KEY TEACHING POINTS
|
Introduction
The subcutaneous implantable cardioverter-defibrillator (ICD) is becoming more widespread.1 Approved by the Food and Drug Administration for use in the United Stated in 2012, the subcutaneous ICD is now being implanted for primary and secondary prevention of sudden cardiac death among individuals meeting conventional ICD implantation criteria but who do not have an indication for cardiac resynchronization therapy or permanent bradycardia pacing, a preexisting unipolar pacemaker, or recurrent ventricular tachycardia that responds to antitachycardia pacing.2, 3, 4 Although it is becoming more evident that the subcutaneous ICD is particularly desirable in patients with venous access issues like those with end-stage renal disease on hemodialysis and in patients at an increased risk of infection, more data on this therapy are needed, especially in relation to long-term outcomes and how those outcomes compare with those of transvenous ICDs.4, 5 Among the clinically important outcomes are inappropriate ICD shocks. In the pivotal randomized clinical trials of the transvenous ICD, the rate of inappropriate shocks was up to 25%.6, 7, 8, 9 This rate is concerning, given the mounting evidence that shocks—both appropriate and inappropriate—are associated with increased mortality and worse quality of life.9, 10, 11 The rate of inappropriate shocks from a subcutaneous ICD is up to 13%, and the most common causes include oversensing as well supraventricular tachycardia.5, 12, 13
Case report
The patient was a 58-year-old man with nonischemic cardiomyopathy who received a primary prevention ICD in 2005 that was replaced in 2012 for low battery. On a few occasions, the patient received ICD therapies for ventricular tachycardia. During follow-up, the patient developed permanent atrial fibrillation for which he was on chronic anticoagulation with warfarin. His other cardiac medication was carvedilol (3.125 mg twice a day). In the setting of diabetes and hypertension, the patient developed end-stage renal disease, for which he had to be started on hemodialysis, and eventually had persistent methicillin-sensitive Staphylococcus aureus bacteremia with sepsis, for which his transvenous ICD had to be removed.
When the patient was screened for a subcutaneous ICD in July 2014, he was determined to be a good candidate for one by passing the screening in primary and secondary, but not alternate, sensing configurations. Therefore, he underwent subcutaneous ICD insertion. He specifically received a Boston Scientific SQ-RX generator with a BSCI CRM electrode, Q-TRAK. The position of the lead and the can was deemed to be appropriate and defibrillation threshold testing during the implantation procedure showed prompt detection of ventricular fibrillation with no evidence of undersensing, successful defibrillation with a 65-joule shock, and a shock impedance of 40 ohms. The patient was in sinus rhythm throughout his procedure, including at the time of defibrillation threshold testing. His procedure took 2 hours and was largely uneventful. Final programmed parameters were as follows: the shock-only zone 220 beats per minute (bpm)/272.7 ms 80.0 J × 5 and the conditional zone 200 bpm/300 ms 80.0 J × 5 with primary sensing configuration.
The patient was seen in follow-up shortly after the implantation, and his device was deemed to be functioning normally with no evidence of undersensing or oversensing and no need to make any changes to the programming of his device.
The patient was in his usual state of health until April 30, 2015, when he awoke to find his hemodialysis catheter, which was in his right subclavian vein, completely displaced. Thus, he presented to our emergency department, where a new hemodialysis catheter was placed in the same vein. He went home and did fine until about 3 AM on May 2, 2015, when as he was turning to his left side, he received an ICD shock. There were no preceding symptoms. He presented to our emergency department where his electrocardiogram demonstrated atrial fibrillation with a narrow QRS complex. He was asymptomatic at the time and thereafter. Interrogation of his device demonstrated undersensing of the R waves with resultant oversensing of atrial fibrillation waves with a resultant inappropriate shock (Figure 1A and B). While he was in the emergency department, the rates for the shock-only zone and the conditional zone were increased to 250 bpm. His anteroposterior and lateral chest radiographs showed good device and lead placement (Figure 2A and B). In-depth analyses of the vector electrograms in the supine and left lateral decubitus positions were done in the primary and secondary sensing configurations, and the same morphology changes seen by the device were observed in the same vectors on the surface electrocardiogram (Figure 3A and B). He showed evidence of oversensing on real-time electrograms in the emergency department and on the inpatient ward, but he had no more shocks. The vector electrograms were examined in the supine and sitting positions in the alternate sensing configuration; however, the R waves were found to be too small to be processed by the device. During his hospital stay, the patient had no bundle branch block or interventricular conduction delay recorded on continuous telemetry. Repeated interrogation of his device in both supine and left lateral decubitus positions on May 3 and May 4 showed a decrease in the R-wave amplitude; however, no further oversensing of atrial fibrillation waves occurred. On the day of discharge, the following changes were made to his device settings: the shock-only zone was reduced from 250 bpm to 230 bpm and the conditional zone was reduced from 250 bpm to 200 bpm. With the suspicion that his R-wave attenuation may be transient and positional, the patient was advised to avoid lying on his left side until his next follow-up appointment in 4 weeks and while awaiting further discussion with the engineers at Boston Scientific. The patient returned to our clinic within about a week with another ICD shock that occurred when the patient again had lain on his left side. The electrograms were identical to the ones downloaded previously. The patient was again advised to avoid lying on his left side. He has abided by this recommendation and has not had any further shocks.
