. The wideness of the QRS (>140 ms) and the slow upstroke of the R wave (pseudo-delta wave), particularly evident in the inferior leads and in the left precordial leads, suggest a possible epicardial origin. B: The postcardioversion sinus rhythm 12-lead ECG shows only minor repolarization abnormalities (inverted T wave in V1 and biphasic T wave in V2) that fail to meet classic T wave changes that help establish the diagnosis of ARVC.
Figure 2.
Electroanatomical mapping of the right ventricle showing A: a complete normal endocardial bipolar voltage (modified right anterior oblique view) and B: a small area of endocardial unipolar abnormality, consistent with mid-myocardial/subepicardial scar, on the anterior free wall (same view). C: A sustained ventricular tachycardia (VT) with 250 ms cycle length, matching the clinical VT, was induced with programmed ventricular stimulation. D: Epicardial mapping (same view) showed bipolar low-voltage areas (color range from blue to red) consistent with epicardial fat distribution; no bipolar abnormality was seen over the endocardial unipolar abnormality, but in this area late potentials during sinus rhythm (D:black dots and E:green arrow) and mid-diastolic potentials during VT (E:red arrow) were mapped and effectively targeted with radiofrequency (RF) with early VT termination and noninducibility at the consequent programmed ventricular stimulation. F: The final RF lesion set is shown.
Discussion
Diagnosis of ARVC is based on a well-defined pool of criteria that sought to identify the genetic and structural abnormalities consequent to the disease with different electrocardiographic and imaging modalities. The diagnosis is documented whenever 2 major, 1 major and 2 minor, or 4 minor criteria are present.2, 3 Nevertheless, diagnosis of ARVC is still challenging owing to the suboptimal sensitivity and specificity of the described criteria, especially in the setting of early-stage disease, in which myocardial abnormalities can be subtle and limited to the epicardium and may remain undetected by currently available noninvasive diagnostic techniques, including echocardiography and cMRI.8, 9 There is a general agreement about cMRI being the gold standard for myocardial scar characterization, but in the setting of VT of RV origin, MRI has been reported to underestimate the presence of scar compared with EAM.10, 11 Moreover, the small amount of scar limited to the epicardial layer may explain the inability of MRI to identify the presence of such a subtle but well-defined substrate.
EAM may contribute to the diagnosis of ARVC, demonstrating either low-voltage areas or the presence of fractionated, split, and late potentials compatible with scar and slow conduction pathways able to sustain reentrant arrhythmias.12, 13, 14, 15 However, because the disease process is known to start from the epicardium and extend toward the endocardium, the substrate may be limited to the epicardial surface and the endocardial bipolar voltage characteristics may be normal. However, it would be too aggressive to perform epicardial voltage mapping for diagnostic purposes. It has been shown that endocardial unipolar voltage could predict the abnormalities in the epicardium and, as such, could be used as an effective tool for predicting epicardial abnormalities, as illustrated by this case, and guide clinicians to selectively undertake epicardial mapping.6
According to the results of recent observations, EAM allows the identification of otherwise unrecognized myocardial structural abnormalities in a significant proportion of patients with apparently idiopathic ventricular arrhythmias.10,15 Distinguishing truly idiopathic VTs from those related to undetected cardiomyopathies is of critical importance for risk stratification and management. Although patients with idiopathic VTs have an excellent prognosis, optimal treatment of the underlying heart disease and sudden cardiac death prevention is required among patients with VTs associated with a cardiomyopathy, particularly one with strong genetic linkage and manifest sustained arrhythmias.
The intensity of investigations required to exclude structural heart disease in patients with idiopathic ventricular arrhythmias is still uncertain. EAM should be considered in the diagnostic work-up of patients with sustained VT and negative routine diagnostic work-up to allow the early identification of subtle structural myocardial abnormalities, which may otherwise remain unrecognized. However, this should be done in a stepwise manner, and an invasive mapping should only be performed when noninvasive results are inconclusive and the diagnostic dilemma remains. Of note, EAM, when undertaken, should be performed carefully, ensuring good tissue contact aided by force sensing information or direct visualization with intracardiac echocardiogram. This case report highlights the utility of EAM as a possible diagnostic tool. We believe this is of particular necessity when VT originated from free wall RVOT sites and peritricuspid valvular sites.
Conclusion
The diagnosis of ARVC is challenging and its prevalence is likely to be underestimated. As in this case, it can often be missed by both echocardiography and cMRI in its early stages. Epicardial mapping can be challenging and may not be available in some centers. However, this case report highlights the utility of endocardial and epicardial voltage mapping in providing valuable information to establish the diagnosis and successful treatment of arrhythmia—in particular, in patients without classic sinus rhythm ECG findings and morphologic changes identified on echo and cMRI. Although we believe that successful VT ablation may be adequate to achieve suppression from VT, we still implant implantable cardioverter-defibrillators because identification of underlying ARVC could be a risk for recurrent arrhythmias and sudden cardiac death.