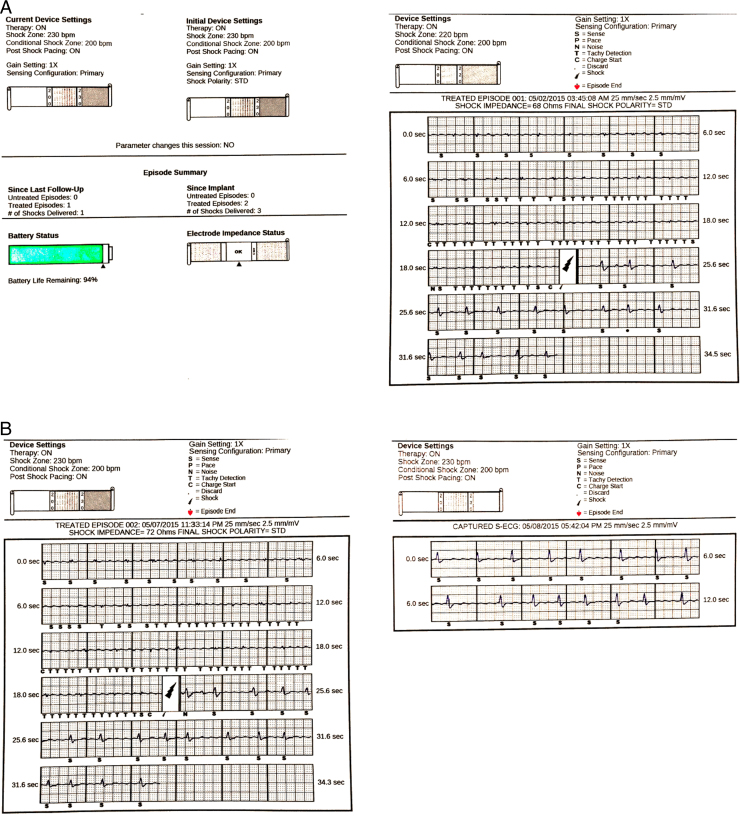
Figure 1.
A: Device settings and electrogram of inappropriate implantable cardioverter-defibrillator (ICD) shock. B: A zoomed-in image of electrogram that shows R-wave attenuation with undersensing and resultant oversensing of atrial fibrillation waves and inappropriate ICD shock.
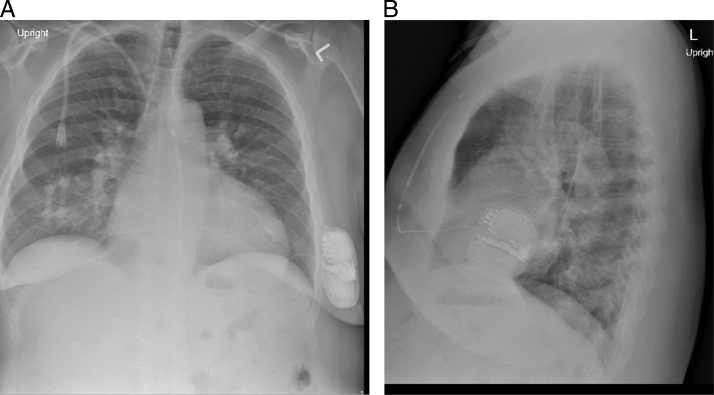
Figure 2.
A: Patient’s posteroanterior chest radiograph. B: Patient’s lateral chest radiograph.
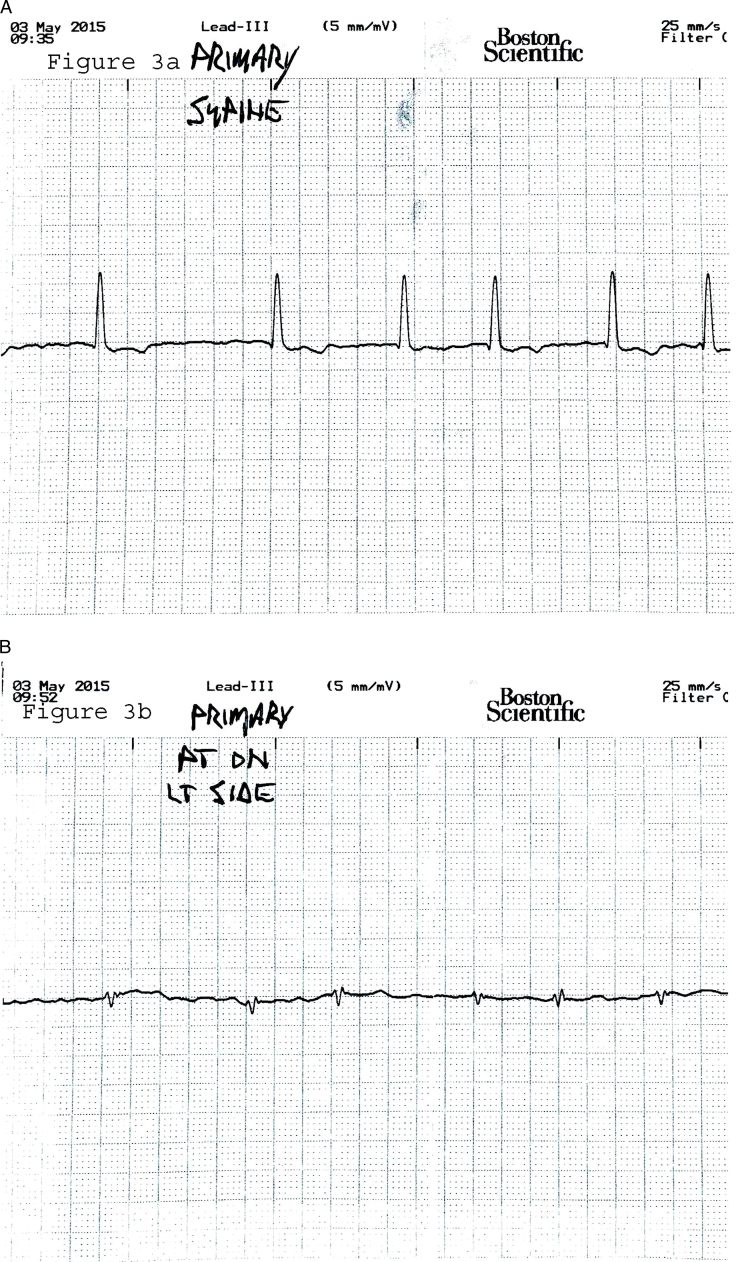
Figure 3.
In-depth analyses of the vector electrograms and posture (A: electrocardiogram [ECG] in supine position; B: ECG in left decubitus position) in the primary sensing configuration demonstrated that the morphology shift seen by the subcutaneous implantable cardioverter-defibrillator is consistent with what is seen in the same vector on the surface ECG.
Discussion
In the available literature, the rate of inappropriate shocks from subcutaneous ICDs has been reported to be up to 13%.5, 12, 13 However, the somewhat small number of patients included in published studies and the relatively short follow-up duration call for more data on this issue.
To our knowledge, this paper is the first to report on inappropriate shocks in a patient with a subcutaneous ICD resulting from positional attenuation of the R waves that activated the algorithm to increase the amplitude of the cardiac signals, resulting in oversensing of atrial fibrillation waves. These atrial fibrillation waves were interpreted by the device as ventricular fibrillation and resulted in an inappropriate shock. One might wonder if more extensive testing at baseline or during implantation might have revealed this issue. This, however, is unlikely, as the patient did very well for about 10 months, during which follow-up interrogations showed no evidence of undersensing of R waves and/or oversensing of atrial fibrillation waves. The timing of the inappropriate shocks raises a question about whether the replacement of the patient’s hemodialysis catheter may have led to R-wave attenuation; however, the mechanism by which this could have happened is uncertain. Subcutaneous edema could have been a possible mechanism. Other potential causes that we ruled out are device/lead dislodgment or migration while the patient is on his left side (a chest radiograph in this position showed no device migration), pleural or pericardial effusion (none present on chest radiograph or echocardiogram), and presence of hematoma (patient had no clinical evidence of that).