Footnotes
Funding: Richard T. and Angela Clark Innovation Fund in Cardiac Electrophysiology.
References
- 1.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. doi: 10.1093/eurheartj/ehq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKenna W.J., Thiene G., Nava A., Fontaliran F., Blomstrom-Lundqvist C., Fontaine G., Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Task Force of the Working Group Myocardial and Pericardial Disease of the European Society of Cardiology and of the Scientific Council on Cardiomyopathies of the International Society and Federation of Cardiology. Br Heart J. 1994;71:215–218. doi: 10.1136/hrt.71.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marcus F.I., McKenna W.J., Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vermes E., Strohm O., Otmani A., Childs H., Duff H., Friedrich M.G. Impact of the revision of arrhythmogenic right ventricular cardiomyopathy/dysplasia task force criteria on its prevalence by CMR criteria. JACC Cardiovasc Imaging. 2011;4:282–287. doi: 10.1016/j.jcmg.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Marchlinski F.E., Callans D.J., Gottlieb C.D., Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–1296. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 6.Polin G.M., Haqqani H., Tzou W., Hutchinson M.D., Garcia F.C., Callans D.J., Zado E.S., Marchlinski F.E. Endocardial unipolar voltage mapping to identify epicardial substrate in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Heart Rhythm. 2011;8:76–83. doi: 10.1016/j.hrthm.2010.09.088. [DOI] [PubMed] [Google Scholar]
- 7.Lazzarini E., Jongbloed J.D.H., Pilichou K., Thiene G., Basso C., Bikker H., Charbon B., Swertz M., van Tintelen J.P., van der Zwaag P.A. The ARVD/C genetic variants database: 2014 update. Hum Mutat. 2015;36:403–410. doi: 10.1002/humu.22765. [DOI] [PubMed] [Google Scholar]
- 8.Basso C., Thiene G., Corrado D., Angelini A., Nava A., Valente M. Arrhythmogenic right ventricular cardiomyopathy. Dysplasia, dystrophy, or myocarditis? Circulation. 1996;94:983–991. doi: 10.1161/01.cir.94.5.983. [DOI] [PubMed] [Google Scholar]
- 9.Corrado D., Basso C., Thiene G., McKenna W.J., Davies M.J., Fontaliran F., Nava A., Silvestri F., Blomstrom-Lundqvist C., Wlodarska E.K., Fontaine G., Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–1520. doi: 10.1016/s0735-1097(97)00332-x. [DOI] [PubMed] [Google Scholar]
- 10.Corrado D., Basso C., Leoni L. Three-dimensional electroanatomical voltage mapping and histologic evaluation of myocardial substrate in right ventricular outflow tract tachycardia. J Am Coll Cardiol. 2008;51:731–739. doi: 10.1016/j.jacc.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Marra M.P., Leoni L., Bauce B. Imaging study of ventricular scar in arrhythmogenic right ventricular cardiomyopathy comparison of 3D standard electroanatomical voltage mapping and contrast-enhanced cardiac magnetic resonance. Circ Arrhythm Electrophysiol. 2012;5:91–100. doi: 10.1161/CIRCEP.111.964635. [DOI] [PubMed] [Google Scholar]
- 12.Corrado D., Basso C., Leoni L. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2005;111:3042–3050. doi: 10.1161/CIRCULATIONAHA.104.486977. [DOI] [PubMed] [Google Scholar]
- 13.Boulos M., Lashevsky I., Reisner S., Gepstein L. Electroanatomic mapping of arrhythmogenic right ventricular dysplasia. J Am Coll Cardiol. 2001;38:2020–2027. doi: 10.1016/s0735-1097(01)01625-4. [DOI] [PubMed] [Google Scholar]
- 14.Garcia F.C., Bazan V., Zado E.S., Ren J.-F., Marchlinski F.E. Epicardial substrate and outcome with epicardial ablation of ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2009;120:366–375. doi: 10.1161/CIRCULATIONAHA.108.834903. [DOI] [PubMed] [Google Scholar]
- 15.Dello Russo A., Pieroni M., Santangeli P. Concealed cardiomyopathies in competitive athletes with ventricular arrhythmias and an apparently normal heart: role of cardiac electroanatomical mapping and biopsy. Heart Rhythm. 2011;8:1915–1922. doi: 10.1016/j.hrthm.2011.07.021. [DOI] [PubMed] [Google Scholar]