An important question is whether periodic defibrillation threshold testing during follow-up may be helpful in revealing inappropriate sensing. However, this certainly has not been proven, and the practicality and impact of this approach are largely questionable. Another vital consideration is whether the detection algorithm in the device itself could be optimized to prevent such events from occurring. To that end, we contacted the subcutaneous ICD engineers at Boston Scientific to make them aware of this case.
Conclusion
Inappropriate ICD shocks have a negative impact on patient outcomes. As a result, every attempt should be made to reduce the risk of inappropriate shocks. Better understanding of reasons for these shocks is important. To that end, clinicians should be aware of the possibility of R-wave attenuation and oversensing of atrial fibrillation as a cause of inappropriate shocks in subcutaneous ICDs that might be avoided by lifestyle modifications until more information emerges on how and whether further optimization of detection algorithms may result in a lower risk of inappropriate shocks.
References
- 1.Friedman D.J., Parzynski C., Curtis J., Varosy P., Russo A., Prutkin J., Patton K., Mithani A., Al-Khatib S.M. Early use of the subcutaneous implantable cardioverter defibrillator in the United States: a report from the National Cardiovascular Data Registry [abstract] J Am Coll Cardiol. 2016;67:685. [Google Scholar]
- 2.FDA approves first subcutaneous heart defibrillator. 2012. Available at: 〈http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm321755.htm〉.Accessed June 30, 2015.
- 3.Subcutaneous implantable defibrillator (s-icd) system - p110042. 2014. Available at: 〈http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/Recently-ApprovedDevices/ucm326541.htm〉.Accessed June 30, 2015.
- 4.Bardy G.H., Smith W.M., Hood M.A. An entirely subcutaneous implantable cardioverter-defibrillator. N Engl J Med. 2010;363:36–44. doi: 10.1056/NEJMoa0909545. [DOI] [PubMed] [Google Scholar]
- 5.Weiss R., Knight B.P., Gold M.R., Leon A.R., Herre J.M., Hood M., Rashtian M., Kremers M., Crozier I., Lee K.L., Smith W., Burke M.C. Safety and efficacy of a totally subcutaneous implantable-cardioverter defibrillator. Circulation. 2013;128:944–953. doi: 10.1161/CIRCULATIONAHA.113.003042. [DOI] [PubMed] [Google Scholar]
- 6.Moss A.J., Zareba W., Hall W.J., Klein H., Wilber D.J., Cannom D.S., Daubert J.P., Higgins S.L., Brown M.W., Andrews M.L. for the Multi-center Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 7.Bardy G.H., Lee K.L., Mark D.B. for the Sudden Cardiac Death in HF Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive HF. N Engl J Med. 2005;352:225–237. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 8.Exner D.V., Pinski S.L., Wyse D.G., Renfroe E.G., Follmann D., Gold M., Beckman K.J., Coromilas J., Lancaster S., Hallstrom A.P. AVID Investigators. Antiarrhythmics versus implantable defibrillators. Electrical storm presages nonsudden death: the antiarrhythmics versus implantable defibrillators (AVID) trial. Circulation. 2001;103:2066–2071. doi: 10.1161/01.cir.103.16.2066. [DOI] [PubMed] [Google Scholar]
- 9.Poole J.E., Johnson G.W., Hellkamp A.S. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sweeney M.O., Sherfesee L., DeGroot P.J., Wathen M.S., Wilkoff B.L. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter-defibrillator patients. Heart Rhythm. 2010;7:353–360. doi: 10.1016/j.hrthm.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Noyes K., Corona E., Veazie P., Dick A.W., Zhao H., Moss A.J. Examination of the effect of implantable cardioverter-defibrillators on health-related quality of life: based on results from the Multicenter Automatic Defibrillator Trial-II. Am J Cardiovasc Drugs. 2009;9(6):393–400. doi: 10.2165/11317980-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke M.C., Gold M.R., Knight B.P. Safety and efficacy of the totally subcutaneous implantable defibrillator 2-year results from a pooled analysis of the IDE study and EFFORTLESS Registry. J Am Coll Cardiol. 2015;65:1605–1615. doi: 10.1016/j.jacc.2015.02.047. [DOI] [PubMed] [Google Scholar]
- 13.Olde Nordkamp L.R., Brouwer T.F., Barr C., Theuns D.A., Boersma L.V., Johansen J.B., Neuzil P., Wilde A.A., Carter N., Husby M., Lambiase P.D., Knops R.E. Inappropriate shocks in the subcutaneous ICD: incidence, predictors and management. Int J Cardiol. 2015;195:126–133. doi: 10.1016/j.ijcard.2015.05.135. [DOI] [PubMed] [Google Scholar]